Abstract
Dehydroepiandrosterone sulfate (DHEAS), is an excitatory neurosteroid synthesized within the CNS that modulates brain function. Effects associated with augmented DHEAS include learning and memory enhancement. Inhibitors of the steroid sulfatase enzyme increase brain DHEAS levels and can also facilitate learning and memory. This study investigated the effect of steroid sulfatase inhibition on learning and memory in rats with selective cholinergic lesion of the septo-hippocampal tract using passive avoidance and delayed matching to position T-maze (DMP) paradigms. The selective cholinergic immunotoxin 192 IgG-saporin (SAP) was infused into the medial septum of animals and then tested using a step-through passive avoidance paradigm or DMP paradigm. Peripheral administration of the steroid sulfatase inhibitor, DU-14, increased step-through latency following footshock in rats with SAP lesion compared to both vehicle treated control and lesioned animals (p < 0.05). However, in the DMP task, steroid sulfatase inhibition impaired acquisition in lesioned rats while having no effect on intact animals. These results suggest that steroid sulfatase inhibition facilitates memory associated with contextual fear, but impairs acquisition of spatial memory tasks in rats with selective lesion of the septo-hippocampal tract.
Keywords: steroid sulfatase inhibitors, medial septum, cholinergic lesion, memory retention, spatial learning
1. Introduction
Neuroactive steroids and neurosteroids play an important role in normal physiology and in the pathogenesis of brain diseases (Kriz, Bicikova, Hill, & Hampl, 2005; Morrow, 2007; Vallee, Mayo & Le Moal, 2001). Neurosteroids possess both rapid non-genomic as well as slow genomic effects (Kriz, Bicikova, Hill, & Hampl, 2005; Akk, et al., 2007; Hosie, Wilkins & Smart, 2007; Melcangi, Garcia-Segura & Mensah-Nyagan, 2008). In contrast to steroids synthesized in classic steroidogenic tissues that activate cytoplasmic receptors, neurosteroids predominantly use signaling pathways common for neuromodulators. It is well known that unconjugated forms of neurosteroids, such as saturated progesterone metabolites 5α-pregnan-3α-ol-20-one (allopregnanolone), its 21-hydroxylated metabolite, androsterone and dehydroepiandrosterone (DHEA) act on GABAA receptors as positive modulators that along with other events, increase the permeability of chloride ion channels (Kriz, Bicikova, Hill, & Hampl, 2005; Hosie, Wilkins, da Silva, Helena & Smart, 2006). Particular sulfated forms of neurosteroids act in the opposite way, as negative GABAA receptor modulators (Kriz, Bicikova, Hill, & Hampl, 2005; Vallee, Mayo & Le Moal, 2001; Twede, Tartaglia, Covey & Bamber, 2007). In addition to modulating GABAA receptors, several studies have shown that neurosteroids can also interact with the sigma-1 receptor, which is believed to modulate intracellular calcium mobilization and extracellular calcium influx, NMDA-mediated responses, acetylcholine release and alter monoaminergic function (Monnet, Mahé, Robel & Baulieu, 1995; Maurice, Roman & Privat, 1996; Debonnel & de Montigny, 1996). Pregnenolone, DHEA and their sulfate esters behave as sigma-1 agonists, while progesterone is a potent sigma-1 antagonist.
Physiological concentrations of DHEA and dehydroepiandrosterone sulfate (DHEAS) are maintained by steroid sulfatase and neurosteroid sulfuryl transferase which are present in the blood and other peripheral tissues, as well as in the brain, (Kriz, Bicikova, Hill, & Hampl, 2005; Wolf & Kirschbaum, 1999; Baulieu, 1997). Steroid sulfatase (estrone sulfatase, arylsulfatase C; E.C. 3.1.6.2) is a ubiquitous membrane-bound, microsomal enzyme localized mainly in the endoplasmic reticulum and the nuclear envelope of cells. In the brain, DHEAS is cleaved to DHEA via steroid sulfatase, and inhibition of the sulfatase has been demonstrated to enhance learning and spatial memory in rats (Li, Pillai, Young, Bender, Martino & Lin, 1993).
Analogues of (p-O-sulfamoyl)-N-alkanoyl tyramine such as (p-O-sulfamoyl)–Tetradecanoyl Tyramine, (DU-14), are effective inhibitors of estrone sulfatase activity (Selcer, DiFrancesca, Chandra & Li, 2007). A single dose of (30 mg/kg) of DU-14 was able to significantly inhibit steroid sulfatase activity within the liver (Baulieu, 1997) and brain (Li, Rhodes, Burke & Johnson, 1997). In rodents, chronic administration of DU-14 (30 mg/kg for 15 days i.p.) increased plasma and brain concentrations of DHEAS, while decreasing plasma DHEA (Rhodes, Li, Burke & Johnson, 1997). DU-14 has also been shown, following chronic administration, to reverse amnesia induced by scopolamine (a muscarinic antagonist), and potentiate the reversal of scopolamine-induced amnesia by DHEAS in passive avoidance and Morris water maze memory tests (Howarth, Purohit, Reed & Potter, 1994; Johnson, Rhodes, Boni & Li, 1997; Li, Rhodes, Burke & Johnson, 1997; Johnson, Wub, Li & Maher, 2000). Moreover, peripheral administration of DU-14 (30 mg/kg, i.p.) has been shown to increase acetylcholine release in the hippocampus, which is consistent with other studies that found that increased DHEAS levels in the brain augment the levels of ACh within the hippocampus (Rhodes, Burke & Johnson, 1997). In the water maze not only did DU-14 reverse the scopolamine induced amnesia, but also enhanced the performance of unimpaired control animals (Johnson, Wub, Li & Maher, 2000).
It has been hypothesized that the memory enhancing effect of steroid sulfatase inhibitors are the result of enhanced cholinergic neurotransmission in brain structures involved in memory such as the hippocampus. If this were the case, one might predict that for animals in which there was loss of cholinergic neurons projecting to the hippocampus, the administration of DU-14 would be relatively ineffective since there would a diminished capacity for neuromodulators such as DHEAS to enhance cholinergic neurotransmission. However, it is possible steroid sulfatase inhibitors may enhance memory utilizing mechanisms that are independent of cholinergic neurotransmission. One possibility is that DU-14 may affect non-cholinergic systems in ways that could compensate for the loss of muscarinic cholinergic activity. If so, then steroid sulfatase inhibitors could potentially be effective for the treatment of cognitive dysfunctions associated with a loss of basal forebrain cholinergic neurons such as in Alzheimer’s disease. Whether or not the cognitive enhancing effects associated with DU-14 are due specifically to changes in ACh release in the hippocampus and cortex is currently unknown.
