Abstract
The investigation of GABAergic systems in learning and extinction has principally focused on ionotropic GABAA receptors. Less well characterized is the metabotropic GABAB receptor, which when activated, induces a more sustained inhibitory effect and has been implicated in regulating oscillatory activity. Few studies have been carried out utilizing GABAB ligands in learning, and investigations of GABAB in extinction have primarily focused on interactions with drugs of abuse. The current study examined changes in GABAB receptor function using the GABAB agonist baclofen (2mg/mL) or the GABAB antagonist phaclofen (0.3mg/mL) on trace cued and contextual fear conditioning and extinction. The compounds were either administered during training and throughout extinction in Experiment 1, or starting 24 hours after training and throughout extinction in Experiment 2. All drugs were administered 1mL/kg via intraperitoneal injection. These studies demonstrated that the administration of baclofen during training and extinction trials impaired animals’ ability to extinguish the fear association to the CS, whereas the animals that were administered baclofen starting 24 hours after training (Experiment 2) did display some extinction. Further, contextual fear extinction was impaired by baclofen in both experiments. Tissue analyses suggest the cued fear extinction deficit may be related to changes in the GABAB2 receptor subunit in the amygdala. The data in the present investigation demonstrate that GABAB receptors play an important role in trace cued and contextual fear extinction, and may function differently than GABAA receptors in learning, memory, and extinction.
Keywords: GABAB, Conditioned fear, Baclofen, Phaclofen
1. Introduction
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the central nervous system and has been implicated in numerous forms of behavior (Baunez & Robbins, 1999; Kim & Richardson, 2007; Mohler, 2012; Wojnicki, Roberts, & Corwin, 2006). GABAergic signaling is mediated through two distinct classes of GABA receptors, the ionotropic GABAA and GABACreceptors, which when activated produce a rapid and very short-lived inhibition via chloride current (Enna, 2007; Olsen, 2001; Watanabe et al., 2002), and the metabotropic GABAB receptor, which is responsible for a slow but sustained inhibitory current (Bettler et al., 2004; Couve, Moss, & Pangalos, 2000). The GABAB receptor is composed of two subunits, GABAB1 and GABAB2. The GABAB1 subunit consists of two isoforms (GABAB1a and GABAB1b) and binds GABA, as well as other ligands (Pinard, Seddik, & Bettler, 2010). Research has suggested that GABAB receptors containing the GABAB1a subunit are primarily located presynaptically and those with GABAB1b subunits are primarily postsynaptic (Vigot et al., 2006). However, this distribution may differ by brain region and whether the presynaptic neuron is GABAergic or glutamatergic (Waldmeier, Kaupmann, & Urwyler, 2008). The GABAB2 subunit is responsible for binding to the intracellular G-protein, which produces presynaptic inhibition of calcium channels (Barral et al., 2000; Bussieres & El Manira, 1999) or postsynaptic activation of inward-rectifying potassium channels (Fernandez-Alacid et al., 2009).
Considerable research investigating GABA systems in learning and memory has demonstrated that the administration of GABAA agonists produces impairments in acquisition (Baunez & Robbins, 1999) and consolidation (Castellano, Cabib, & Puglisi-Allegra, 1996; Chapouthier & Venault, 2002; Myhrer, 2003), while GABAA antagonists and inverse agonists have been demonstrated to consistently enhance acquisition and consolidation in several learning and memory paradigms (Castellano, Cabib, & Puglisi-Allegra, 1996; Chapouthier & Venault, 2002; Collinson et al., 2006; McNally, Augustyn, & Richardson, 2008; Myhrer, 2003), including Pavlovian conditioning (Akirav, Raizel, & Maroun, 2006; McEown & Treit, 2010; Wilensky, Schafe, & LeDoux, 2000). While these data contribute to the understanding of rapid inhibitory currents in learning and memory, GABAA receptors represent only a portion of overall GABAergic signaling.
The data from investigations of GABAB receptor function in learning and memory have been mixed. For instance, rats exhibited impaired acquisition and consolidation in several learning and memory tasks following the administration of baclofen, a GABAB agonist (Castellano, Cabib, & Puglisi-Allegra, 1996; McNamara & Skelton, 1996; Myhrer, 2003; Stuchlik & Vales, 2009). However, Myhrer (2003) reviewed four studies investigating the effects of baclofen on the same passive avoidance task, and each study reported different results, including that baclofen improved (Georgiev, Yonkov, & Kambourova, 1988), impaired (Swartzwelder et al., 1987), or did not alter performance (Car & Wisniewski, 1998; Kuziemka-Leska, Car, & Wisniewski, 1999). These differences may reflect the complexity of metabotropic-mediated inhibitory currents in simple learning and memory tasks.
The results among studies that utilize GABAB antagonists in learning and memory tasks are also mixed. Studies have reported enhanced learning and memory after the administration of several GABAB antagonists (Castellano et al., 1993; Getova & Bowery, 1998); however, these results are not uniform. For instance, Mondadori, Mobius, & Borkowski (1996) administered a GABAB antagonist, CGP 36742, after a passive avoidance task and found that the compound enhanced memory for the task. An alternative investigation (Zarrindast et al. 2002) also administered a GABAB antagonist, CGP 35348, after a passive avoidance task and found that high doses actually decreased memory, whereas low doses had no effect on behavior.
Examination of transgenic animals has also yielded differing interpretations of the role of GABAB in learning and memory. For instance, Jacobson et al. (2006) used a conditioned taste aversion task and found that GABAB1a knockout (KO) mice demonstrated impaired acquisition of the taste aversion, while GABAB1b KO mice acquired the taste aversion as well as the controls. However, Shaban et al. (2006) utilized a two tone Pavlovian conditioning task and found that GABAB1a KO mice demonstrated an over-generalization of fear, or an inability to learn the predictive association; further, GABAB1b KO mice were unable to acquire the task at all.
The results from the above studies using GABAB ligands demonstrate that the findings are difficult to merge into a coherent understanding of the role of GABAB receptors in simple learning and memory tasks. A careful examination of a more difficult version of a Pavlovian task such as cued and contextual fear (CCF) conditioning, a task in which discrete brain regions mediate the acquisition of the associations, may provide additional useful information about the role of GABAB in learning and memory. CCF conditioning allows the investigation of the association between a conditioned stimulus (CS; cued fear) and an unconditioned stimulus (US; a foot shock), as well as the association between the US and the original context in which it was presented (contextual fear). Distinct brain regions, including the amygdala and hippocampus, differentially mediate each aspect of CCF (Phillips & LeDoux, 1992). Research has demonstrated that when the CS and US temporally overlap and co-terminate (delay CCF) the cued fear component requires an intact amygdala (Goosens & Maren, 2001; Vazdarjanova & McGaugh, 1998), whereas contextual fear depends on the hippocampus (Esclassan et al., 2009; Kim & Jung 2006; Phillips & LeDoux 1992).
The acquisition of the associations can be made more difficult by inserting a brief interval between the offset of the CS and the onset of the US (trace CCF; see Figure 1). This change results in the cued fear association being dependent on both the hippocampus and the amygdala (Chowdhury, Quinn, & Fanselow, 2005; Makkar, Zhang, & Cranney 2010; McEchron, et al., 1998; Misane, et al., 2005; Moyer, Deyo, & Disterhoft, 1990; Phillips & LeDoux, 1992). Trace conditioning does not affect contextual fear because all other environmental cues remain unchanged during the US presentation, thus the hippocampus still mediates the contextual fear component (Chowdhury, Quinn, & Fanselow, 2005; McEchron, et al., 1998; Misane et al., 2005). The use of the trace CCF procedure has reliably been used to evaluate more subtle learning and memory alterations based on the increased difficulty of this protocol (Bolton et al., 2012; Holmes et al., 2002; Kinney et al., 2002).
