Abstract
Background
Adenosine infusion after pulmonary vein isolation (PVI) with radiofrequency energy reveals dormant muscular sleeves and predicts atrial fibrillation (AF) recurrence. The aim of our study was to determine whether adenosine could reveal dormant PV sleeves after cryoballoon isolation and study its effect on long-term recurrence of AF.
Methods
Patients with paroxysmal AF underwent cryoballoon PVI. After PVI, adenosine 25 mg was infused to test for dormant muscular sleeves in each vein. If reconnection under adenosine was shown, further cryoballoon ablation was performed until no more reconnection occurred. Follow-up was performed with ECG, 24-h Holter recording, and a symptom questionnaire at three monthly intervals. Transtelephonic Holter monitoring was performed for 1 month before and 3 months after PVI. Patients who underwent cryoballoon PVI without adenosine administration were used as controls for comparison.
Results
In the study group (n = 34, 24 males), adenosine revealed dormant sleeves in 9/132 (8 %) veins, and 7/34 (21 %) patients. All but one vein was further treated until the dormant sleeves were isolated. During a mean follow-up of 520 ± 147 days, 23/34 (68 %) patients were free of AF without antiarrhythmic drugs (AADs). In the control group (n = 65, 46 males), 29/65 (46 %) were free of AF without AADs. There were significantly less AF recurrences in the study group (p = 0.04).
Conclusions
Adenosine administration after cryoballoon PVI reveals dormant muscular sleeves in 21 % of patients. Clinical follow-up shows that adenosine testing is effective in reducing AF recurrence after cryoballoon ablation.
Keywords: Atrial fibrillation, Ablation, Pulmonary veins, Cryoballoon, Adenosine
Introduction
Pulmonary vein isolation (PVI) has become the cornerstone of invasive treatment of atrial fibrillation (AF). Previous studies have shown that there is a high percentage of electrical reconduction from the atria to the pulmonary veins after circumferential ablation using radiofrequency current (RF) and that resumption of conduction to previously ablated pulmonary veins is responsible for recurrence of AF [1, 2]. It has also been proven that adenosine infusion after PVI reveals dormant muscular sleeves, which are a predictor of late reconduction [3].
Adenosine activates adenosine-sensitive potassium channels, restoring the resting potential to its normal value in myocytes with reversible thermal injury [4, 5]. Cryoballoon ablation has been proven to be effective in pulmonary vein isolation [6, 7], but reconduction to the pulmonary veins is invariably present (100 %) in patients who develop recurrences of AF [8]. The scope of our study was to determine whether adenosine could reveal dormant PV sleeves after cryoballoon isolation and gain insight into the long-term outcome.
Methods
Study population
Patients referred to our centre for ablation of symptomatic paroxysmal AF resistant to antiarrhythmic drugs (AADs), at two or more occasions, were included. Patients with obstructive pulmonary disease and severe valvular disease were excluded. Informed consent was obtained in all patients.
A control population with similar demographic characteristics was selected from patients who were ablated for AF with the cryoballoon during the same period as the study population. They were used to compare the freedom of AF after long-term follow-up. For comparison the groups were labelled ‘adenosine’ and ‘no adenosine’.
Pulmonary vein isolation procedure
The procedure was performed under conscious sedation or general anaesthesia according to patient preference. Both femoral veins were used for venous access. A 10 Fr intracardiac echocardiography (ICE) catheter (Flexview, EP Med Systems, New Jersey, USA) was introduced through the left femoral vein and positioned in the right atrium. A decapolar catheter was placed in the coronary sinus. A single transseptal puncture was performed using a transseptal needle (BKR1, St Jude Medical, Minnesota, USA) and an 8F sheath (Fastcath SL1, St Jude Medical, Minnesota, USA), guided by both ICE and fluoroscopy. A 6 Fr angiocatheter (Mach 1 MP2, Boston Scientific, Massachusetts, USA) was used to perform a selective angiography of each pulmonary vein to locate the ostium. A circular mapping catheter was advanced and positioned in the antrum of each pulmonary vein to record the presence of PV potentials. The sheath was exchanged for a 14 Fr steerable sheath (Flexcath, Medtronic, Minneapolis, USA), through which a 28 mm, 12 Fr cryoballoon catheter (Arctic Front, Medtronic, Minneapolis, USA) was inserted, and positioned over an exchange wire to occlude the ostium of each PV. Cryoablation was performed for 5 min per application. A minimum of two applications per vein were given. Before targeting the right superior pulmonary vein (RSPV), a quadripolar catheter was positioned in the superior caval vein for continuous phrenic nerve stimulation during cryoapplication. At loss of capture, ablation was instantaneously terminated. After targeting all the PVs, the cryocatheter was exchanged for a circular mapping catheter to register any remaining electrical activity. If this registration showed persistence of PV potentials, the cryoballoon was introduced again and an additional two applications of 5 min were given. If PV potentials remained present after this second ablation attempt, a conventional cryocatheter (Freezor Max, Medtronic, Minneapolis, USA) was used to perform a segmental isolation through the same transseptal puncture. After isolation of all the veins, the electrical activity was registered at the ostia during bolus administration of 25 mg of adenosine. If reconduction to the pulmonary vein was confirmed during adenosine administration, additional cryoablation was performed until this was no longer the case. Throughout the procedure, the activated clotting time was monitored every 30 min and maintained between 275 and 300 s.