Previous studies have demonstrated that the administration of the cholinergic neurotoxin 192 IgG-Saporin (SAP) into the medial septum (MS), produced selective lesion of cholinergic neurons that project from the MS to the hippocampus. Lesion of this tract resulted in a significant reduction in hippocampal ACh and impaired cognitive function as measured by significant delays in acquisition of a DMP T-maze task (Gibbs & Johnson, 2007; Johnson, Zambon & Gibbs, 2002; Fitz, Gibbs & Johnson, 2008; Fitz, Gibbs & Johnson, 2006).
The intent of the current investigation is to determine the effect of the steroid sulfatase inhibition on learning and memory in rats with a selective cholinergic lesion of the septo-hippocampal tract.
2. Methodology and procedures
2.1. Animal Condition
All experiments followed NIH guidelines for the care and use of laboratory animals and were approved by the Duquesne University Institutional Animal Care and Use Committee. All chemicals were purchased through Sigma Inc. (St. Louis, MO) unless stated otherwise. Male Sprague-Dawley rats weighing (275–300 g) were purchased from Hilltop Lab Animal Inc. (Scottdale, PA) and individually housed in a well ventilated, temperature and humidity controlled facility (22–25° C, 50–75% humidity). A standard 12:12 h light:dark cycle was maintained and rodents had access to standard laboratory rat chow and water ad libitum. Animals were allowed a minimum of five days to acclimate to the housing conditions, before any experiments were performed. DU-14 was prepared as previously described (Li, P.-K., et al., 1993)
2.2. Animal Surgery
Rats weighing approximately 300 grams were anesthetized with pentobarbital (50 mg/kg: IP of a 50 mg/ml stock solution, Ovation Pharmaceuticals, Deerfield, IL), shaved and then placed into a stereotaxic frame (Stoelting, Wood Dale, IL). An incision was made exposing the dorsal aspect of the skull and a small hole (2 mm in diameter) was drilled, through which a stainless steel cannula (28 gauge, Plastics One Inc., Roanoke, VA) was lowered into the medial septum (+0.2 mm bregma 0.0 mm lateral, −5.4 mm dorsal ventral). Animals were infused with either 1 μl of vehicle (artificial cerebrospinal fluid (CSF); CMA Inc., North Chelmsford, MA) or SAP (0.20 μg in 1 μl of artificial cerebrospinal fluid; Advanced Targeting Systems, San Diego, CA, Lot # 24–87) over 5 min at 0.2 μl/min using a syringe pump (Harvard Apparatus, Holliston, MA). The dose of SAP was selected based on previous studies that demonstrated a substantial loss of cholinergic neurons in the MS with little non-selective damage to GABAergic neurons (Johnson, Zambon & Gibbs, 2002). Following infusion, the cannula was left in place for 5 min to allow for diffusion of the solution into the tissue. The incision was closed, rats were administered Ibuprofen (1 ml/kg IP, of a 10 mg/ml stock solution) and allowed to recover for 14 days prior to behavioral testing.
2.3. Behavioral testing
2.3.1. Passive Avoidance Testing
To assess passive avoidance memory retention, fourteen days following infusion of SAP or artificial CSF into the MS, a Gemini Avoidance System (San Diego Instruments, San Diego, CA) was used in a modified passive avoidance paradigm. The avoidance apparatus consisted of a box (53 × 53 × 32 cm) with 2 compartments connected by an opening with a computer controlled sliding door. The compartment in which the rats were placed was illuminated, while the other compartment remained dark throughout the experiment.
There were three stages of the behavioral testing: acclimation, acquisition, and retention. During acclimation trials rats were allowed to explore the apparatus and the latency period, the time it took for the rat to cross into the dark compartment, was recorded. During the acquisition trials, rats were placed in the lighted compartment. After five seconds of adaptation, the rats were given a maximum trial duration of 5 min to cross to the darkened chamber. If the animal entered the dark compartment, a sliding door closed and a mild footshock of either 0 mA, 1.0 mA or 1.25 mA for 1 sec was delivered. The 1.0 mA footshock was selected based on previous studies in our laboratory, 1.25 mA was a mild increase while the 0 mA was used as a control. Individual rats were exposed to only one level of footshock. The rat was then removed from the chamber and tested again after 5 min. This procedure was repeated until the animal spent 5 min in the lighted chamber two consecutive times with a maximum of 5 trials. The number of trials to reach criterion was recorded. One day following the acquisition trial, the animals were administered either DU-14 (30 mg/kg, IP) or corn oil (vehicle, 1 ml/kg, IP) for 6 consecutive days. Three hours after the final treatment, the animals were again placed in the lighted compartment and the crossover latency recorded. If an animal did not enter the dark compartment within 10 min, it was removed from the apparatus, and the latency recorded as 10 min. Memory retention was assessed as an increase in crossover latency period during the retention trial.
2.3.2. Delayed match-to-position (DMP) T-maze paradigm
A separate cohort of animals that had not undergone passive avoidance testing was utilized for the DMP T-maze paradigm. Fourteen days following SAP or CSF infusion, rats were randomly separated into DU-14 treatment and control groups. DU-14 (30 mg/kg, IP) or corn oil (vehicle, 1ml/kg, IP) was administered daily until the study was completed. To minimize introduction of an aversive stimulus before testing, DU-14 was administered 4 hours after completion of the days testing procedure.
The DMP task was performed as previously described (Johnson, et al., 2002). Briefly, rats were food deprived to 85% of their normal body weight and then adapted to the T-maze by placing them into the maze with sweetened reward pellets (45 mg Noyes pellets; Research Diets; New Brunswick, NJ) once per day for 5 days. Rats were then trained to run to the ends of each goal arm by using a series of six forced choices per day for 3 days. To avoid the introduction of a side bias, right and left goal arms were alternated in a random and balanced fashion. Animals then began DMP training. Each rat received eight trial pairs per day. The first trial consisted of a forced choice in which one of the goal arms was closed, forcing the animal to enter the open goal arm to receive a reward (two pellets). The rat was immediately returned to the approach alley for an open choice trial during which both goal arms could be entered. Returning to the same goal arm entered during the forced trial resulted in a reward (four pellets). Entering the opposite arm resulted in no reward and confinement in the goal arm for 10s. Rats were run in groups of three or four. After each trial pair, rats were returned to their cages for 5–10 min, while training proceeded on the remaining animals of the group. Goal arms were varied in a random balanced fashion. Rats continued to receive eight trial pairs per day until a criterion of 15 correct choices out of 16 consecutive open choice trials was met. The number of days required to reach criterion was recorded.