Figure 1.
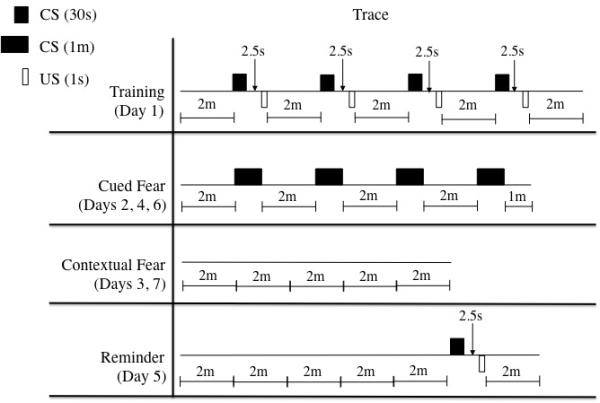
Schematic representation of the trace CCF protocol. Animals in Experiment 1 were administered drugs beginning Day 1 of the protocol. Those in Experiment 2 were administered drugs beginning Day 2 of the protocol.
As with other learning and memory tasks, CCF conditioning also allows for an examination of the extinction of the learned associations, either by presenting the CS without the US, or by placing the animal into the original context without any CS or US presentations. Several studies have demonstrated that GABAergic neurotransmission is likely to play a crucial role in extinction. For instance, infusing muscimol, a GABAA agonist, prior to extinction training into either the basolateral amygdala (Akirav & Maroun, 2007) or dorsal hippocampus (Corcoran & Maren, 2001) enhanced fear extinction. Bicuculline, a GABAA antagonist, infused into the basolateral amygdala after extinction training also produced enhanced fear extinction (Berlau & McGaugh, 2006). Further, Yee et al. (2004) demonstrated that mice lacking the GABAAα5 receptor exhibited impaired fear extinction (i.e. lack of reduction in freezing behavior).
The data for GABAB in extinction are much less extensive, with the majority of the investigations designed to primarily investigate drugs of abuse (e.g. Heinrichs et al., 2010; Lasseter et al., 2009; Voigt, Herrold, & Napier, 2011). These types of studies complicate the understanding of GABAB in extinction as GABAB is not investigated in isolation. Alternatively, other studies have utilized transgenic GABAB1 knockouts (Jacobson et al., 2006), which provide valuable information but at the cost of an altered GABAergic system throughout development. There exists, then, the considerable need for a better characterization of the involvement of GABAB receptors in extinction.
In order to better understand the receptor’s involvement in learning and extinction, we investigated the effects of pharmacologically altering GABAB receptors on learning and extinction in the below studies. We utilized trace CCF, a more difficult variation of the standard delay Pavlovian conditioning that has not been utilized in the examination of GABAB receptor function. Further, trace conditioning has the added utility of being able to investigate local neural network interactions as the cued fear association relies on an interaction of the hippocampus and amygdala. Considerable evidence has indicated that GABAB receptors play a large role in coordinated network function (Brown, Davies, & Randall, 2007), therefore a better understanding of the effect of GABAB receptors in a learning and memory task mediated by connections between brain regions is warranted. In the experiments below, we investigated the effects of baclofen (GABAB agonist) and phaclofen (GABAB antagonist) administration on learning and extinction in trace CCF. The dosage of baclofen selected was based on preliminary testing (unpublished data) and previous reports of altered learning and memory in other tasks (Nakagawa & Takashima, 1997; Swartzwelder et al., 1987; Wilson et al., 2011), and investigations of sub-anesthetic concentrations in nociception studies (see De Luca & Massotti, 1990). Considerably fewer studies have investigated phaclofen in behavior and the dose was selected based on preliminary testing identifying the minimal dose that induced behavioral or tissue differences (unpublished data). In order to evaluate if any learning deficits observed may be associated with alterations in sensory function by the drug treatments we performed two control tests. The animals were tested for their ability to detect auditory stimuli and to confirm normal startle reflexes using an acoustic startle task; additionally, the tail flick test was utilized to ensure that the administered ligands did not alter the perception of pain. We then examined brain tissue for alterations to GABAergic proteins.
2. Materials and Methods
2.1. Subjects
Sixty male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) approximately three months of age and weighing 250-300g were used. Rats were housed in a temperature and humidity controlled facility, and food and water were provided ad libitum. Animals were housed in pairs and kept on a 12:12 light:dark cycle, lights on at 7:00am. All procedures were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with NIH guidelines for the care and use of animals.
2.2. Drug Treatments
R(+)-Baclofen hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in0.9% physiological saline vehicle at a concentration of 2mg/mL. Phaclofen (Sigma-Aldrich) was dissolved in 0.9% physiological saline vehicle at a concentration of 0.3mg/mL. Compounds were administered 1mL/kg body weight via intraperitoneal (i.p.) injection 15 minutes before behavioral testing. Drug treatment started on Day 1 for all groups in Experiment 1 and on Day 2 for all groups in Experiment 2, and continued daily until tissue collection. Animals were randomly assigned to one of three treatment groups (n=10) for a total of n=30 per experiment. Due to a complication that arose during the experiment, one of the animals in the baclofen treatment group in Experiment 2 was removed.
2.3. Trace Cued and Contextual Fear Conditioning
In order to examine the effects of altered GABAB tone on learning and extinction, we utilized a common procedural variant of CCF and methods are as previously described (Bolton et al., 2012; Kinney et al., 2002). Animals were trained in the trace CCF procedure, as described in Section 2.3.2. In Experiment 1, drug treatment began 15 minutes before training on Day 1; for Experiment 2, animals did not receive any drug injections during training and the first administration of drug was on Day 2, 15 minutes before testing began. Drug treatment continued throughout the entire protocol for both experiments.
2.3.1. Apparatus
Fear conditioning training and contextual fear testing were conducted in a 25.4cm × 25.4cm × 19.05cm (L×W×H) acrylic chamber (San Diego Instruments, San Diego, CA). The floor of the chamber consisted of a stainless steel grid made of 0.64cm rods spaced at1.43cm. The chamber was cleaned between subjects using a common household cleaner, Formula 409 (Clorox Company).
Cued fear testing was conducted in an altered context 43.18cm × 26.67cm × 12.7cm (L×W×H) made of opaque plastic. In addition to differences in material and shape, a novel scent cue (vanilla extract) was added to one of the walls. The chamber was cleaned between animals using a 1% ethanol solution to ensure no olfactory overlap with the training chamber.
All sessions were programmed and run with Freeze Monitor software (San Diego Instruments) and freezing behavior (defined as no movement from the animal except for breathing) was collected by visual inspection every 10 seconds by a researcher. The data collected for each animal included proportion time freezing in each session.
2.3.2. Training
Subjects were taken individually from the colony room to a testing room containing the fear conditioning chamber. Animals were allowed to explore the chamber for two minutes. After the two-minute acclimatization period, the CS, a 2.9kHz 88dB tone, was presented for 30 seconds. Two and a half seconds following the termination of the CS, a one-second mild foot shock (0.5mA; US) was presented (see Figure 1). Once the US terminated, the animals were given another two minutes to explore the chamber before the next CS-US presentation. The CS-US pairing was presented three more times, for a total of four pairings. The animals were given a final two minutes in the chamber after the last CS-US pairing. Data collected from this session consisted of freezing during the first (PreCSUS) and last two minutes (PostCSUS) of the session.