The control population was ablated using the same method, except for the administration of adenosine.
Rhythm evaluation
Patients were instructed to submit daily rhythm strips, and additional strips when symptomatic, for 1 month before the ablation and 3 months after, with a transtelephonic Holter monitoring system. These recordings were used to calculate the AF burden, defined as the ratio of transmitted strips revealing AF and the number of days the patient was in possession of the recording device. Twenty-four hour Holter monitoring was performed before the ablation and repeated at three monthly intervals after ablation. The patients were evaluated at three monthly intervals by a cardiologist at the outpatient clinic, at which time an electrocardiogram (ECG) was performed, until at least 1 year after ablation. Unsolicited ECG tracings, performed for any reason outside the routine follow-up, were also taken into consideration. No blanking period for AF recurrence was applied.
A questionnaire was used to score palpitation symptoms for frequency and duration. Patients were asked to score this at baseline and at each outpatient visit during the follow-up. Frequency categories were subdivided into: none, daily, weekly, monthly, and yearly; duration categories into: none, minutes, hours, and days.
Drug management
The AAD regime was discontinued 5 days before the procedure and restarted the day after the procedure, until 3 months after ablation. If no recurrences were recorded during this period, the AADs were discontinued.
Oral anticoagulation (INR between 2.0 and 3.0) was stopped 3 days before the ablation. The day before the procedure a transoesophageal echocardiography was performed to exclude the presence of a left atrial thrombus. Oral anticoagulation was restarted the day after the procedure, until at least 6 months after ablation.
Statistical analysis
Continuous variables are expressed as mean ± SD if normally distributed, or otherwise by median and interquartile range. Continuous variables were analysed using Student’s t-test or the Mann–Whitney U test in case of non-normal distribution of data. Categorical data were summarised as frequency (percentage) and compared using a Chi-square test. A two-sided p value < 0.05 was used for declaring statistical significance. Analyses were performed with SPSS for Windows (version 16.0, SPSS Inc, Chicago, Illinois, USA).
Results
Demographics
Patients with drug-resistant, paroxysmal and symptomatic AF (n = 34, 24 males, 57 ± 12 years) were included in the study. The control population was a similar group of patients with drug-resistant, paroxysmal and symptomatic AF, ablated during the same period with the cryoballoon (n = 65, 46 males, 58 ± 9 years). Demographic data are represented in Table 1.