2.4. ChAT (Choline Acetyltransferase) Assay
At the completion of behavioral testing, all animals were processed for the quantification of ChAT activity in the frontal cortex and hippocampus. Animals were anesthetized with 5% halothane or isoflurane, decapitated and the brains removed. Tissues from the frontal cortex (FX), and hippocampus (HIPP) dissected, frozen at 80°C and processed at a later time. Tissues were not pooled. On the day of the assay, frozen tissues were thawed at 4°C and dissociated by sonication in a medium containing EDTA (10 mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 μl/mg tissue. An aliquot of each sample was used for the determination of total protein (Fitz, Gibbs & Johnson, 2006). Three 5 μl aliquots of each sample were incubated for 30 min in a medium containing 0.25 mM [H3]-Acetyl-CoA, 50 mM sodium phosphate buffer (pH 7.4); 300 mM sodium chloride, 10 mM choline chloride, 10 mM EDTA and 0.2 mM physostigmine sulfate. The reaction was stopped by adding 4 ml of 10 mM sodium phosphate buffer (pH 7.4) followed by the addition of 1.6 ml of acetonitrile containing 5 mg/ml tetraphenylboron in scintillation vials. The amount of [H3] – acetylcholine produced was determined by adding 8 ml of EconoFluor scintillation cocktail and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, the three reaction tubes containing sample were averaged and the difference between total cpm and background cpm was used to estimate the total amount of ACh produced per sample. The ChAT enzyme activity was expressed as pmol acetylcholine produced/h/μg protein.
The goal of study was to compare rats with a substantial selective cholinergic lesion of the hippocampus to non-lesion control animals. Therefore, results of hippocampal ChAT enzyme activity that indicated a partial lesion of the hippocampus (ChAT activity values within two standard divisions of the mean ChAT activity of non-lesion controls) were excluded from further analysis. Similarly, for lesioned rats with ChAT activity in the frontal cortex indicating a cholinergic lesion (loss of ChAT activity greater than two standard deviations below the mean ChAT activity in the frontal cortex of control animals) rats were excluded from further analysis.
2.5. Statistical Analyses
All analyses were performed using the GraphPad Prism 3.02 version or SPSS v16. Differences in ChAT activity in the HIPP and FX of SAP lesioned and control animals were compared using the one-way ANOVA with a Newman-Keuls post-hoc test. Differences in crossover latency in the passive avoidance tests were determined by statistical analysis utilizing the Student’s t-test, Kruskal-Wallis analysis with a Dunn’s test post-hoc or a two-way ANOVA using lesion and footshock intensity as variables. The effect of frontal cortex lesion on acclimation crossover latency was examined using the Spearmen’s correlation analysis.
For the DMP task, days to criterion were analyzed using two-way ANOVA, lesion and DU-14 drug treatment as factors, with a Bonferroni post-hoc test for individual comparisons of the treatment groups. To compare treatment effects during different stages of acquisition, performance data was blocked into 3-day periods of training. After an animal had reached criterion, a value of 93.75% (15/16 correct choices/trial, criterion) was recorded for performance on subsequent days. Performance during acquisition testing was analyzed using two-way repeated measures ANOVA (General Liner Model, RM-ANOVA) for overall effects of block and treatment and one-way ANOVA with a Newman-Keuls post-hoc test for treatment effects within blocks. The data for both the rate of performance and days to reach criterion in the DMP t-maze task were determined to be parametric. The turning strategy (whereby they consistently turned to the right or left goal arm of the maze) observed in animals throughout the early stages of DMP training was quantified by counting the total number of days the animals utilized the strategy during the testing process. Any animal that entered 7 out of 8 times into the same goal arm was defined as using a turning strategy. A two-way ANOVA, lesion and DU-14 drug treatment as factors, with Bonferroni post hoc test was used to analyze the number of animals that adopted this strategy and also the number of days that an animal utilized the strategy. Significant differences was defined as p<0.05 for all studies. The particular statistical analyses utilized to compare groups during the different stages of testing are described in the corresponding figure legends.
3. Results
3.1 ChAT Assays
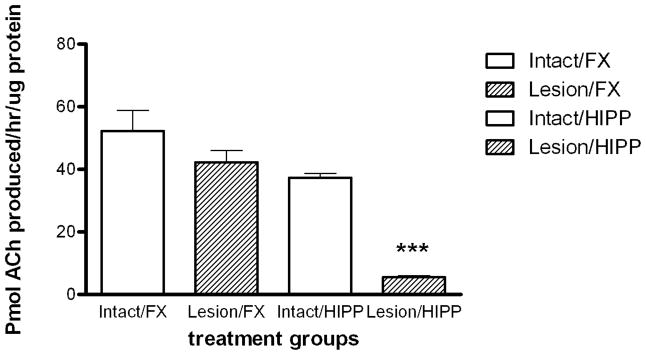
There were 90 non-lesioned and 56 lesioned rats following the exclusion of animals with either a partial cholinergic lesion of the hippocampus or indications of lesion of the frontal cortex. ChAT activity was measured to assess the degree of cholinergic denervation of the frontal cortex and hippocampus following SAP lesion of the remaining animals. There was a significant decrease in ChAT activity of 85.3% in hippocampal tissues for lesioned rats compared to intact animals (p<0.0001 Newman-Keuls post-hoc test; Fig. 1). There was no significant change in ChAT activity in frontal cortex tissues of lesioned compared to intact control rats (p > 0.05; Fig. 1).
Figure 1. ChAT activity.
The effect of SAP lesions on ChAT activity in the frontal cortex (Lesion/FX) and hippocampus (Lesion/HIPP) relative to control (Intact/FX and Intact/HIPP). Bars show group mean ± SEM. Data were analyzed by one-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test (*** = p<0.001). There was an 80.3% decrease in ChAT activity in hippocampus of lesioned versus intact rats (7.07 ± 0.98 versus 35.90 ± 1.26 pmol ACh produced/hr/μg protein; p<0.001) n=30–97.