2.3.3. Cued Fear Test
Animals were placed into the altered context and allowed to explore for two minutes; after the two-minute exploration, the CS was presented for one minute. Following the offset of the CS, the animal had another two minutes to explore before the CS was presented again. The CS was presented in this manner for a total of four presentations during the cued fear test session (see Figure 1). The first two minutes of the session (PreCS1), freezing during each CS presentation, and freezing in-between each CS presentation were separately binned for analyses in order to determine initial fear (freezing) to the novel context, fear to the CS, and fear expressed between CS presentations. Animals were tested for cued fear on Days 2, 4, and 6.
2.3.4. Contextual Fear Test
Animals were placed in the original training chamber for 10 minutes; neither the CS nor the US was presented during this time (see Figure 1). Freezing behavior during the 10-minute session was averaged for each animal. Contextual fear was tested on Days 3, 5, and 7.
2.3.5. Reminder
On Day 5, after the completion of the second contextual fear test session, the animals underwent a single reminder trial (see Figure 1). The reminder trial consisted of a single presentation of the 30-second CS, followed 2.5 seconds later by the one-second US, and then a two-minute observation period. The data collected were analyzed separately as the contextual fear test session and the reminder trial (binned into the 30- second CS and the final two minutes).
2.4. Acoustic Startle
The animals were tested for their ability to detect the auditory stimulus, as well as to confirm normal startle reflexes, using a sound attenuation chamber consisting of a transparent Plexiglas tube (10cm × 20cm) mounted on an accelerometer (San Diego Instruments). Trials were programmed and run with the Startle software from San Diego Instruments. Rats were placed into the chambers (28cm × 28cm × 28cm (L×W×H)) and given five minutes to acclimate with only background noise (65dB) present. Ten millisecond white noise bursts were presented at 90, 100, 110, and 120dB throughout the session. Each stimulus was presented five times in random order with inter-trial intervals randomized between 10 and 55 seconds. At the end of the session, animals were returned to their home cage. This test was done 24 hours after the completion of the fear conditioning procedure. For evaluation, the data for the first presentation of each stimulus were removed and the remaining four trials were averaged.
2.5. Tail Flick
After acoustic startle testing, the animals underwent a nociception test to ensure that the administered ligands did not alter the perception of pain. The tip (end 2cm) of the animal’s tail was placed into water heated to 55°C; researchers timed and recorded the latency for the animal to react to (flick) or remove its tail from the water. A maximum of 10 seconds was set for the animal to react to or remove its tail from the heated water to ensure no damage occurred, and no animals ever reached this criterion. Fifteen minutes following the tail flick test, tissue was collected from the animals.
2.6. Tissue Collection
Following the tail flick test, rats were individually euthanized via CO2 asphyxiation and hippocampi, cortices, and amygdalae were rapidly dissected out and flash frozen for SDS-PAGE western blotting analysis.
2.7. SDS-PAGE Western Blotting
Each region of tissue was homogenized in RIPA lysis buffer (Cell Signaling, Danvers, MA). Lysates were centrifuged at 15,000xg for 15 minutes at 4°C; the supernatants were then collected and protein concentrations were determined using the bicinchoninic acid assay (Pierce, Rockford, IL). Samples were loaded at a concentration of 20μg into 8% SDS-PAGE gels and separated via electrophoresis (Laemmli, 1970). Proteins were then transferred to a nitrocellulose membrane and blocked in 5% BSA mixed in TBS-Tween-20 with 0.02% sodium azide. Membranes were incubated in primary antibody overnight mixed in 5% BSA-TBS-Tween-20 (rabbit anti-GABAB1, 1:2000, Cell Signaling; rabbit anti-GABAB2, 1:1000, Cell Signaling; rabbit anti-GABAAα5, 1:1000, Millipore, Billerica, MA; mouse or rabbit anti-β-actin, 1:10,000, Cell Signaling). The next day, membranes were incubated in HRP-conjugated secondary antibodies mixed in 5% milk-TBS-Tween-20 (goat anti-mouse or goat anti-rabbit, 1:5000, Vector Laboratories, Burlingame, CA) and then probed with Amersham ECL Plus (GE Healthcare Life Sciences, Piscataway, NJ) and imaged with a Typhoon 9410 Variable Mode Imager (GE Healthcare Life Sciences). Band intensity was determined via ImageQuant 5.2 (GE Healthcare Life Sciences). Western blots were analyzed by normalizing the densities of the protein of interest to the density of -actin for each individual sample. A proportion was determined for each normalized value of the treatment group protein bands compared to the averaged normalized values for saline control groups run in the same gel. These proportional values were used for analysis.
2.8. Statistical Analyses
Analyses were performed using SPSS. Between and within group training, cued and contextual fear data, and between group PreCS1 data were analyzed using analysis of variance (ANOVA). Data from tail flick latencies, and western blot analyses were analyzed via ANOVA. Startle amplitudes were analyzed via repeated measures ANOVA (RMANOVA), and an ANOVA was completed for each individual startle amplitude if the RMANOVA was significant. Tukey post-hoc comparisons were performed following a significant result where applicable.
3. Results
3.1. Trace Cued and Contextual Fear Conditioning
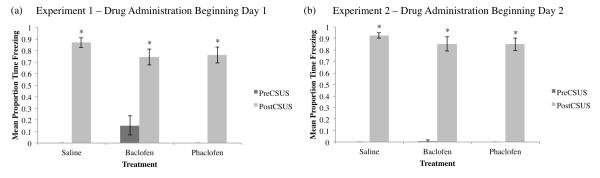
In Experiment 1, drug treatment began on Day 1. One-way ANOVA of each group’s average proportion time freezing during the first two minutes of training before any CS-US pairings (PreCSUS, see Figure 2a) did not reveal any significant differences between groups (F2,27=0.263, p>0.05). Further, there were no differences between groups during the last two minutes of training (PostCSUS, F2,27=1.259, p>0.05). All groups demonstrated significantly increased freezing during the last two minutes of training compared to the first two minutes before any CS-US pairings (PreCSUS vs PostCSUS freezing; saline F1,18=434.571; baclofen F1,18=30.552; phaclofen F1,18=122.379; p<0.01 for all groups). In Experiment 2, drug treatment began on Day 2. There were no significant differences between groups during the first (PreCSUS, F2,26=1.121, p>0.05) or last (PostCSUS, F2,26=0.783, p>0.05) two minutes of training (see Figure 2b). All groups demonstrated significantly increased freezing during the last two minutes of training compared to the first two minutes before any CS-US pairings (PreCSUS vs PostCSUS; saline F1,18=1607.087; baclofen F1,17=182; phaclofen F1,18=236.455; p<0.01 for all groups).
Figure 2.
Mean proportion time freezing (±SEM) during the initial two minutes before the first CS-US pairing (PreCSUS) and the final two minutes after the last CS-US pairing (PostCSUS) during training on Day 1 for Experiment 1, drug administration beginning Day 1 (a) and Experiment 2, drug administration beginning Day 2 (b). All groups froze significantly more in the final two minutes of training (*=significant difference between PreCSUS and PostCSUS, p<0.05). No significant differences appeared between groups in the first and last two minutes of training for Experiment 1 or Experiment 2.
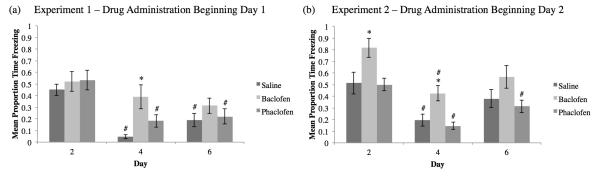
Cued Fear was tested on Days 2, 4, and 6 in the altered context chamber to determine the strength of the association between the CS and the US (Figure 3a,b). As demonstrated in Figure 3a, on Day 2 for Experiment 1, there were no significant differences in freezing between groups during the CS presentations (F2,27=0.366, p>0.05); however on Day 4, the baclofen-treated animals demonstrated significantly higher freezing compared to saline-treated group (F2,27=6.407, p<0.01; Tukey post-hoc saline vs baclofen (SvB) p<0.01). On Day 6, which occurred 24 hours after the Reminder Trial, this difference disappeared and no significant differences between groups was observed (F2,27=1.07, p>0.05). In Experiment 2 (Figure 3b), the baclofen-treated animals froze significantly more than the saline-treated group during the CS presentations on Days 2 (F2,26=5.187, p<0.05; Tukey post-hoc SvB p<0.05) and 4 (F2,26=8.033, p<0.05; Tukey post-hoc SvB p<0.05). On Day 6, this difference disappeared and no significant differences between groups were observed (F2,26=2.846, p>0.05).