Table 1.
Demographic, procedure and follow-up data: comparison between the study group (adenosine) and the control group (no adenosine)
| Adenosine | No adenosine | p | |
|---|---|---|---|
| Demographic data | |||
| Male/Female (n) | 24/10 | 46/19 | NS |
| Age (years) | 57 ± 12 | 58 ± 9 | NS |
| LA diameter (mm) | 45 ± 7 | 42 ± 6 | 0.05 |
| Body mass index | 28 ± 5 | 26 ± 5 | NS |
| Years of AF (years) | 7 ± 5 | 7 ± 6 | NS |
| AF burden (%) | 12 ± 23 | 20 ± 21 | NS |
| Procedure data | |||
| Procedure time (min) | 202 ± 68 | 193 ± 57 | NS |
| Fluoroscopy time (min) | 41 ± 24 | 46 ± 23 | NS |
| Balloon applications (n) | 11 (9–13) | 9 (8–11) | 0.013 |
| Follow-up data | |||
| Follow-up (days) | 520 ± 147 | 539 ± 214 | NS |
| No AF, no AAD (n (%)) | 23 (68) | 29 (46) | 0.04 |
| AF recurrence (n (%)) | 11 (32) | 34 (54) | 0.04 |
| Reduced burden and/or AAD (n (%)) | 6 (18) | 16 (25) | NS |
| Re-intervention (n (%)) | 5 (14) | 18 (29) | NS |
AAD antiarrhythmic drugs; AF atrial fibrillation; LA left atrium
Adenosine revealing dormant conduction to the pulmonary veins: adenosine group
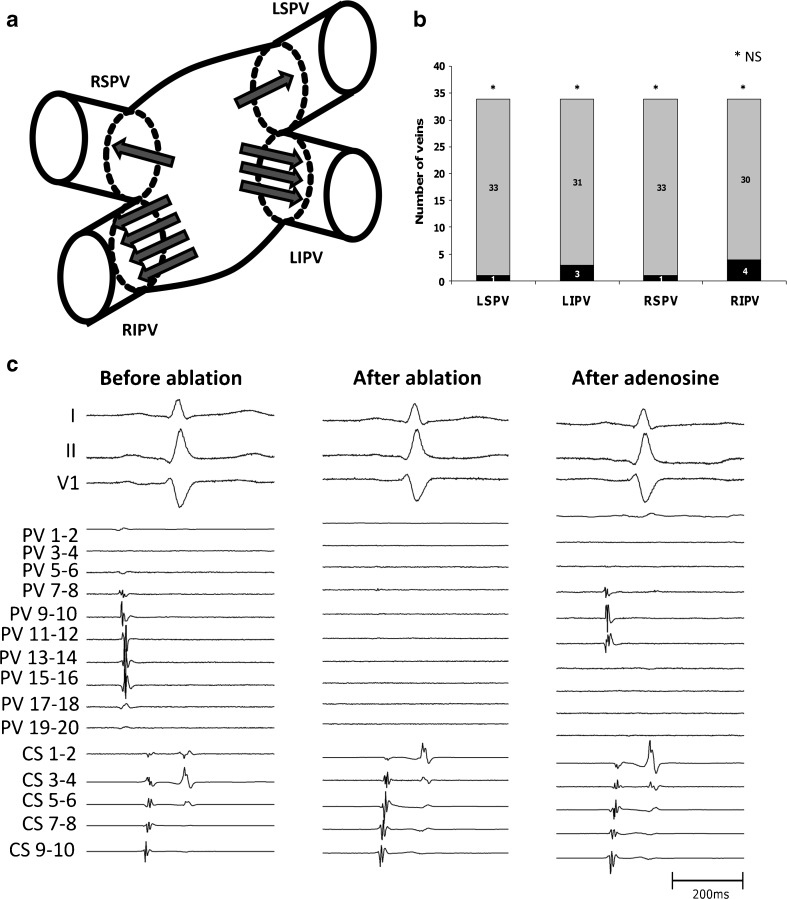
Administration of adenosine revealed dormant conduction to one or more PVs in seven patients (21 %) and nine veins (8 %): left superior pulmonary vein (LSPV) 1 (3 %); left inferior pulmonary vein (LIPV) 3 (9 %); right superior pulmonary vein (RSPV) 1 (3 %); and right inferior pulmonary vein (RIPV) 4 (12 %). These results are represented in Fig. 1a and b. The average dose of adenosine administered per vein was 27 ± 2 mg. There was no significant difference when considering reconduction in right versus left veins (NS), inferior versus superior veins and/or the RIPV versus other veins. All veins with dormant conduction were additionally ablated until adenosine could not reveal reconduction. The electrical activity revealed by adenosine in one vein (RIPV) could not be ablated by the operating physician within a reasonable timeframe, and the procedure was terminated before isolation under adenosine was achieved. These findings are summarised in Table 2.
Fig. 1.
Reconnection of dormant pulmonary vein sleeves under adenosine administration after cryoballoon ablation. A. Schematic representation of the absolute number of reconnecting veins at the respective ostium. Each arrow represents a reconnecting sleeve. B. Bar graph representing the ratio of reconnection under adenosine in each vein. C. An example of signals present in a right inferior pulmonary vein as measured with a 20-pole catheter at the ostium. Before ablation, electrical activity is present in dipoles PV7-8 to PV15-16. After ablation no more electrical signals are present. After adenosine administration, electrical activity reappears on dipoles PV7-8 to 11-12
Table 2.