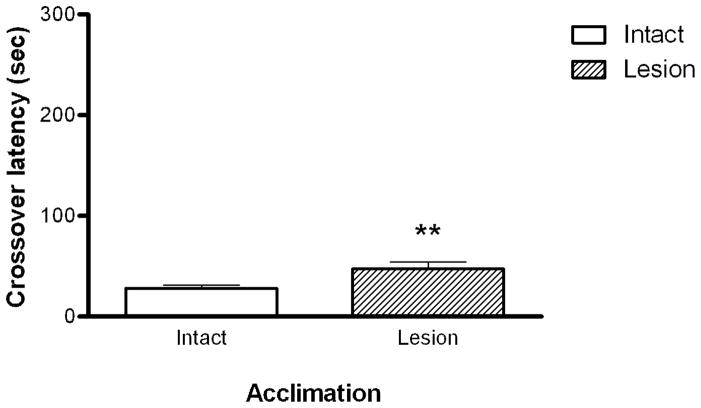
3.2 Passive avoidance: Acclimation
To assess whether cholinergic lesion of the septo-hippocampal tract had an effect on basal exploratory behavior, crossover latency during the acclimation phase was measured. There was a small but significant increase (mean values 33.2 vs 52.8 sec; p < 0.01 Student t-test), in crossover latency in lesioned animals during acclimation to the passive avoidance apparatus (Fig. 2). A Spearman’s correlation analysis was performed to determine whether there was a relationship between ChAT activity and behavioral performance in SAP treated animals. There was no correlation between the magnitude of the lesion as reflected in differences in ChAT activity and acclimation crossover latency (r = 0.1036, 0.2000 and −0.4524 Spearman’s Correlation, data not shown).
Figure 2.
The effect of lesion on acclimation crossover latency. A significant increase in crossover latency was seen for lesion animals (n=16) compared to intact animals (n=24). Bars represent the mean ± SEM. Data were analyzed using Student’s t-test (** = p<0.05).
Passive Avoidance: Acquisition
To determine the effect of footshock intensity on acquisition in lesioned and intact animals, following acclimation, rats were randomly assigned to (0 mA, 1.0 mA, and 1.25 mA) footshock treatment groups. There were no significant differences in footshock acquisition between lesioned and intact rats at 1.0 mA or 1.25 mA based on the number of footshocks required to reach criterion (data not shown).
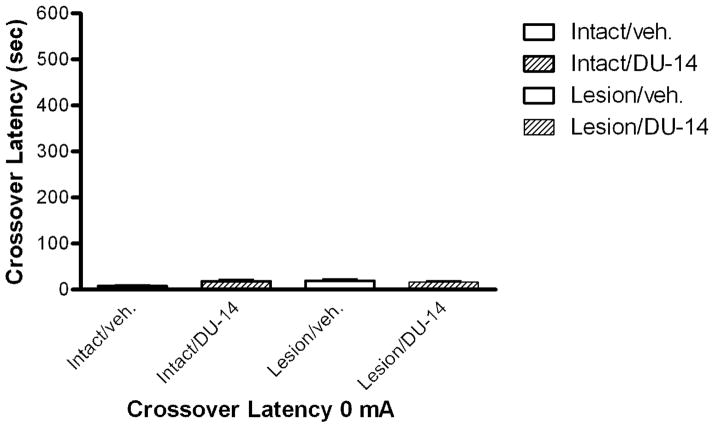
Crossover Latency in Treated Rats Without Footshock
Rats were dosed for six days with either corn oil (vehicle) or DU-14 and tested for crossover latency without footshock in a fashion similar to the footshock groups for three trials. Non-footshock rats had crossover latencies that were similar to latencies recorded during the acclimation phase. There were no significant differences in crossover latencies between intact and lesioned rats treated with DU-14 or vehicle (Fig. 3).
Figure 3.
The effect of DU-14 and vehicle after six days of dosing on crossover latency with 0 mA footshock. The crossover latency following treatment was similar to acclimation crossover latency prior to drug treatment. Bars represent the mean ± SEM. Kruskal-Wallis analysis (p=0.15; h=5.37) shows there were no significant differences in crossover latency between treatment groups (p >0.05 Dunn’s post hoc test.) n=3–5.
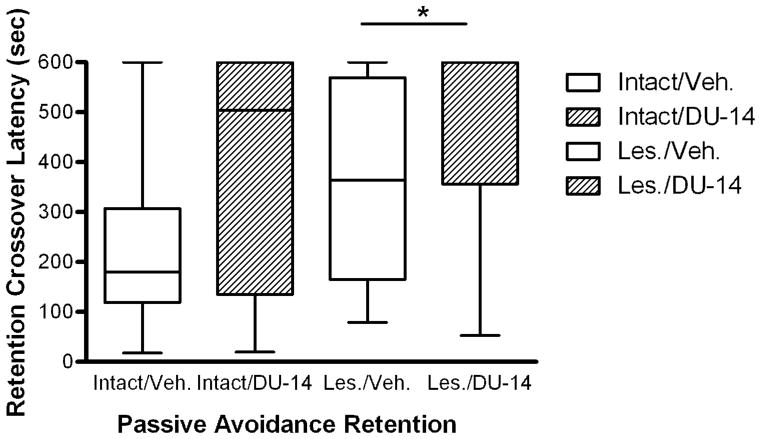
3.3.1 Effect of DU-14 on memory retention after footshock administration
For animals dosed six days with vehicle or DU-14, administered 1.0mA footshock, treatment with DU-14 significantly increased crossover latency in lesioned animals (p=0.04 Dunn’s post-hoc test) compared to lesioned vehicle rats (Fig. 4). Moreover, there was a non-significant increase in the crossover latency in intact animals treated with DU-14 (p=0.15) compared to vehicle. With 1.25 mA foot shock, DU-14 produced no significant differences compared to vehicle in the crossover latency compared in either intact or lesioned animals (data not shown).
Figure 4.
