Abstract
Background
Children born to HIV-infected women are susceptible to undernutrition, but modifiable risk factors and the time course of the development of undernutrition have not been well characterized.
Objective
To identify maternal, socioeconomic, and child characteristics that are associated with stunting, wasting, and underweight among Tanzanian children born to HIV-infected mothers, followed from 6 weeks for 24 months.
Methods
Maternal and socioeconomic characteristics were recorded during pregnancy, data pertaining to the infant’s birth were collected immediately after delivery, morbidity histories and anthropometric measurements were performed monthly. Multivariate Cox proportional hazards methods were used to assess the association between potential predictors and the time to first episode of stunting, wasting, and underweight.
Results
2387 infants (54.0% male) were enrolled and followed for a median duration of 21.2 months. The respective prevalence of prematurity (<37 weeks) and low birthweight (<2500g) was 15.2% and 7.0%; 11.3% of infants were HIV-positive at 6 weeks. Median time to first episode of stunting, wasting, and underweight was 8.7, 7.2, and 7.0 months, respectively. Low maternal education, few household possessions, low infant birthweight, child HIV infection and male sex were all independent predictors of stunting, wasting, and underweight. In addition, preterm infants were more likely to become wasted and underweight, whereas those with a low Apgar score at birth were more likely to become stunted.
Conclusion
Interventions to improve maternal education and nutritional status, reduce mother-to-child transmission of HIV, and increase birth weight may lower the risk of undernutrition among children born to HIV-infected women.
Keywords: Child undernutrition, child growth, HIV
Introduction
One of the most common manifestations of HIV infection is undernutrition, and poor growth has been reported in as many as 50% of children with HIV (1). Although differences in weight and length between HIV-infected and uninfected infants may not be striking at birth (2), they often become apparent by three months of age, and tend to worsen over time (3, 4). In a recent review of growth patterns among HIV-infected children, 9 out of 10 studies conducted in resource-constrained countries reported negative associations between HIV infection and height-for-age, and all ten studies reported a negative association with weight gain (5).
While there is consistent evidence of impaired growth among HIV-infected children, fewer data are available on HIV-exposed, uninfected children (henceforth referred to as HIV-exposed children). The size of this sub-population of children is growing due to the increasing availability of anti-retroviral (ARV) therapy for the prophylaxis of mother-to-child transmission of HIV. Few studies have examined growth patterns of HIV-exposed (4, 5), and risk factors for undernutrition among this vulnerable population have not been well characterized. Children born to HIV-infected women are at risk of deficiencies in multiple micronutrients that play an important role in child growth and development (6–8). Furthermore, poor socioeconomic conditions in resource-limited settings may limit the quality of care the child receives, resulting in impaired growth and health (9).
The objective of this study was to identify the maternal, socioeconomic and child predictors of stunting, wasting, and underweight among Tanzanian children born to HIV-infected women who were followed from 6 weeks of age for 24 months.
Subjects and Methods
Subjects included in this analysis were part of a randomized, double-blind, placebo-controlled trial designed to determine whether the daily administration of multiple micronutrients (vitamins B, C, and E) to infants born to HIV-infected women reduced the risk of mortality and infectious disease morbidity, compared with placebo. Women ≥ 18 years who tested HIV-positive at the 32nd week of gestation or earlier in one of eight antenatal clinics in Dar es Salaam, Tanzania were invited to participate. Written informed consent for participation was obtained from all women. Maternal and socioeconomic characteristics were assessed prior to delivery. Eligibility for infant participation in the trial included: 1) singleton birth and; 2) 5–7 weeks of age at randomization. Infants with serious congenital anomalies or other conditions that would have interfered with study procedures were excluded. Infants were then randomly assigned to receive a daily oral dose of multivitamins or placebo for 24 months after enrollment.