Figure 3.
Mean proportion time freezing (±SEM) during Cued Fear Extinction for Experiment 1 (a) and Experiment 2 (b). In Experiment 1, drug administration beginning Day 1, all groups froze equivalently on Days 2 and 6, but the baclofen group did not exhibit a decline in freezing on Day 4 indicative of extinction (a; *=significant difference compared to the saline group, p<0.05). Both the saline and phaclofen groups significantly decreased freezing on Days 4 and 6 compared to Day 2, indicative of the extinction of the fear behavior (# = significant difference compared to Day 2, p<0.05). For Experiment 2, drug administration beginning Day 2, the baclofen group froze significantly more than the saline group on Days 2 and 4, but not on Day 6 (b). However, all groups significantly decreased freezing from Day 2 to Day 4.
Cued fear data were also analyzed to determine if there were any differences in freezing within treatment group across sessions (Days 2, 4, and 6) as an indication of extinction (Figure 3a,b). The baclofen group from Experiment 1 did not show any significant change in freezing behavior across days (see Figure 3a). The saline (F2,27=21.304, p<0.01; Tukey post-hoc Day 2 vs Day 4 and Day 2 vs Day 6 p<0.01) and phaclofen (F2,27=7.781, p<0.01; Tukey post-hoc Day 2 vs Day 4 and Day 2 vs Day 6 p<0.01) groups displayed a significant decrease in freezing across days. In Experiment 2 (see Figure 3b), however, all groups demonstrated a significant decrease across days (saline: F2,27=4.372, p<0.01, Tukey post-hoc Day 2 vs Day 4 p<0.05; baclofen: F2,24=5.739, p<0.05, Tukey post-hoc Day 2 vs Day 4 p<0.05; phaclofen: F2,27=14.235, p<0.01; Tukey post-hoc Day 2 vs Day 4 and Day 2 vs Day 6 p<0.05).
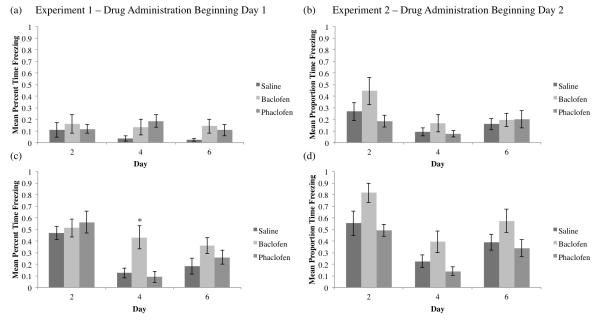
In order to determine if the altered context induced any fear (neophobia), we analyzed the first two minutes before any CS presentations of the Cued Fear sessions (see Figure 4a,b). There were no significant differences between groups during the first two minutes before the CS presentations on any day for Experiment 1 (Day 2: F2,27=0184; Day 4: F2,27=2.286; Day 6: F2,27=1.706; p>0.05 for all Days) nor Experiment 2 (Day 2: F2,26=2.486; Day 4: F2,26=1.018; Day 6: F2,26=0.14; p>0.05 for all Days).
Figure 4.
Mean proportion time freezing (±SEM) during the first two minutes before a CS presentation by day (a, b) and in between CS presentations by day (c, d) for Experiment 1 (a, c) and Experiment 2 (b, d). There were no significant differences between groups for either Experiment 1, drug administration beginning Day 1 (a) or Experiment 2, drug administration beginning Day 2 (b) for freezing during the first two minutes before a CS presentation. In Experiment 1, there were no significant differences between groups on Days 2 or 6 for freezing in between CS presentations, however the baclofen group froze significantly more than the saline group on Day 4 (c; *=significant difference compared to the saline group, p<0.05). In Experiment 2, there were no significant differences in freezing in between CS presentations for any day (d).
Additionally, we analyzed the time blocks between the CS presentations (Figure 4c,d). While there were no differences in freezing in between CS presentations among the groups on Days 2 or 6 in Experiment 1 (Figure 4c), the baclofen group exhibited significantly greater freezing compared to the saline-treated group on Day 4 (F2,27=7.614, p<0.01, Tukey post-hoc SvB p<0.05). In Experiment 2 (Figure 4d) there were no significant differences between groups for freezing behavior in between CS presentations for any day (Day 2: F2,26=4.184, p<0.05, Tukey post-hoc did not reveal any significant differences compared to saline; Day 4: F2,26=3.947, p<0.05, Tukey post-hoc did not reveal any significant differences compared to saline; Day 6: F2,26=2.247, p>0.05).
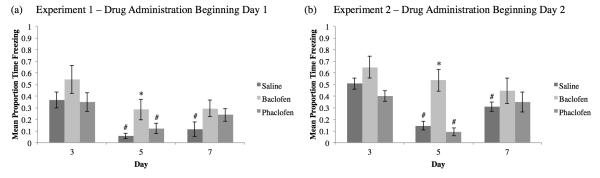
Contextual Fear was tested on Days 3, 5, and 7 when the animals were placed back in the original training context and observed for ten minutes, with no CS or US presentations (Figure 5). No significant differences were found compared to the saline group in either Experiment on Days 3 (Experiment 1: F2,27=0.274, p>0.05; Experiment 2: F2,26=3.676, p<0.05, Tukey post-hoc did not reveal any significant differences compared to saline) or 7 (Experiment 1: F2,27=2.188, p>0.05; Experiment 2: F2,26=0.737, p>0.05). However, in both experiments (see Figure 5a,b), the baclofen group displayed significantly greater freezing versus saline controls on Day 5 (Experiment 1: F2,27=4.045, p<0.05; Tukey post-hoc SvB p<0.05; Experiment 2: F2,26=16.531, p<0.01; Tukey post-hoc SvB p<0.01).
Figure 5.
Mean proportion time freezing (±SEM) during Contextual Fear Extinction for Experiment 1 (a) and Experiment 2 (b). In Experiment 1, drug administration beginning Day 1, all groups froze equivalently on Days 3 and 5, but the baclofen group did not exhibit a decline in freezing on Day 4 indicative of extinction (a; *=significant difference compared to the saline group, p<0.05). Both the saline and phaclofen groups significantly decreased freezing on Days 5 and 7 compared to Day 3, indicative of the extinction of the fear behavior (# = significant difference compared to Day 2, p<0.05). For Experiment 2, drug administration beginning Day 2, all groups froze equivalently on Days 3 and 5, but the baclofen group froze significantly more than the saline group on Day 5 (b). Further, only the saline and phaclofen groups exhibited significantly decreased freezing on Day 5 compared to Day 3.