The effect of DU-14 and vehicle treatment on retention crossover latency. Kruskal-Wallis analysis (p=0.05; h=7.92) shows that crossover latency was significantly longer in the lesioned DU-14 treatment group (n=6) compared to the lesioned vehicle group (n=8; * = p< 0.05 Dunn’s post-hoc test). There was a non-significant trend for an increase in retention crossover latency in the intact DU-14 treatment group (n=11) compared to intact vehicle (n=9).
3.4 DMP-T-maze task
3.4.1 Effect of DU-14 treatment on time and rate of DMP-T-maze acquisition
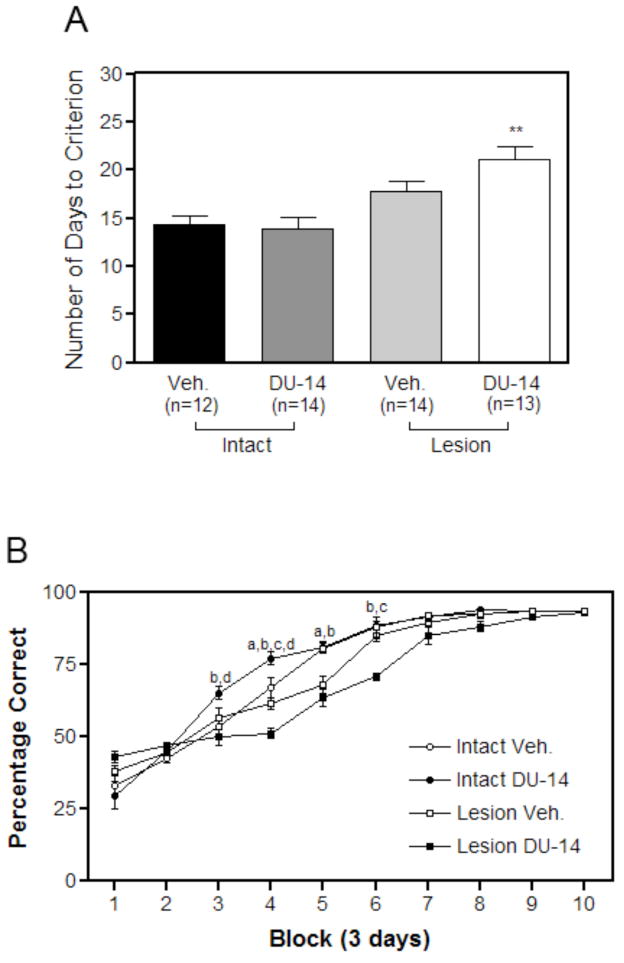
Of the original 56 animals tested in the DMP paradigm, all but three reached criterion or displayed a preference for one goal arm of the T-maze (side bias) and were excluded from the study. Two of the three animals were intact vehicle treated control animals while the other was a lesioned DU-14 treated animal. Analysis with a two-way ANOVA of the remaining animals indicated a significant main effect on lesion (F=20.57, p<0.001) but not drug treatment (F=2.19, p>0.05) and no interaction (F=1.42, p>0.05), indicating that lesioned animals required more days to reach criterion than intact rats. Surprisingly, drug treatment with DU-14 further increased the number of days required for lesioned animals to reach criterion (p < 0.001 Bonferroni post-hoc test; Fig. 5A). Lesioned vehicle treated animals took an average of 17.8 ± 3.5 days to complete the DMP task while drug treatment with DU-14 increased days to criterion to an average of 21.2 ± 4.5 in the lesioned group. Intact vehicle treated animals took an average of 14.3 ± 3.2 days to reach criterion, while lesioned vehicle treated animals took an average of 17.9 ± 3.5 to complete the DMP task.
Figure 5.
(A) The effect of DU-14 treatment on the number of days to reach criterion in the DMP T-maze task. Bar graph representing the effect of DU-14 treatment on spatial acquisition of MS cholinergic lesioned rats. Bars represents the mean number of days to reach criterion ± SEM. Data analyzed by two-way ANOVA with Boneferroni post-hoc test reveals a significant effect of lesion (p<0.001) but no significant effect of treatment or interaction. DU-14 treated lesioned animals (n=13) required significantly more days to reach criterion than the lesioned vehicle treated animals (n=14 ** p < 0.01 Bonferroni post-hoc test). DU-14 had no effect on days to criterion of intact control animals (n=12 intact vehicle; n=14 intact DU-14). (B) The effect of DU-14 treatment on the rate of acquisition in the DMP T-maze task for lesioned rats: Points represent the mean percentage correct for each treatment group during a 3 day training period. Note that all groups showed improved performance over time; however during blocks 4 and 6 the performance of the lesioned DU-14 treated animals was significantly worse than the corresponding lesioned vehicle treated animals. Also during blocks 3 and 4 intact vehicle treated animals performed significantly worse than intact DU-14 treated animals. Performance during acquisition testing was analyzed using two-way repeated measures ANOVA (General Liner Model, RM-ANOVA) for overall effects of block and treatment and one-way ANOVA with a Newman-Keuls post-hoc test for treatments within blocks. a: p< 0.005 for intact vehicle treated animals relative to lesioned vehicle treated animals. b: p< 0.005 for intact DU-14 treated animals relative to lesioned DU-14 treated animals. c: p< 0.005 for lesioned vehicle treated animals relative to lesioned DU-14 treated animals. d: p< 0.005 for intact vehicle treated animals relative to intact DU-14 treated animals.