Mothers and children were asked to return to the study clinics for monthly follow-up visits. As part of standard medical care, all children received growth monitoring, immunizations, routine medical care for illnesses, and large periodic doses of vitamin A. Mothers were counseled on the risks and benefits of exclusive breastfeeding. Nevirapine prophylaxis for mother-to-child transmission of HIV was provided to the mother at the onset of labor and to the child within 72 hours of birth. Part way through the trial, the availability of ARV medication increased and mothers and children in the study were screened for ARV eligibility and treated according to Tanzanian Ministry of Health guidelines. The standard first line regimen was stavudine (d4T), lamivudine (3TC), and nevirapine (NVP) for mothers and zidovudine (AZT), 3TC, and NVP for children. All children were tested for HIV infection at six weeks of age using Amplicor HIV-1 DNA PCR assay version 1.5 (Roche Molecular Systems, Branchburg, NJ, USA), and then again at 18 months using ELISAs. If a child tested positive at 18 months, his or her blood samples were back-tested to estimate the time of transmission, which was defined as the mid-point between the last negative and first positive HIV test. Children who remained HIV-negative throughout the follow-up period were considered HIV-exposed. A complete blood count was also obtained at 6 weeks of age and every six months thereafter.
Data pertaining to the infant’s birth were collected immediately after delivery. At each monthly follow-up visit, a study nurse conducted a morbidity assessment, which was supported by a pictorial diary that mothers were asked to complete at home to record when their child had certain symptoms. Diarrhea was defined as the presence of three or more loose or watery stools in the prior 24 hours. Body temperature, measured using a digital thermometer to the nearest 0.1°C, and respiratory rate, measured for 1 min using a stopwatch, were recorded on the day of the clinic visit. Rapid respiratory rate was defined as ≥ 50 breaths/min for infants and ≥ 40 breaths/min for children over 12 months. Respiratory conditions were defined as follows: the occurrence of cough alone; cough and fever; or cough plus one or more of these events: difficulty breathing; rapid respiratory rate; refusal to eat, breastfeed or drink liquids. At each monthly follow-up visit, the study nurse inquired about infant feeding in the past seven days. Exclusive breastfeeding was defined as feeding a child with breast milk only and no additional foods. We calculated the duration of exclusive breastfeeding as the mean infant age at which the last time the mother reported the child was still exclusively breastfeeding and the first time the mother reported the child was given other foods in addition to breast milk. The study nurse also performed anthropometric measurements at each monthly clinic visit. Weight was measured to the nearest 10 g using a digital infant balance (TANITA) and length was measured to the nearest 1 mm using a rigid length board with a movable foot piece (Shorr Productions). Anthropometric equipment was calibrated on a regular basis.
Statistical Analyses
For this analysis, we included all infants enrolled in the trial who were not stunted, wasted, or underweight at the time of enrollment (i.e. 6 weeks of age) and who had at least one additional set of anthropometric measurements taken after enrollment and before 24 months of age. Length-for-age (LAZ), weight-for-length (WLZ), and weight-for-age (WAZ) Z scores were calculated using the 2006 WHO Child Growth Standards (10). Stunting, wasting, and underweight were defined as binary outcomes using a cut-off of <-2 SD.
Descriptive statistics were used to summarize the baseline characteristics of the study population. Hazard ratios and 95% CI for each of the potential predictors were obtained from separate Cox proportional hazards models, with time to first episode of stunting, wasting, or underweight as the outcome and age in months as the time metameter. The mother’s vital status and the child’s HIV status, exclusive breastfeeding, anemia, and morbidity history varied over the course of follow-up; therefore, we included all available time-updated data on these variables. Time-varying data was carried forward until the next observation except for hemoglobin concentrations, which were carried forward for a maximum of six months. Given the well-known inter-relationship between undernutrition and infection (11) and the potential for a child’s declining nutritional status to influence acute morbidity, we stopped updating the time-varying morbidity variables 8 weeks before the end of each time interval of analysis.
Each outcome was modeled separately, and the first time the child reached a score of <-2 SD signified an “event”. Follow-up time was censored at the time of event, or time of last anthropometric assessment. First, we ran separate univariate models between each potential predictor and the time to each event. We then constructed multivariate models that included all predictors that were significant at p <0.10 in univariate analyses. A priori, we decided to retain child sex, time-varying HIV status, and assigned study treatment regimen in the multivariate models, regardless of their significance, since these variables are important from a biological perspective. We used missing indicator variables in the multivariate models for covariates with missing data.
We constructed curves that depicted all subjects’ LAZ, WLZ, and WAZ, by age, over the entire follow-up period, and stratified these by HIV status at 6 weeks using unadjusted, restricted cubic spline models (12). Automated stepwise selection with entry and retain criteria of p<0.20 was used for the placement of 10 knots in each curve (13). We used the likelihood ratio test to test for non-linearity, comparing the model with only the linear term to the model with the linear and restricted cubic spline terms.