Procedure characteristics in the adenosine group
| LSPV | LIPV | RSPV | RIPV | Total | |
|---|---|---|---|---|---|
| Balloon applications : median (range) | 3 (2–6) | 3 (2–7) | 2 (2–4) | 2 (1–6) | 11 (8–16) |
| Linear touch-up : n (%) | 5 (15) | 4 (12) | 2 (6) | 3 (9) | 14 (10) |
| Early reconduction adenosine: n (%) | 1 (3) | 3 (9) | 1 (3) | 4 (12) | 9 (8) |
LIPV left inferior pulmonary vein; LSPV left superior pulmonary vein; RIPV right inferior pulmonary vein; RSPV right superior pulmonary vein
Outcome of the ablation procedure: adenosine group
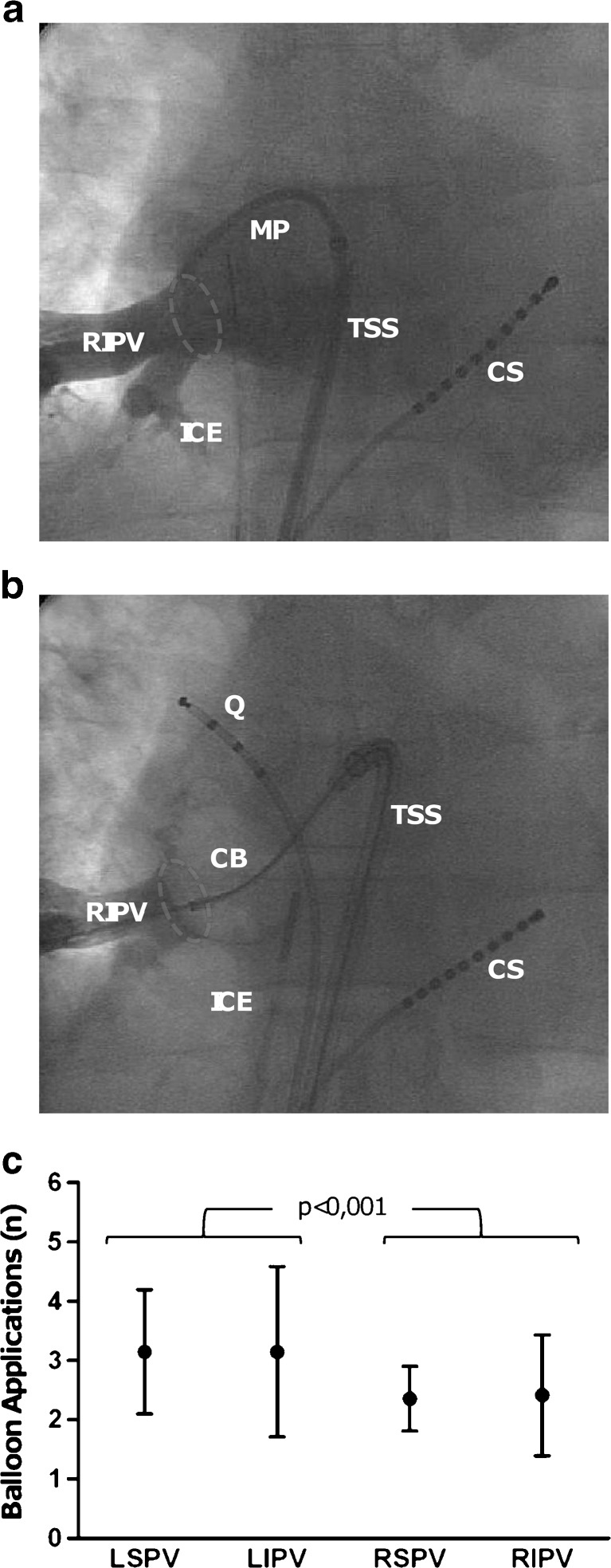
All study patients underwent successful pulmonary vein isolation with a median procedure time of 202 ± 68 min and a fluoroscopy time of 41 ± 24 min. General anaesthesia was given in 13 (38 %) patients, 21 (62 %) were consciously sedated. The median number of balloon applications was 11 [9–13], and the left-sided veins needed significantly more balloon applications than the right-sided veins to achieve isolation: 3 (2–7) versus 2 (1–6) (p < 0.001). An example of a cryoballoon occlusion of the right inferior pulmonary vein is shown in Fig. 2. The use of an additional linear cryocatheter was necessary in nine patients (26 %) and/or 14 veins (10 %); in one patient, however, the linear catheter was used due to failure of the balloon console. There were significantly more linear catheters used in left-sided than in right-sided veins to complete the circular lesions: 9 (13 %) versus 5 (7 %) (p = 0.01) (Table 2). Complete isolation of the four veins was achieved in all patients.
Fig. 2.