Examination of learning curves (Fig. 5B) found that all animals performed at similar, below chance, levels at the start of DMP training. Lesioned vehicle animals improved at a slower rate during training when compared to intact vehicle treated controls. Lesioned DU-14 treated animals improved at the slowest rate. By day 21 of training, all intact vehicle treated animals and all but one intact DU-14 treated rat had reached criterion. On the other hand, only six of 13 lesioned DU-14 treated animals had reached criterion. By day 30 all animals reached criterion. Repeated measures-ANOVA analysis revealed a significant main effect of “Treatment” (F [27, 88] = 6.88, p< 0.0001), a significant effect of “Block” (F[9,5425] = 425.90, p< 0.0001 ), and a significant ‘Treatment’ X ‘Block’ interaction (F[27,88] = 6.88, p< 0.0001). A separate analysis of performance within block revealed vehicle treated intact animals performed significantly better than lesioned vehicle animals during blocks 4 and 5 of training. Intact DU-14 treated animals performed significantly better during blocks 3 and 4 when compared to intact vehicle treated animals. Lesioned vehicle animals performed significantly better during blocks 4 and 6 when compared to lesioned DU-14 treated animals (Fig. 5B)
3.4.2 Effect of DU-14 treatment on persistent turning strategy
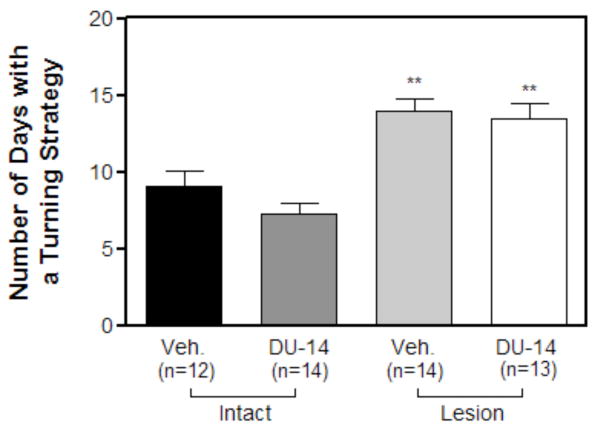
During early stages of training many of the animals adopted a turning strategy. There was no significant difference in the number of animals in each treatment group that adopted the persistent turning strategy (data not shown). Furthermore, there was no significant difference in the number of days before each of the treatment groups adopted the turning strategy (data not shown). Two-way ANOVA analysis of the number of days with a persistent turning strategy reveals that there was a significant main effect on lesion (F=62.47, p<0.001), but not drug treatment (F=1.11, p>0.05) nor interaction (F=0.26, p>0.05), indicating that lesioned animals utilized a persistent turning strategy for longer period of time. Lesioned vehicle treated animals engaged in a turning strategy for 14.50 ± 1.18 days while intact vehicle treated controls engaged in this strategy for 8.33 ± .80 days. Lesioned DU-14 treated animals engaged in a turning strategy for 14.08 ± 1.25 days while intact DU-14 treated animals engaged in this strategy for 7.35 ± 0.71 days. There was no significant difference in the number of days lesioned or intact animals used the turning strategy following DU-14 treatment. To determine whether the percentage of animals that adopted the turning strategy had a significant effect on the number of days animals engaged in the turning strategy, those animals that never adopted a turning strategy were excluded. After excluding these animals, there was still no significant difference in the number of days lesioned and intact control animals used the turning strategy following DU-14 treatment (data not shown). This suggests that the difference in days to criterion between lesioned vehicle and lesioned DU-14 treated animals cannot be explained by an increased utilization of a turning strategy following DU-14 treatment.
4. Discussion
The decrease in hippocampal ChAT activity by 85% indicates that infusion of the SAP neurotoxin into the medial septum substantially decreased cholinergic innervation of the hippocampus. This result is consistent with previous studies that used the same approach to model memory impairments associated with cholinergic lesions (Johnson, Zambon & Gibbs, 2002; Rossner, Schliebs, Hartig & Bigl, 1995).
Steroid sulfatase inhibition significantly increased retention latency in the passive avoidance test in rats with selective cholinergic lesion of the MS indicating enhanced memory for foot shock compared to lesioned animals that did not receive the steroid sulfatase inhibitor. However, while intact rats treated with DU-14 performed better than control intact animals during specific learning blocks of the DMP task, steroid sulfatase inhibition by DU-14 significantly increased the average number of days required for lesioned rats to reach criterion in the DMP T-maze task compared to vehicle lesioned animals, indicating that sulfatase inhibition further impaired spatial working memory in lesioned rats.
Footshock Acquisition
For rats that did not receive footshock, the retention crossover latency was not significantly different than latencies observed during the acclimation phase for any of the treatment groups. This result suggests that the increase in retention crossover latency following footshock was the result of memory for the aversive stimulus not alterations in exploratory behavior resulting from treatment with DU-14 or vehicle.
Cholinergic Lesion and Crossover Latency
Cholinergic lesion of the MS caused a small but significant increase in acclimation crossover latency in lesioned rats compared to intact animals. This result suggests a possibility of either a slight increase in anxiety or diminished motivation to explore for lesioned animals. However, repeated exposure to the passive avoidance apparatus without footshock eliminated the difference in crossover latency.
The Effect of Steroid Sulfatase Inhibition on Retention Crossover Latency
Lesioned rats administered 1.0 mA footshock and DU-14 had a significant increase in crossover latency compared to lesioned rats administered vehicle. This result suggests that decreased hippocampal cholinergic tone affects retention of contextual fear memory and that steroid sulfatase inhibition could facilitate passive avoidance retention in rats with selective cholinergic lesion of the septo-hippocampal tract. It is possible that the enhanced passive avoidance performance in lesioned animals following treatment with DU-14 was the result of increased activation of hippocampal cholinergic neurons that were not lesioned, or possibly facilitation of hippocampal function via other neurotransmitter systems such as glutamate. It is also possible that other structures involved in contextual fear memory such as the amygdala might also have been facilitated via enhanced excitatory neurosteroid mediated inhibition of GABAA receptors or activation of NMDA or other receptor types (Laszlo et al., 2012).
Previous studies (Johnson, Rhodes, Boni, & Li, 1997; Rhodes, Li, Burke, & Johnson, 1997), demonstrated that animals chronically treated with DU-14 increased plasma DHEAS and enhanced hippocampal ACh release. Furthermore, DU-14 also attenuated scopolamine induced amnesia using the same passive avoidance paradigm (Johnson, Rhodes, Boni & Li, 1997; Rhodes, Li, Burke, & Johnson, 1997) and in the Morris water maze test (Johnson, Wub, Li & Maher, 2000). By inhibiting the metabolism of DHEAS to DHEA, DU-14 increased endogenous levels of DHEAS, which could then decrease GABAergic inhibitory tone on cholinergic neurons of the basal forebrain (Li, Rhodes, Jagannathan, & Johnson, 1995). This would account for the increased hippocampal ACh and attenuation of scopolamine induced amnesia observed in the previous studies (Rhodes, Li, Burke, & Johnson, 1997), as well as facilitation of passive avoidance retention in the current study. Inhibition of GABAA receptors and facilitation of NMDA receptor activity could result in activation of cholinergic neurons modulated by those receptors outside the hippocampus, however, there are currently no studies demonstrating steroid sulfatase inhibition mediated facilitation of ACh release outside of that structure.