All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC). Institutional review board approval was granted by the Harvard School of Public Health Human Subjects Committee, and the Muhimbili University of Health and Allied Sciences Senate Research and Publications Committee.
Results
Study population
Between August 2004 and November 2007, 2387 infants were enrolled. Table 1 shows baseline characteristics of this study population. Of the 2,387 infants who were enrolled in the parent study, 206 were excluded from the stunting analysis, 183 from the wasting analysis, and 217 from the underweight analysis because they had the outcome at six weeks or did not have at least one additional anthropometric measurement over follow-up. The remaining infants contributed a total of 31,040; 30,531; and 31,564 person-months of follow-up to the analysis of stunting, wasting, and underweight, respectively.
Table 1.
Baseline characteristics
| Maternal characteristics | N1 (%) or Mean ± SD |
|---|---|
| Age at delivery (years) | |
| <20 | 83 (3.6) |
| 20–24 | 540 (23.6) |
| ≥ 25 | 1666 (72.8) |
| Marital status | |
| Single/Widowed/Divorced/Other | 310 (13.1) |
| Married | 2051 (86.9) |
| Number of prior pregnancies | |
| 0 | 539 (22.8) |
| 1–3 | 1632 (69.1) |
| ≥ 4 | 191 (8.1) |
| Formal education (years) | |
| 0 | 158 (6.7) |
| 1–7 | 1700 (71.9) |
| ≥ 8 | 507 (21.4) |
| Occupation | |
| Housewife without income | 1528 (66.5) |
| Housewife with income | 200 (8.7) |
| Business woman | 130 (5.7) |
| Other | 440 (19.2) |
| Mid-Upper Arm Circumference during pregnancy (cm) | 25.9 ± 3.2 |
| Height (cm) | 155.2 ± 6.1 |
| WHO HIV disease stage | |
| 1 | 1040 (72.8) |
| 2 | 215 (15.1) |
| ≥ 3 | 174 (12.2) |
| Received ARVs during pregnancy2 | 429 (20.2) |
|
| |
|
Socioeconomic characteristics
| |
| Daily food expenditure per person in household <500 TShs | 304 (13.6) |
| Household possessions3 | |
| 0 | 339 (14.4) |
| 1–2 | 1091 (46.2) |
| 3–4 | 931 (39.4) |
|
| |
|
Child characteristics
| |
| Female sex | 1098 (46.0) |
| Low birthweight (<2,500 g) | 161 (7.0) |
| Preterm birth (gestational age < 37 weeks) | 357 (15.2) |
| Place of birth | |
| Home | 50 (2.2) |
| Hospital/clinic | 2233 (97.8) |
| Apgar score ≤ 7 at 5 min | 87 (4.1) |
| Child fed colostrum at birth | 1861 (90.3) |
| Child received BCG immunization as a newborn | 1859 (90.2) |
| HIV-positive at 6 weeks | 264 (11.3) |
Total N=2387
The standard first line regimen for mothers was stavudine, lamivudine, and nevirapine
From a list that includes fan, refrigerator, sofa, radio/television
Predictors of stunting
The median age at which the first episode of stunting occurred among the 666 (30.5%) infants who became stunted was 8.7 months. Maternal education and height, as well as more household possessions were inversely independently associated with the risk of child stunting (Table 2). The risk of becoming stunted was 28% greater among males vs. females (HR = 1.28; 95% CI=1.09, 1.49; p=0.002) and 49% greater among infants with a low Apgar score (HR = 1.49; 95% CI = 1.04, 2.13; p=0.03). Low birthweight infants were 2.5 times more likely to become stunted than those with a birth weight ≥2500 g (HR=2.50; 95% CI=1.80, 3.47; p<0.0001). Infants who were HIV-positive had more than 2.5 times the risk of stunting than those who were HIV-negative (HR=2.79; 95% CI: 2.29, 3.40; p<0.0001). The number of cumulative episodes of diarrhea, fever, common cold, vomiting, loss of appetite, and cough were not significantly associated with the risk of stunting.
Table 2.