These data were also analyzed to determine if there were any differences in freezing within treatment groups across days (extinction). As illustrated in Figure 5a, only the saline and phaclofen groups demonstrated a significant decrease in freezing across days in Experiment 1 (saline: F2,27=9.137, p<0.01, Tukey post-hoc Day 2 vs Day 4 and Day 2 vs Day 6 p<0.01; baclofen: F2,27=2.354, p>0.05; phaclofen: F2,27=3.453, p<0.05, Tukey post-hoc Day 2 vs Day 4 p<0.05). Similarly in Experiment 2 (Figure 5b), only the saline and phaclofen groups exhibited extinction across days (saline: F2,27=19.192, Tukey post-hoc Day 2 vs Day 4 and Day 2 vs Day 6 p<0.01; baclofen: F2,24=1.045, p>0.05; phaclofen: F2,27=7.667, p<0.01, Tukey post-hoc Day 2 vs Day 4 p<0.01). No differences were found between groups during the two minutes after the reminder on Day 5 for Experiment 1 (F2,27=1.069, p>0.05) or Experiment 2 (F2,26=0.46, p>0.05).
3.2. Acoustic Startle
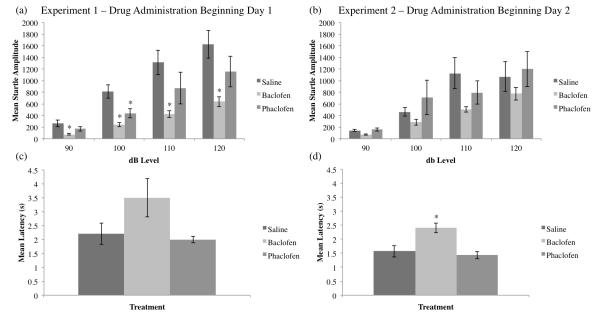
To determine whether baclofen or phaclofen altered auditory sensitivity, we tested startle amplitudes to pulses at several decibel levels (Figure 6a,b). For Experiment 1 (Figure 6a), RMANOVA revealed a significant between-subjects effect (F2,117=8.559, p<0.01, Tukey post-hoc SvB p<0.01), and the one-way ANOVA revealed significant differences between the saline and baclofen groups for all startle amplitudes, and between the saline and phaclofen groups for the 100db startle only (90db: F2,117=5.466, p<0.01, Tukey post-hoc SvB p<0.01; 100db: F2,117=12.726, p<0.01, Tukey post-hoc SvB and saline vs phaclofen (SvP) p<0.01; 110dB: F2,177=4.828, p<0.05, Tukey post-hoc SvB p<0.01; 120db: F2,117=5.428, p<0.01; Tukey post-hoc SvB p<0.01). For Experiment 2 (Figure 6b), drug administration beginning Day 2, the RMANOVA did not reveal any significant between-subjects effects for the startle amplitudes (F2,113=1.393, p>0.05).
Figure 6.
Mean startle amplitude (±SEM) to pulses at several decibel levels and for Experiment 1, drug beginning Day 1 (a) and Experiment 2, drug beginning Day 2 (b). The baclofen group in Experiment 1 (a) startled significantly less than the saline group at all decibel levels, and the phaclofen group at 100dB only (*=significantly different from saline, p<0.05). All groups in Experiment 2 startled equivalently (b). Mean latency (±SEM) for animals to remove their tails from heated water for Experiment 1 (c) and Experiment 2 (d). All groups in Experiment 1 have statistically equivalent tail flick latencies (c), whereas the baclofen group in Experiment 2 had a significantly increased tail flick latency compared to the saline group (d).
3.3. Tail Flick
To determine whether baclofen or phaclofen altered sensitivity to noxious stimuli, the tail flick test was used to measure analgesia (Figure 6c,d). One-way ANOVA did not reveal any significant differences between groups for Experiment 1 (F2,27=3.126, p>0.05; see Figure 6c). However, in Experiment 2, drug administration beginning Day 2, the baclofen-treated animals had a significantly longer tail flick latency compared to the saline-treated animals (F2,26=9.122, p<0.01, Tukey post-hoc SvB p<0.01; see Figure 6d).
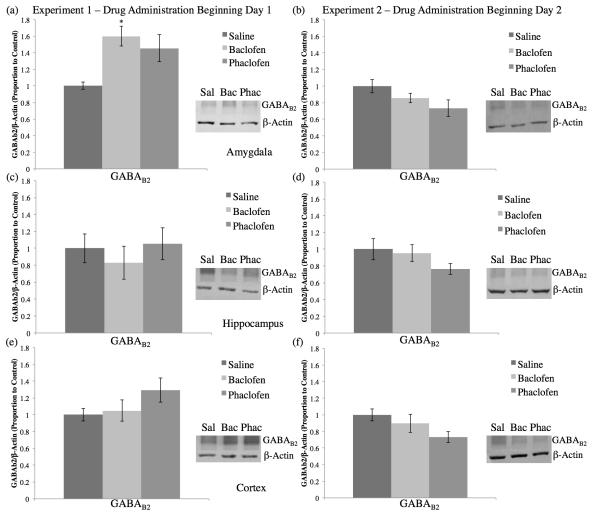
3.4. SDS-PAGE Western Blotting
A significant increase in GABAB2 protein levels in the amygdala was observed in the baclofen-treated group compared to the saline controls in Experiment 1 (F2,19=5.178, p<0.05, Tukey post-hoc SvB p<0.05; see Figure 7a). No significant differences in GABAB2 protein levels in the amygdala were found in Experiment 2 (F2,25=2.682, p>0.05; see Figure 7b). No significant differences were found in hippocampal GABAB2 protein levels for either Experiment 1 (F2,27=0.395, p>0.05; see Figure 7c) or Experiment 2 (F2,25=1.633, p>0.05; see Figure 7d). Further, there were no significant differences in cortical GABAB2 protein levels for Experiment 1 (F2,27=1.787, p>0.05; see Figure 7e) or Experiment 2 (F2,25=2.956, p>0.05; see Figure 7f).
Figure 7.
Average proportion of GABAB2/ -actin to control (±SEM) for tissue from the amygdala (a, b), hippocampus (c, d), and cortex (e, f) for Experiment 1 (a, c, e) and Experiment 2 (b, d, f). The baclofen group in Experiment 1, drug administration beginning Day 1, had demonstrated significantly more GABAB2 total protein levels compared to the saline group in the amygdala (a; *=significantly different from saline, p<0.05). There were no significant differences in GABAB2 total protein levels compared to the saline group for Experiment 1 in the hippocampus (c) or cortex (e), nor for Experiment 2 in the amygdala (b), hippocampus (d), or cortex (f).
Evaluation of GABAB1 protein levels in several brain regions did not indicate a significant difference. In the amygdala, there were no significant differences between groups in GABAB1a protein levels for Experiment 1 (F2,19 =0.986, p>0.05) or Experiment 2 (F2,25=2.696, p>0.05), nor were there any significant differences between groups in GABAB1b protein levels for Experiment 1 (F2,19=1.063, p>0.05). In Experiment 2, however, there was a significant reduction of GABAB1b protein levels in the amygdala for the phaclofen-treated group compared to the saline controls (F2,25=5.039, p<0.05, Tukey post-hoc SvP p<0.05). In the hippocampus, no significant differences were found in GABAB1 protein levels for Experiment 1 (GABAB1a: F2,27=0.168; GABAB1b: F2,27=0.409; p>0.05 for both subunits) nor in Experiment 2 (GABAB1a: F2,25=0.176; GABAB1b: F2,25=0.154; p>0.05 for both subunits). Finally, no significant differences were found in cortical GABAB1 protein levels in Experiment 1 (GABAB1a: F2,27=0.680; GABAB1b: F2,27=0.638; p>0.05 for both subunits) nor in Experiment 2 (GABAB1a: F2,25=6.712, p<0.01; GABAB1b: F2,25=4.574, p<0.05, Tukey post-hoc did not reveal any significant differences compared to saline for either protein).