At the 1.25 mA footshock level, DU-14 had no significant effect on retention crossover latency in either control or lesioned animals. This result suggests that the level of shock intensity affects the cognitive response to steroid sulfatase inhibition. It is well established that moderate stress can facilitate memory function while severe stress impairs memory (Sandi, 1998; Luine, Villegas, Martinez & McEwen, 1994; Bodnoff, et al. 1995). Thus, the results of this study are consistent with the literature that demonstrates that the magnitude of stress associated with a stimulus affects memory retention.
Spatial Memory
As in previous studies, cholinergic lesion of the septohippocampal tract resulted in a delay in acquisition of the DMP task (Johnson, et al., 2002; Fitz et al., 2006; Fitz et al. 2008). The time it took the rats to reach criterion in the DMP task was, in part, a function of perseveration in maintaining an egocentric response strategy (bias) before adopting a more efficient allocentric place strategy (Fitz et al., 2008). The enhanced perseveration in lesioned animals for utilizing the response strategy in the DMP T-maze task is consistent with other studies demonstrating behavioral perseverations in rats with impaired hippocampal function (Arkhipov et al., 2008). This result is also consistent with an increased role for the striatum in behavioral responses in animals with impaired hippocampal function (Chang et al., 2003). Steroid sulfatase inhibition by DU-14 facilitated performance during several time blocks of the learning curve for the DMP task in intact rats. However, treatment with DU-14 further impaired acquisition of the task with the lesioned rodents. Specifically, lesioned rats that received DU-14 had an increase in the number of days to reach criterion compared lesioned control animals. The impairment was associated with decreased performance during blocks 4 and 6 of the learning curve compared to vehicle treated lesioned animals (Fig. 5B), but was not associated with an increase in perseveration. These findings do not support the initial hypothesis that chronic pretreatment with DU-14 would improve acquisition of the DMP paradigm for animals with cholinergic lesion of the MS. It is possible that treatment with a steroid sulfatase inhibitor could increase ACh release in neuronal systems that do not receive projections from the MS, and fail to increase ACh in the hippocampus due to the septo-hippocampal lesion. Chang et al. (2003), showed that changes in cholinergic tone in the hippocampus and striatum were associated with different learning strategies. Moreover, the results of the current study are consistent with studies of diencephalic amnesia in rats that sustained damage to cholinergic neurons of the septo-hippocampal tract, but retained intact cholinergic neurons projecting from the nucleus basalis to the amygdala. The rats had reduced ACh efflux in the hippocampus and were impaired in a spontaneous alternation task (Savage et al., 2007; Vetreno et al., 2008). However, in contrast to the results of the current study, a recent study of diencephalic amnesia using the same experimental model as the studies above found that the administration of the acetylcholinesterase inhibitor Tacrine, either peripherally or directly into the MS, could reverse an impairment in performance in the spontaneous alternation task. The differing outcomes in the studies of diencephalic amnesia and the current study may be due to differing degrees of cholinergic lesion, different testing paradigms, or the more indirect effects of excitatory neurosteroids on cholinergic tone (Rhodes et al. 1997). Unlike the reversal of memory impairments by steroid sulfatase inhibitors resulting from muscarinic antagonists (Johnson, Wub, Li & Maher, 2000), treatments in animals with actual lesion of cholinergic inputs to the hippocampus may not be able to reverse deficits in hippocampal function due to the loss of cholinergic enervation. This would account for the inability of DU-14 to reverse the impairment of DMP performance in lesioned animals, but facilitate performance in intact animals. Additionally, steroid sulfatase inhibition in animals with cholinergic lesion of the hippocampus could result in a differential increase in ACh levels in other brain structures such as the striatum, while hippocampal levels remained low (Savage, Roland & Klintsova, 2007). The result would cause an imbalance in cholinergic tone that could disrupt performance in the DMP task (Chang & Gold, 2003). This could account for increased impairment for the DMP task in lesioned animals administered DU-14.
Passive avoidance vs Delayed Matching to Position
Rats in both testing paradigms were administered DU-14, but rats in the passive avoidance experiments received doses of the steroid sulfatase inhibitor for six days, while rats in the DMP task received the same dose (30 mg/kg/day) for up to 30 days. Previous studies in rats have demonstrated a significant inhibition of steroid sulfatase in the liver and brain and reversal of scopolamine induced impairment of passive avoidance after a single injection of DU-14 (Li et al., 1997). Moreover, pretreatment with the same dose of DU-14 for 15 days could reverse scopolamine induced impairment and facilitate performance in control rats in the Morris Water Maze (Johnson et al., 2000). How varying the days of treatment with DU-14 might affect performance in rats with selective cholinergic lesion in passive avoidance and DMP tasks remains to be determined.
In summary, steroid sulfatase inhibition facilitated contextual fear memory in a passive avoidance test in rats with selective cholinergic lesion of the septo-hippocampal tract and aspects of DMP performance in intact animals, but enhanced impairment in the DMP task of rats with selective cholinergic lesion of the septo-hippocampal tract. The results of this study suggest that the administration of a steroid sulfatase inhibitor may not be effective in facilitating working memory in instances of deficits resulting from substantial loss of cholinergic inputs to the hippocampus.
Figure 6.
The effect of daily DU-14 injections on the utilization of a persistent turning strategy. Bars represent the mean number of days using a turning strategy ± SEM. Analysis by two-way ANOVA and Bonferroni post hoc-test reveals that there is a main effect on lesion (p<0.001) but no significant effect of treatment or an interaction. Note that Lesioned vehicle treated animals adopted a turning strategy for more days compared to Intact vehicle treated controls (Bonferroni post-hoc test, ** p < 0.01). Also note that there was no significance difference between intact DU-14 and intact vehicle animals. Furthermore lesioned DU-14 animals did not utilize a turning strategy for significantly more days when compared to vehicle treated lesioned animals.
Highlights.
Steroid sulfatase inhibition and cholinergic lesion of the hippocampus was tested.
SSI enhanced retention memory in lesioned rats in the passive avoidance task.
SSI enhanced learning in intact rats in the delayed matching to position task.
SSI further impaired learning in the DMP task in lesioned animals.