Cryoballoon pulmonary vein isolation. a Fluoroscopic image (anteroposterior projection) of a selective contrast injection in a right inferior pulmonary vein through a multipurpose catheter positioned at its ostium (circle) in the left atrium. Also visible are a decapolar catheter in the coronary sinus, and an intracardiac echocatheter in the right atrium. b Fluoroscopic image (anteroposterior projection) of an occlusion at the ostium of the right inferior pulmonary vein by a 28 mm cryoballoon catheter, with distal contrast injection. Also visible are a decapolar catheter in the coronary sinus, a quadripolar catheter in the superior caval vein (used for phrenic nerve pacing during ablation), and an intracardiac echocatheter in the right atrium. c Graph showing the average number of balloon applications necessary for isolation of the respective veins. CS coronary sinus catheter, CB inflated cryoballoon catheter, ICE intracardiac echography catheter, MP multipurpose angiography catheter, Q quadripolar catheter positioned for phrenic nerve capture in the superior caval vein, RIPV right inferior pulmonary vein, TSS transseptal sheath
Long-term outcome after cryoballoon ablation: adenosine group
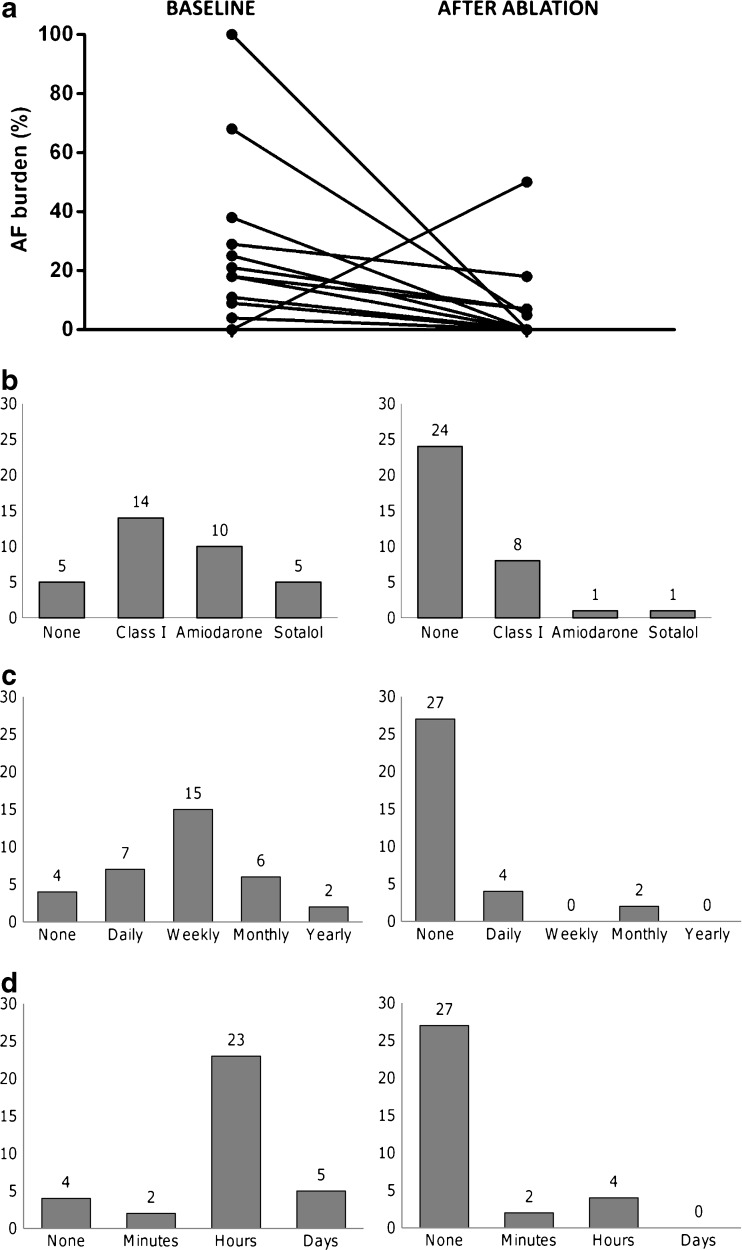
The follow-up period was 17 ± 5 months. All results are graphically depicted in Fig. 3. At the end of follow-up, 23 (68 %) patients were free of AF episodes, without AADs. The average AF burden of the entire group decreased from 12 % to 3 % (p = 0.01). Of the 11 patients who had a recurrence of AF, 6 (18 %) had a reduced AF burden of arrhythmia under the previously ineffective AAD regime, and were not reconsidered for reintervention (Fig. 3A shows paired data of AF burden before and after the procedure). The remaining five patients with recurrence of AF despite AAD (14 %) were planned for reintervention. The one patient in whom dormant reconduction under adenosine had not been eliminated at the end of the ablation procedure was also scheduled for reintervention. These results are summarised in Table 1. The median time until the first recurrence after ablation was 9 (2–84) days. As shown in Fig. 3a, one patient exhibited an artificial increase in burden due to poor compliance with transtelephonic Holter recording before the ablation (Fig. 3a).
Fig. 3.
One-year follow-up after cryoballoon pulmonary vein ablation for paroxysmal atrial fibrillation, with ablation of dormant pulmonary vein sleeves revealed by adenosine administration. a Paired graph showing the baseline atrial fibrillation burden (%), paired with the burden after ablation. b Bar graphs showing the antiarrhythmic drug use before and after ablation. The absolute number of patients is indicated above the respective bar. c Symptom frequency of atrial fibrillation related complaints, as scored by a questionnaire. The two graphs show the frequency before and after ablation. d Symptom duration of atrial fibrillation related complaints, as scored by a questionnaire. The two graphs show the duration before and after ablation
Antiarrhythmic drugs were used by 29 patients (85 %) at baseline: 14 (41 %) class I AAD, 10 (29 %) amiodarone, and 5 (15 %) sotalol. One year after ablation, only ten patients (29 %) were still on AAD: nine patients due to recurrence of AF, and one patient because of symptomatic supraventricular extrasystoles. Of the 24 (71 %) patients who were free of AAD, 22 (65 %) had no more AF, and 2 (6 %) had stopped the AAD because they had a drastic reduction in AF burden (Fig. 3b).