4. Discussion
In the above studies, we were interested in determining the effect of altered GABAB function on learning and extinction using the trace cued and contextual fear conditioning paradigm. In Experiment 1, the GABAB agonist baclofen and GABAB antagonist phaclofen were administered throughout the entirety of the experiment. Interestingly, the baclofen-treated animals did not exhibit extinction to the CS or to the context as did the saline- and phaclofen-treated groups. These extinction deficits are evidenced by the lack of a significant decrease in freezing to the CS from Day 2 to 4 and by the lack of a significant decrease in freezing to the original context from Day 3 to 5. Further, we did not observe any significant differences between groups 24 hours following training, suggesting that these differences cannot be attributed to alterations in initial learning.
In Experiment 2, we investigated the effects of altered GABAB function in extinction after subjects had already learned the association (drug starting Day 2). Similar to Experiment 1, the baclofen-treated animals in Experiment 2 also exhibited an extinction deficit, but only to the context, as demonstrated by the lack of significantly decreased freezing from Day 3 to 5. Contrary to Experiment 1, the baclofen group did demonstrate significantly greater freezing to the CS presentations compared to the saline group on Days 2 and 4. This difference suggests that enhanced GABAB receptor activity after the initial fear conditioning may create an exaggerated fear response (association) compared to the control group. The baclofen group in Experiment 2 also demonstrated a significant reduction in freezing to the CS from Day 2 to 4, similar to the saline group, thus demonstrating some extinction to the CS. When this result is taken into consideration with the baclofen-induced cued fear extinction deficit from Experiment 1, these data suggest that increased GABAB receptor function during the initial training impairs the ability to extinguish a fear response to the CS. This difference is interesting because it suggests that facilitating GABAB receptor activity during training and not after training may induce an association that is more resistant to extinction. The baclofen treated animals did display equivalent learning of the association 24 hours post-training; however, this conditioned fear did not dissipate after repeated CS presentations analogous to controls. Similar to Experiment 1, the phaclofen-treated animals showed no behavioral differences compared to the saline control group. Future investigations will help further clarify whether the extinction deficits observed in this study are inducible via baclofen administration during training only or whether they require the administration of baclofen during training and throughout testing.
In order to ensure the above change is tied to extinction, we analyzed the data for the first two minutes of each Cued Fear test session, as well as the time between CS presentations, in order to determine if the treatment groups were simply freezing throughout the entire session. Neither the baclofen or phaclofen group demonstrated increased freezing during the first two minutes of the Cued Fear sessions compared to the saline groups, which suggests that the increased freezing is specific to the CS presentations. Furthermore, there were no significant differences between groups in Experiment 2 for freezing between CS presentations, demonstrating that all of the treatment groups were behaving equivalently when the CS was not present. However, in Experiment 1 the baclofen group froze significantly more than the saline group between CS presentations only on Day 4. This result, taken in conjunction with the above data, suggests that the baclofen group in this experiment did not benefit from the extinction training on Day 2. As this difference was only observed during the session when the baclofen group displayed a lack of reduction in freezing to the CS (that is, not during Days 2 or 6), we are confident this behavior is not representative of a general elevated anxiety phenotype due to the administration of baclofen.
For contextual fear, all groups demonstrated equivalent freezing to the context on Day 3 (the first exposure to the original training context after training), demonstrating equivalent learning of the association between the context and US. The phaclofen-treated groups in both experiments had no behavioral differences compared to saline controls. However, the baclofen groups in both experiments demonstrated a contextual fear extinction deficit, as evidenced by a lack of a significant reduction in freezing from Day 3 to Day 5. These results suggest that increased GABAB function impairs the extinction of the hippocampally-mediated contextual fear, regardless of whether or not the drug is present during the initial training. Further, these differences between groups disappear on Day 7, which occurred 48 hours after the reminder trial, suggesting the reinstatement of the fear association between the US and the context in the saline and phaclofen groups without any elevation in the baclofen groups.
The amygdala has been demonstrated to play a role in cued fear association (Goosens & Maren, 2001; Phillips & LeDoux, 1992; Vazdarjanova & McGaugh, 1998), and would be a likely site for changes impacting extinction. Additionally, previous investigations of GABAA systems in extinction have argued that cortical GABAergic neurons projecting to the amygdala mediate some aspects of extinction (Akirav & Maroun, 2007; Akirav, Raizel, & Maroun, 2006; Makkar, Zhang, & Cranney, 2010). The observed cued fear extinction deficit in Experiment 1 may be related to altered receptivity to GABA in the amygdala. In Experiment 1, there was a significant increase in the total protein levels of the GABAB2 subunit in the amygdala of the baclofen group, the only group that failed to display cued fear extinction. The possibility exists that an increase in the GABAB2 subunit may have altered network connectivity in the amygdala and may be related to the lack of cued fear extinction seen in Experiment 1. Since the baclofen group in Experiment 2 did show a significant decease in freezing from Day 2 to Day 4, the lack of altered GABAB2 protein levels in the amygdala in this experiment may further support the hypothesis that the GABAB2 subunit is linked with cued fear extinction.
The phaclofen-treated group from Experiment 1 also demonstrated a non-significant increase in total GABAB2 protein levels (see Figure 7a), but there were no behavioral differences observed. It is possible, therefore, that the increases in total protein level observed (significant for baclofen, non-significant for phaclofen) in this experiment are in response to alterations in GABAB tone, and are not necessarily linked to the behavioral differences. Equally interesting is that this increase in total protein was not observed in either treatment group in Experiment 2. It is possible that altering GABAB signaling during the training session induced a different effect as compared to altering GABAB tone during other sessions. Specifically, these data suggest that alterations in GABAB signaling coupled with the delivery of the US appears to produce different alterations in total GABAB receptor protein levels than when the drug treatments are administered after training. The extent to which this difference directly impacts brain function and behavior is unclear and certainly merits further investigation.
Data from the tissue analyses cannot account for all of the behavioral differences mentioned above. None of the protein markers we examined in the hippocampus indicated a difference that may be related to the contextual fear extinction deficit seen in both baclofen groups, suggesting the administration of baclofen may have altered other transmitter systems, or other GABAergic targets (see below). However, it is clear that increasing GABAB function via baclofen does inhibit the extinction of a hippocampally-mediated association. Research indicates that the dorsal hippocampus mediates contextual fear conditioning (Esclassan et al., 2009; Kim & Fanselow, 1992; Quinn et al., 2005), so it is possible that analyzing whole hippocampus GABAergic protein levels hindered the discovery of a significant target related to the observed contextual fear extinction deficits. Alternatively, it is also possible that the total protein changes in the amygdala could contribute to these observed behavioral changes. For instance, it has been established that amygdala activity is capable of mediating performance of hippocampal-dependent behavioral tasks (Galliot et al., 2010; McIntyre, Marriott, & Gold, 2003; Vafaei et al., 2007). Considering the extent to which amygdala function contributes to hippocampal-dependent processes, the protein changes in the present study may have relevance to the behavioral data. The increase in total GABAB2 protein levels found in the amygdala could have had a direct effect on the hippocampally-mediated contextual fear extinction deficit seen in Experiment 1. However, the lack of significant changes in total protein levels of the targets we examined in the amygdala or in the hippocampus of any of the treatment groups in Experiment 2 make a direct connection impossible. Thus, while it is possible for the amygdala to be contributing to the contextual fear extinction deficit in Experiment 1, this explanation does not sufficiently address the contextual fear extinction deficit observed in Experiment 2.
The extinction deficits associated with the administration of baclofen found in these studies are surprising considering the extensive literature that suggests extinction is largely facilitated by inhibitory mechanisms (see Makkar, Zhang, & Cranney, 2010). Possibly because the bulk of the data are derived from research that used compounds that target GABAA receptors, GABAB-driven alterations of extinction may just need to be characterized further. Alternatively, it is possible that altering the GABAB system may alter existing circuits through autoinhibition. By preventing the presynaptic release of GABA via enhancing the function of autoreceptors, baclofen administration could result in the lack of inhibition on the circuits responsible for maintaining the fear associations, thus the associations stay intact.