Acknowledgments
The authors wish to acknowledge the excellent technical skills of Mrs. Christine Close and Mr. Douglas Nelson. This study was supported by NIH grants: AG16261 (DAJ) and AG021471 (RBG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–37. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipov V, Kulesskaja N, Lebedev D. Behavioral perseveration and impairment of long-term memory in rats after intrahippocampal injection of kainic acid in subconvulsive dose. Pharmacology, Biochem Behav. 2008;88:299–305. doi: 10.1016/j.pbb.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. Journal of Neuroscience. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. Journal of Neuroscience. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, de Montigny C. Modulation of nmda and dopaminergic neurotransmissions by sigma ligands: Possible implications for the treatment of psychiatric disorders. Life Sciences. 1996;58:721–734. doi: 10.1016/0024-3205(95)02248-1. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain research bulletin. 2008;77:356–360. doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Aversive stimulus attenuates impairment of acquisition in a delayed match to position T-maze task caused by a selective lesion of septo-hippocampal cholinergic projections. Brain research bulletin. 2006;69:660–665. doi: 10.1016/j.brainresbull.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiology of Learning and Memory. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva, Helena MA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacology & Therapeutics. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Howarth NM, Purohit A, Reed MJ, Potter BV. Estrone sulfamates: potent inhibitors of estrone sulfatase with therapeutic potential. Journal of Medicinal Chemistry. 1994;37:219–221. doi: 10.1021/jm00028a002. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Rhodes ME, Boni RL, Li P. Chronic steroid sulfatase inhibition by (p-O-sulfamoyl)-N-tetradecanoyl tyramine increases dehydroepiandrosterone sulfate in whole brain. Life Sciences. 1997;61:355–359. doi: 10.1016/s0024-3205(97)00961-2. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain research. 2002;943:132–141. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Wub T, Li P, Maher TJ. The effect of steroid sulfatase inhibition on learning and spatial memory. Brain Research. 2000;865:286–289. doi: 10.1016/s0006-8993(00)02372-6. [DOI] [PubMed] [Google Scholar]
- Kriz L, Bicikova M, Hill M, Hampl R. Steroid sulfatase and sulfuryl transferase activity in monkey brain tissue. Steroids. 2005;70:960–969. doi: 10.1016/j.steroids.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Laszlo K, Toth K, Kertes E, Peczely L, Ollmann T, Madarassy-Szucs A, Lenard L. The role of neurotensin in passive avoidance learning in the rat central nucleus of amygdala. Behavioural Brain Research. 2012;226:597–600. doi: 10.1016/j.bbr.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Li PK, Pillai R, Young BL, Bender WH, Martino DM, Lin FT. Synthesis and biochemical studies of estrone sulfatase inhibitors. Steroids. 1993;58:106–111. doi: 10.1016/0039-128x(93)90045-o. [DOI] [PubMed] [Google Scholar]
- Li P, Rhodes ME, Burke AM, Johnson DA. Memory enhancement mediated by the steroid sulfatase inhibitor (p-O-sulfamoyl)-N-tetradecanoyl tyramine. Life Sciences. 1997;60:PL45–PL51. doi: 10.1016/s0024-3205(96)00621-2. [DOI] [PubMed] [Google Scholar]
- Li PK, Rhodes ME, Jagannathan S, Johnson DA. Reversal of scopolamine induced amnesia in rats by the steroid sulfatase inhibitor estrone-3-O-sulfamate. Cognitive Brain Research. 1995;2:251–254. doi: 10.1016/0926-6410(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Maurice T, Roman FJ, Privat A. Modulation by Neurosteroids of the In Vivo (+)-[3H]SKF-10,047 Binding to sigma1 Receptors in the Mouse Forebrain. Journal of Neuroscience Research. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci. 2008;65:777–797. doi: 10.1007/s00018-007-7403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Mahé V, Robel P, Baulieu EE. Neurosteroids, via cr receptors, modulate the [3lHlnorepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL. Recent Developments in the Significance and Therapeutic Relevance of Neuroactive Steroids – Introduction to the Special Issue. Pharmacol Ther. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokala VN, Witt-Enderby PA, Gibbs RB, Johnson DA. Selective septohippocampal cholinergic lesioning in rat brain: Effects on muscarinic receptor function and secretase activity. Current Topics in Pharmacology. 2010;14:73–78. [Google Scholar]
- Rhodes ME, Li P, Burke AM, Johnson DA. Enhanced plasma DHEAS, brain acetylcholine and memory mediated by steroid sulfatase inhibition. Brain Research. 1997;773:28–32. doi: 10.1016/s0006-8993(97)00867-6. [DOI] [PubMed] [Google Scholar]
- Roland JJ, Levinson M, Vetreno RP, Savage L. Differential effects of systemic and intraseptal administration of the acetylcholinesterase inhibitor tacrine on the recovery of spatial behavior in an animal model of diencephalic amnesia. European Journal of Pharmacology. 2010;629:1–3. 31–39. doi: 10.1016/j.ejphar.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner S, Schliebs R, Hartig W, Bigl V. 192IGG-saporin-induced selective lesion of cholinergic basal forebrain system: neurochemical effects on cholinergic neurotransmission in rat cerebral cortex and hippocampus. Brain research bulletin. 1995;38:371–381. doi: 10.1016/0361-9230(95)02002-9. [DOI] [PubMed] [Google Scholar]
- Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural plasticity. 1998;6:41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Roland J, Klintsova A. Selective septohippocampal - but not forebrain amygdalar - cholinergic dysfunction in diencephalic amnesia. Brain research. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcer KW, DiFrancesca HM, Chandra AB, Li P. Immunohistochemical analysis of steroid sulfatase in human tissues. Journal of Steroid Biochemistry & Molecular Biology. 2007;105:115–123. doi: 10.1016/j.jsbmb.2006.12.105. [DOI] [PubMed] [Google Scholar]
- Twede V, Tartaglia AL, Covey DF, Bamber BA. The neurosteroids dehydroepiandrosterone sulfate and pregnenolone sulfate inhibit the UNC-49 GABA receptor through a common set of residues. Mol Pharmacol. 2007;72:1322–1329. doi: 10.1124/mol.107.034058. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Research Reviews. 2001;37:301–312. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Anzalone SJ, Savage LM. Impaired, spared, and enhanced ACh efflux across the hippocampus and striatum in diencephalic amnesia is dependent on task demands. Neurobiology of Learning & Memory. 2008;90:237–244. doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Research Reviews. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]