The baseline symptom score showed that the average patient had a symptom frequency of weekly complaints, with a duration of hours. One year after the procedure, the average patient had no more symptoms (Fig. 3c and d).
Comparison with the control population: adenosine vs. no adenosine group
The adenosine group had a significantly larger left atrial diameter compared with the no adenosine group: 45 ± 7 vs. 42 ± 6 (p = 0.05). There were no other significant differences between the groups at baseline. In both the adenosine and no adenosine groups, all patients were ablated until pulmonary vein isolation was achieved (NS). The number of balloon applications in the adenosine group was significantly higher than in the no adenosine group: 11 (9–13) vs. 9 (8–11) (p = 0.013). No difference in procedure and fluoroscopy times was observed (NS).
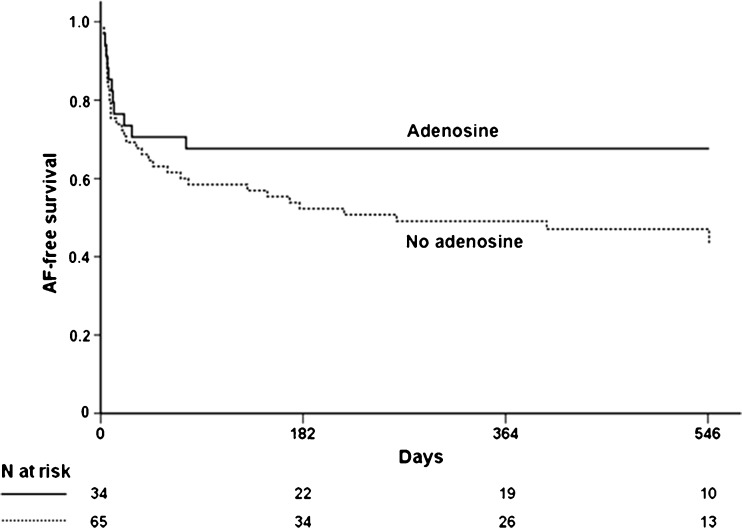
Both groups had a comparable follow-up period. At the end of the follow-up, the freedom of AF recurrence showed significantly less recurrence in the adenosine group: 68 % vs. 46 % (p = 0.04) (Table 1). Figure 4 shows a Kaplan-Meier graph of AF-free survival in both groups.
Fig. 4.
Kaplan-Meier graph representing the AF-free survival after pulmonary vein isolation without AAD in the adenosine vs. the no adenosine group
Adverse events
In the adenosine group, one patient experienced a right phrenic nerve paralysis after cryoballoon ablation of the RSPV: this resulted in slight dyspnoea which resolved spontaneously within 3 months. Another patient experienced transient worsening of preexisting migraine during the first 2 weeks after the ablation. An extensive neurological diagnostic work-up could not reveal a procedure-related cerebral event.
In the no adenosine group one patient experienced a pericardial tamponade after the procedure, which was successfully treated with a percutaneous drain. A second patient experienced an asymptomatic phrenic nerve paralysis that spontaneously resolved within 6 months.
No other adverse events were noted in the two groups.
Discussion
Our report shows that administration of adenosine after cryoballoon pulmonary vein isolation demonstrates reconduction to the PV sleeves in 9 out of 132 (8 %) of the veins, and is useful in 7 out of 34 patients (21 %). The 1-year follow-up after one procedure showed a freedom of AF without AAD of 68 % (23/34 patients). There is a significant reduction in AF recurrence during the long-term follow-up as compared with a control group, ablated without adenosine testing.
It has been proven that in pulmonary vein isolation by radiofrequency energy, transient conduction after administration of adenosine occurs in 25 % to 35 % of veins [3, 9]. Since resumption of electrical activity in the muscular sleeves seems to be one of the most important factors for recurrence of AF after isolation [1], it remains a challenge to achieve both continuous and permanent lesions during the first procedure. Ablation of dormant PV sleeves has proven to reduce recurrence of AF during follow-up [10–12]. Most of the currently available studies have been performed using radiofrequency energy. It is therefore unclear what the clinical implications are for cryothermal ablation. Our study is in accordance with a recent report about a comparable number of patients, also demonstrating a lower number of dormant PVs demonstrated with adenosine after cryoballoon PVI than one would expect in RF ablation [13]; our study in addition shows a clear clinical benefit of adenosine testing. In cryoballoon ablation, however, it has also been shown that reconduction is an important factor for recurrences of AF after PVI, with on average 3 PVs showing recovery during a second procedure [8]. Therefore, elimination of dormant PV sleeves could improve long-term results. Building on these findings, we designed our study to answer the question whether adenosine could have the same predictive value in cryoballoon ablation as it has in radiofrequency ablation.