It is possible the baclofen-induced extinction deficits in Experiment 1 may be influenced by altered auditory processing based on data from the acoustic startle experiments. However, we believe this is unlikely based on equivalent performance in the fear conditioning experiments between groups 24 hours post-training. If baclofen impaired detection of the auditory CS, we would expect differences in the initial cued fear session (e.g. decreased freezing compared to the saline controls). Given the data of equivalent performance between groups it seems likely the baclofen group detected the auditory CS consistent with controls. Further, the differences in acoustic startle were not observed in Experiment 2. A closer evaluation of the acoustic startle data between the two experiments suggests that the saline controls in Experiment 1 displayed a much larger startle response than controls in Experiment 2. This is in contrast to consistent startle amplitudes for both drug treatment groups in Experiment 1 and 2, suggesting the significant difference may be related to an elevated response in a subset of controls. These data merit additional investigations and could be evaluated using both acoustic and tactile startle approaches. Similarly, we believe the differences in the tail flick nociception task observed only in Experiment 2 are not directly related to the above extinction deficits. If a true nociceptive reduction were induced by baclofen, we would expect differences in freezing (decreased compared to the saline controls) in the initial cued and contextual fear sessions on Days 2 and 3 as reported elsewhere with drugs associated with analgesia (see Abbot, Melzack, & Leber, 1982). No such reduction was observed in the above experiments, and data from other investigations have demonstrated that systemic administration of baclofen at the dosage used in the current study does not inhibit nociception (De Luca & Massotti, 1990; Smith et al., 1994). As the difference in tail flick was only significant in one experiment and we have not observed a similar difference in preliminary studies with baclofen and phaclofen (unpublished results) we do not believe the extinction deficits are related to altered nociception. These data do merit additional investigation to determine why the administration of baclofen induced changes in the above tests; however, given equivalent learning between all groups it seems unlikely that the extinction deficits observed could be mediated by these differences.
The administration of phaclofen did not alter performance in either experiment, but it did produce a significant decrease in total protein levels of the GABAB1b subunit compared to the saline group in the amygdala tissue from Experiment 2. This result suggests that although phaclofen used at the above concentration may not be altering behavior in CCF, the drug did induce a physiological effect at the cellular level. The decrease in total protein may be an initial response to the drug, but these changes do not appear to specifically impact the fear conditioning as examined in these studies. It is possible that the alteration of total GABAB1 protein levels may impact performance in other tasks and/or that a higher dosage of phaclofen may be sufficient to alter performance in trace CCF. The novel finding that altering GABAB tone impairs extinction merits substantial additional investigations, some of which include examining alternative doses of phaclofen and baclofen, determining if phaclofen is able to reverse the baclofen-induced extinction deficits, as well as extending these investigations into additional learning and memory tasks.
Future experiments are also needed to help elucidate the protein correlates to these behavioral differences. It is possible that other GABAergic targets (such as inward-rectifying potassium (GIRK) channels, GAD65/67, or GABA transporters) were altered. Studies have demonstrated that the GABAB receptors do not respond in a typical manner seen with other G-protein coupled receptors. For instance, Wetherington and Lambert (2002) demonstrated that GIRK-associated postsynaptic GABAB receptors rapidly desensitized to agonist treatment, whereas GIRK-associated presynaptic GABAB receptors failed to desensitize even after 24 hours of agonist treatment. Therefore, it is possible that presynaptic GABAB receptors are less sensitive to prolonged ligand treatment. Other research has demonstrated that GABAB receptors do not internalize in response to agonist treatment (Fairfax et al., 2004; Mutneja et al., 2005; Perroy et al., 2003), but that agonist treatment does produce a decrease in cell surface-expressed receptors (Fairfax et al., 2004). Further, it would be interesting to determine if the total protein level differences observed translate to functional or even membrane-expressed differences in GABAB receptors. Additional studies are also needed to help determine if altering GABAB receptor function using GABAB antagonists can affect fear learning and extinction.
Overall, the data presented in the above studies suggest a significant role of GABAB receptor function in fear learning and extinction. In particular, enhanced GABAB function during training appears to influence the ability to extinguish the fear response to a conditioned stimulus, and may be mediated by the GABAB2 subunit in the amygdala. Regardless of when the drug was administered, baclofen impaired the extinction of the fear association to the original context. It appears that the GABAB receptor may play an alternative role in learning and extinction compared to the GABAA receptor. Generally, GABAA agonists tend to impair learning, but enhance extinction. The current study found that using a GABAB agonist did not impair learning, but that it did prevent extinction. Future experiments are needed to determine if the administration of phaclofen is capable of rescuing the extinction deficit induced by baclofen, as well as if a higher dose of phaclofen alone may be able to affect a change in behavior, and further characterize GABAergic targets that may mediate these extinction deficits.
Highlights (limited to 3-5 points, max 85 characters per point).
Baclofen administered during training impaired extinction to the CS.
Regardless of time of administration, baclofen impaired contextual extinction.
GABAB2 subunit in amygdala may mediate extinction to the CS.
Acknowledgements
This publication was made possible by grants from the National Center for Research Resources (5P20RR016464-11) and the National Institute of General Medical Sciences (8 P20 GM103440-11) from the National Institutes of Health.
Abbreviations
- ANOVA
analysis of variance
- CCF
cued and contextual fear conditioning
- CR
conditioned response
- CS
conditioned stimulus
- GABA
gamma-aminobutyric acid
- US
unconditioned response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbot FV, Melzack R, Leber BF. Morphine analgesia and tolerance in the tail-flick and formalin tests: dose-response relationships. Pharmacology Biochemistry & Behavior. 1982;17(6):1213–1219. doi: 10.1016/0091-3057(82)90123-x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. European Journal of Neuroscience. 2006;23(3):758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Barral J, Toro S, Galarraga E, Bargas J. GABAergic presynaptic inhibition of rat neostriatal afferents is mediated by Q-type Ca2+ channels. Neuroscience Letters. 2000;283(1):33–36. doi: 10.1016/s0304-3940(00)00909-5. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time in rats. Psychopharmacology. 1999;141(1):57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiology of Learning and Memory. 2006;86(2):123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiology of GABAB receptors. Physiological Reviews. 2004;84(3):835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bolton MM, Heaney CF, Sabbagh JJ, Murtishaw AS, Magcalas CM, Kinney JW. Deficits in emotional learning and memory in an animal model of schizophrenia. Behavioural Brain Research. 2012;233(1):35–44. doi: 10.1016/j.bbr.2012.04.049. doi:10.1016/j.bbr.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JT, Davies CH, Randall AD. Synaptic activation of GABAB receptors regulates neuronal network activity and entrainment. European Journal of Neuroscience. 2007;25(10):2982–2990. doi: 10.1111/j.1460-9568.2007.05544.x. [DOI] [PubMed] [Google Scholar]
- Bussieres N, El Manira A. GABAB receptor activation inhibits N- and P/Q- type calcium channels in culture lamprey sensory neurons. Brain Research. 1999;847(2):175–185. doi: 10.1016/s0006-8993(99)02002-8. [DOI] [PubMed] [Google Scholar]
- Car H, Wisniewski K. The effect of baclofen and AP-7 on selected behavior in rats. Pharmacology, Biochemistry, and Behavior. 1998;59(3):685–689. doi: 10.1016/s0091-3057(97)00462-0. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Cabib S, Puglisi-Allegra S. Strain-dependent effects of post-training GABA receptor agonists and antagonists on memory storage in mice. Psychopharmacology. 1993;111(2):134–138. doi: 10.1007/BF02245514. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cabib S, Puglisi-Allegra S. Psychopharmacology of memory modulation: Evident for multiple interaction among neurotransmitters and hormones. Behavioural Brain Research. 1996;77(1-2):1–21. doi: 10.1016/0166-4328(96)00200-8. [DOI] [PubMed] [Google Scholar]