The mechanism by which adenosine causes reconnection of apparently isolated PVs is by activating an outward potassium current through activation of a purginergic A1-membrane receptor [5]. Reversibly damaged myocytes show a higher resting potential, deactivating the depolarising voltage-dependent sodium channels and thus inhibiting fast depolarisation. By hyperpolarising the muscular PV cells that underwent reversible damage due to ablation, normal function of the sodium channels is restored, normalising the conduction properties and revealing viable excitable tissue [4]. A possible explanation for the difference in incidence of reconnection between radiofrequency and cryothermal PV isolation is the difference in the amount of reversible damage around the permanent lesion. Radiofrequency ablation causes a temperature-specific zone of reversible lesion around the ablation point [14–16]. Cryothermal energy has been proven to cause little or no surrounding reversible injury after ablation [17, 18]. This is probably caused by the fact that the cryomapping effect at temperatures around −30 °C is immediately reversible upon cessation of the application [19]. The substrate that is sensitive to adenosine would therefore be absent or present in small amounts, resulting in the lower incidence.
We report a freedom of AF without AAD after one procedure of 68 %, which is a significant increase in success compared with the 46 % in the control group without adenosine testing. Since adenosine testing (with subsequent ablation) is a proven method for increasing freedom of AF after radiofrequency ablation, it seems to prove that the same is true for cryoballoon ablation. There is a high discrepancy in published literature on recurrence of AF after cryoballoon ablation: success rates after a single procedure range from 49 % (8) to 74 % (6). Two major differences in follow-up method are apparent in published literature on cryoballoon ablation: the use of a blanking period, and the modalities of rhythm monitoring after ablation. In this study, no blanking period was employed since early AF recurrence is a proven predictor of late recurrence [8, 20]. HRS/EHRA/ECAS recommendations for follow-up after AF ablation still state that a blanking period of 3 months should be employed [21], but this is based on studies performed with radiofrequency energy, which has a delayed effect, rendering up to 60 % of patients free of AF during long-term follow-up after early recurrence [22]. It is hypothesised that either the thermal injury of radiofrequency ablation [23], or a transient autonomic imbalance [24] is responsible for this. Unlike RF, cryothermal ablation causes tissue injury with preservation of tissue architecture [25], so it remains unclear at present whether a blanking period should be adopted for cryoballoon PVI.
As a follow-up method our study combines daily transtelephonic ECG, 24-h Holter monitoring and a symptom-based questionnaire. Studies have shown that transtelephonic ECG and 7-day Holter have the same sensitivity for detecting AF episodes of about 70 % [26]. The addition of both 24-h Holter monitoring and a symptom-based questionnaire should increase the sensitivity and therefore decrease the reported long-term freedom of AF [27], compared with reports using less follow-up modalities.
Limitations
This study focused on demonstrating the potential of adenosine for revealing dormant pulmonary vein sleeves after cryoballoon ablation. No blinded randomisation was performed; instead a control population was used to assess the difference in outcome. No repeat procedures were performed in these patients to assess long-term durability of the pulmonary vein isolation, to prove the predictive value of reconnection of sleeves under adenosine for late reconduction.
Conclusion
Adenosine administration after cryoballoon pulmonary vein isolation reveals dormant connections from the left atrium to the PV in 21 % of patients. Additional ablation of dormant PVs gives a long-term freedom of AF in 68 % of patients after a single procedure. This is a significant increase compared with cryoballoon PVI without ablation of dormant PVs.
Acknowledgements
We would like to thank Yvette van Gestel for her assistance in preparing Figs. 2c and 3a.
Footnotes
Industry relations: There are no relationships with industry to be disclosed.