- Chapouthier G, Venault P. GABAA receptor complex and memory processes. Current Topics in Medicinal Chemistry. 2002;2(8):841–851. doi: 10.2174/1568026023393552. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience. 2005;119(5):1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for 5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Pscyhopharmacology. 2006;188(4):619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21(5):1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: A new paradigm in G protein signaling. Molecular and Cellular Neurosciences. 2000;16(4):296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- De Luca C, Massotti M. Phaclofen antagonizes the antinociceptive but not the sedative effects of (-)-baclofen. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1990;14(4):597–607. doi: 10.1016/0278-5846(90)90011-5. [DOI] [PubMed] [Google Scholar]
- Enna SJ. The GABA receptors. In: Enna SJ, Mohler H, editors. The GABA Receptors. 3rd ed Humana Press; Totowa, New Jersey: 2007. pp. 1–21. [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009;19(1):33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Fairfax BP, Pitcher JA, Scott MGH, Calver AR, Pangalos MN, Moss SJ, Couve A. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. Journal of Biological Chemistry. 2004;279(13):12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alacid L, Aguado C, Ciruela F, Martin R, Colon J, Cabanero MJ, Gassmann M, Watanabe M, Shigemoto R, Wickman K, Bettler B, Sanchez-Prieto J, Lujan R. Subcellular compartment-specific molecular diversity of pre-and post-synaptic GABA-activated GIRK channels in Purkinje cells. Journal of Neurochemistry. 2009;110(4):1363–1376. doi: 10.1111/j.1471-4159.2009.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliot E, Levaillant M, Beard E, Millot JL, Pourie G. Enhancement of spatial learning by predator odor in mice: involvement of amygdala and hippocampus. Neurobiology of Learning and Memory. 2010;93(2):196–202. doi: 10.1016/j.nlm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Georgiev VP, Yonkov DI, Kambourova TS. Interactions between angiotensin II and baclofen in shuttle-box and passive avoidance performance. Neuropeptides. 1988;12(3):155–158. doi: 10.1016/0143-4179(88)90047-9. [DOI] [PubMed] [Google Scholar]
- Getova DP, Bowery NG. The modulatory effects of high affinity GABAB receptor antagonists in an active avoidance learning paradigm in rats. Psychopharmacology. 1998;137(4):369–373. doi: 10.1007/s002130050632. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & Memory. 2001;8(3):148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Leite-Morris KA, Carey RJ, Kaplan GB. Baclofen enhances extinction of opiate conditioned place preference. Behavioural Brain Research. 2010;207(2):353–359. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes, Brain, and Behavior. 2002;1(1):55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABAB(1) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. Journal of Neuroscience. 2006;26(34):8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behavioral Neuroscience. 2007;121(1):131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Starosta G, Holmes A, Wrenn CC, Yang RJ, Harris AP, Long KC, Crawley JN. Deficits in trace cued fear conditioning in galanin-treated rats and galanin-overexpressing transgenic mice. Learning & Memory. 2002;9(4):178–190. doi: 10.1101/m.49502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziemka-Leska M, Car H, Wisniewski K. Baclofen and AII 3-7 on learning and memory processes in rats chronically treated with ethanol. Pharmacology, Biochemistry, and Behavior. 1999;62(1):39–43. doi: 10.1016/s0091-3057(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. European Journal of Neuroscience. 2009;30(7):1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8(6):638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. Inactivation of the dorsal or ventral hippocampus with muscimol differentially affects fear and memory. Brain Research. 2010;1353:145–151. doi: 10.1016/j.brainres.2010.07.030. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriot LK, Gold PE. Cooperation between memory systems: acetylcholine release in the amygdala correlates positively with performance on a hippocampus-dependent task. Behavioral Neurosciencei. 2003;117(2):320–326. doi: 10.1037/0735-7044.117.2.320. [DOI] [PubMed] [Google Scholar]
- McNally GP, Augustyn K, Richardson R. GABAA receptors determine the temporal dynamics of memory retention. Learning & Memory. 2008;15(3):106–111. doi: 10.1101/lm.806008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacology, Biochemistry, and Behavior. 1996;53(2):303–308. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15(4):418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62(1):42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Mondadori CG, Mobius HJ, Borkowski J. The GABAB receptor antagonist CGP 36742 and the nootropic oxiracetam facilitate the formation of long-term memory. Behavioural Brain Research. 1996;77(1-2):223–225. doi: 10.1016/0166-4328(95)00222-7. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104(2):243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Mutneja M, Berton F, Suen KF, Luscher C, Slesinger PA. Endogenous RGS proteins enhance acute desensitization of GABAB receptor-activated GIRK currents in HEK-293T cells. European Journal of Physiology. 2005;450(1):61–73. doi: 10.1007/s00424-004-1367-1. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: A meta-analysis based on studies of four behavioral tasks. Brain Research Reviews. 2003;41(2-3):268–287. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Takashima T. The GABAB receptor antagonist CGP36742 attenuates the baclofen- and scopolamine- induced deficit in Morris water maze task in rats. Brain Research. 1997;766(1997):101–106. doi: 10.1016/s0006-8993(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Olsen RW. GABA. In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2001. pp. 159–168. [Google Scholar]
- Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M. Phosphorylation-independent desensitization of GABAB receptor by GRK4. The EMBO Journal. 2003;22(15):3816–3824. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. GABAB receptors: Physiological functions and mechanisms of diversity. Advances in Pharmacology. 2010;58:231–255. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Faneslow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15(5):665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nature Neuroscience. 2006;9(8):1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Smith GD, Harrison SM, Birch PJ, Elliott PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/-)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33(9):1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Vales K. Baclofen dose-dependently disrupts learning in a place avoidance task requiring cognitive coordination. Physiology & Behavior. 2009;97(3-4):507–511. doi: 10.1016/j.physbeh.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Tilson HA, McLamb RL, Wilson WA. Baclofen disrupts passive avoidance retention in rats. Psychopharmacology. 1987;92(3):398–401. doi: 10.1007/BF00210851. [DOI] [PubMed] [Google Scholar]
- Vafaei AA, Jezek K, Bures J, Fenton A, Rashidy-Pour A. Post-training reversible inactivation of the rat’s basolateral amygdala interferes with hippocampus-dependent place avoidance memory in a time-dependent manner. Neurobiology of Learning and Memory. 2007;88(1):87–93. doi: 10.1016/j.nlm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50(4):589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Herrold AA, Napier TC. Baclofen facilitates the extinction of methamphetamine-induced conditioned place preference in rats. Behavioral Neuroscience. 2011;125(2):261–267. doi: 10.1037/a0022893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC, Kaupmann K, Urwyler S. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulation GABA and glutamate release. Journal of Neural Transmission. 2008;115(10):1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. International Review of Cytology. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. Journal of Neuroscience. 2002;22(4):1248–1255. doi: 10.1523/JNEUROSCI.22-04-01248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. Journal of Neuroscience. 2000;20(18):7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GN, Biesan OR, Remus JL, Mickley GA. Baclofen alters gustatory discrimination capabilities and induces a conditioned taste aversion (CTA) BMC Research Notes. 2011;4(1):527. doi: 10.1186/1756-0500-4-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Roberts DCS, Corwin RLW. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacology, Biochemistry, and Behavior. 2006;84(2):197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, Feldon J. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitve conditioning and extinction of conditioned fear. European Journal of Neuroscience. 2004;20(7):1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. Journal of Psychopharmacology. 2002;16(4):313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]