References
- 1.Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108(13):1599–1604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 2.Lemola K, Hall B, Cheung P, et al. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004;1(2):197–202. doi: 10.1016/j.hrthm.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 3.Arentz T, Macle L, Kalusche D, et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15(9):1041–1047. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 4.Datino T, Macle L, Comtois P, et al. Mechanisms by which adenosine reveals “dormant conduction” in pulmonary veins. Heart Rhythm. 2009;6(5):S297. [Google Scholar]
- 5.Freilich A, Tepper D. Adenosine and its cardiovascular effects. Am Heart J. 1992;123(5):1324–1328. doi: 10.1016/0002-8703(92)91040-8. [DOI] [PubMed] [Google Scholar]
- 6.Neumann T, Vogt J, Schumacher B, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52(4):273–278. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Belle Y, Janse P, Rivero-Ayerza MJ, et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28(18):2231–2237. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 8.Belle Y, Janse P, Theuns D, et al. One year follow-up after cryoballoon isolation of the pulmonary veins in patients with paroxysmal atrial fibrillation. Europace. 2008;10(11):1271–1276. doi: 10.1093/europace/eun218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tritto M, Ponti R, Salerno-Uriarte JA, et al. Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. Eur Heart J. 2004;25(23):2155–2163. doi: 10.1016/j.ehj.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Hachiya H, Hirao K, Takahashi A, et al. Clinical implications of reconnection between the left atrium and isolated pulmonary veins provoked by adenosine triphosphate after extensive encircling pulmonary vein isolation. J Cardiovasc Electrophysiol. 2007;18(4):392–398. doi: 10.1111/j.1540-8167.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 11.Jiang CY, Jiang RH, Matsuo S, et al. Early detection of pulmonary vein reconnection after isolation in patients with paroxysmal atrial fibrillation: a comparison of ATP-induction and reassessment at 30 minutes postisolation. J Cardiovasc Electrophysiol. 2009 Jul 28. [DOI] [PubMed]
- 12.Matsuo S, Yamane T, Date T, et al. Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. J Cardiovasc Electrophysiol. 2007;18(7):704–708. doi: 10.1111/j.1540-8167.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 13.Chierchia GB, Yazaki Y, Sorgente A, et al. Transient atriovenous reconnection induced by adenosine after successful pulmonary vein isolation with the cryothermal energy balloon. Europace. 2009 Oct 31. [DOI] [PubMed]
- 14.Nath S, Lynch C, 3rd, Whayne JG, et al. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation. 1993;88(4 Pt 1):1826–31. doi: 10.1161/01.CIR.88.4.1826. [DOI] [PubMed] [Google Scholar]
- 15.Wood MA, Fuller IA. Acute and chronic electrophysiologic changes surrounding radiofrequency lesions. J Cardiovasc Electrophysiol. 2002;13(1):56–61. doi: 10.1046/j.1540-8167.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, Fasciano RW, 2nd, Calkins H, et al. Sequential change in action potential of rabbit epicardium during and following radiofrequency ablation. J Cardiovasc Electrophysiol. 1999;10(9):1252–1261. doi: 10.1111/j.1540-8167.1999.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 17.Klein GJ, Harrison L, Ideker RF, et al. Reaction of the myocardium to cryosurgery: electrophysiology and arrhythmogenic potential. Circulation. 1979;59(2):364–372. doi: 10.1161/01.CIR.59.2.364. [DOI] [PubMed] [Google Scholar]
- 18.Lustgarten DL, Keane D, Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis. 1999;41(6):481–98. doi: 10.1016/S0033-0620(99)70024-1. [DOI] [PubMed] [Google Scholar]
- 19.Theuns DA, Kimman GP, Szili-Torok T, et al. Ice mapping during cryothermal ablation of accessory pathways in WPW: the role of the temperature time constant. Europace. 2004;6(2):116–122. doi: 10.1016/j.eupc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Lellouche N, Jais P, Nault I, et al. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J Cardiovasc Electrophysiol. 2008;19(6):599–605. doi: 10.1111/j.1540-8167.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 21.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9(6):335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell D, Furniss SS, Dunuwille A, et al. Delayed cure despite early recurrence after pulmonary vein isolation for atrial fibrillation. Am J Cardiol. 2003;91(1):83–85. doi: 10.1016/S0002-9149(02)03005-9. [DOI] [PubMed] [Google Scholar]
- 23.Tanno K, Kobayashi Y, Kurano K, et al. Histopathology of canine hearts subjected to catheter ablation using radiofrequency energy. Jpn Circ J. 1994;58(2):123–135. doi: 10.1253/jcj.58.123. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh MH, Chiou CW, Wen ZC, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. 1999;100(22):2237–2243. doi: 10.1161/01.CIR.100.22.2237. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez LM, Leunissen J, Hoekstra A, et al. Transvenous cold mapping and cryoablation of the AV node in dogs: observations of chronic lesions and comparison to those obtained using radiofrequency ablation. J Cardiovasc Electrophysiol. 1998;9(10):1055–1061. doi: 10.1111/j.1540-8167.1998.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 26.Brignole M, Vardas P, Hoffman E, et al. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11(5):671–687. doi: 10.1093/europace/eup097. [DOI] [PubMed] [Google Scholar]
- 27.Piorkowski C, Kottkamp H, Tanner H, et al. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16(12):1286–1292. doi: 10.1111/j.1540-8167.2005.00245.x. [DOI] [PubMed] [Google Scholar]