Maternal, socioeconomic and child risk factors for time to stunting
| N1 | Events | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI)2 | p | HR (95% CI)2,3 | p | |||
|
Maternal Characteristics
| ||||||
| Age at delivery (years) | ||||||
| <20 | 72 | 24 | 1.00 | 0.12 | ||
| 20–24 | 498 | 162 | 1.01 (0.66, 1.54) | |||
| ≥ 25 | 1477 | 453 | 0.87 (0.58, 1.31) | |||
| Marital status | ||||||
| Single/Widowed/Divorced/Other | 280 | 96 | 1.00 | 0.17 | ||
| Married | 1831 | 564 | 0.86 (0.69, 1.07) | |||
| Number of prior pregnancies | ||||||
| 0 | 490 | 131 | 1.00 | 0.61 | ||
| 1–3 | 1460 | 486 | 1.21 (1.00, 1.47) | |||
| ≥ 4 | 162 | 41 | 0.90 (0.63, 1.27) | |||
| Formal education (years) | ||||||
| 0 | 139 | 53 | 1.00 | <0.0001 | 1.00 | 0.04 |
| 1–7 | 1521 | 509 | 0.92 (0.69, 1.22) | 1.01 (0.75, 1.34) | ||
| ≥ 8 | 455 | 98 | 0.55 (0.40, 0.77) | 0.77 (0.54, 1.08) | ||
| Occupation | ||||||
| Housewife without income | 1377 | 444 | 1.00 | |||
| Housewife with income | 175 | 54 | 0.90 (0.68, 1.20) | 0.31 | ||
| Business woman | 118 | 28 | 0.72 (0.49, 1.05) | |||
| Other | 388 | 121 | 0.94 (0.77, 1.15) | |||
| Mid-Upper Arm Circumference during pregnancy (cm) | ||||||
| < 22.0 | 135 | 53 | 1.00 | 0.06 | 1.00 | 0.72 |
| ≥ 22.0 | 1976 | 606 | 0.76 (0.58, 1.01) | 0.95 (0.71, 1.26) | ||
| Height (cm) | ||||||
| < 155.0 | 1027 | 410 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| ≥ 155.0 | 1107 | 255 | 0.51 (0.43, 0.59) | 0.52 (0.45, 0.61) | ||
| WHO HIV disease stage | ||||||
| 1 | 947 | 288 | 1.00 | 0.41 | ||
| 2 | 195 | 65 | 1.15 (0.88, 1.50) | |||
| ≥ 3 | 152 | 49 | 1.08 (0.80, 1.47) | |||
| Received ARVs during pregnancy4 | ||||||
| No | 1549 | 511 | 1.00 | 0.46 | ||
| Yes | 376 | 115 | 0.93 (0.76, 1.14) | |||
| Vital status5 | ||||||
| Alive | 30914 | 665 | 1.00 | 0.44 | ||
| Dead | 126 | 1 | 0.44 (0.06, 3.11) | |||
|
| ||||||
|
Socioeconomic Characteristics
| ||||||
| Household food expenditure (TShs/person/day) | ||||||
| < 500 | 276 | 109 | 1.00 | 0.005 | 1.00 | 0.08 |
| ≥ 500 | 1725 | 516 | 0.74 (0.60, 0.91) | 0.32 (0.67, 1.02) | ||
| Household possessions6 | ||||||
| 0 | 292 | 117 | 1.00 | <0.0001 | 1.00 | 0.002 |
| 1–2 | 977 | 327 | 0.82 (0.66, 1.01) | 0.91 (0.73, 1.13) | ||
| 3–4 | 843 | 215 | 0.59 (0.47, 0.73) | 0.71 (0.56, 0.90) | ||
|
| ||||||
|
Child Characteristics
| ||||||
| Sex | ||||||
| Female | 987 | 284 | 1.00 | 0.02 | 1.00 | 0.002 |
| Male | 1148 | 382 | 1.20 (1.03, 1.40) | 1.28 (1.09, 1.49) | ||
| Birth weight (g) | ||||||
| ≥ 2,500 | 1988 | 598 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| < 2,500 | 71 | 40 | 2.76 (2.00, 3.80) | 2.50 (1.80, 3.47) | ||
| Gestational age at birth (weeks) | ||||||
| ≥ 37 | 1830 | 556 | 1.00 | 0.03 | 1.00 | 0.15 |
| < 37 | 272 | 98 | 1.26 (1.02, 1.56) | 1.18 (0.94, 1.46) | ||
| Place of birth | ||||||
| Home | 39 | 13 | 1.00 | 0.39 | ||
| Hospital/clinic | 2010 | 625 | 0.79 (0.45, 1.36) | |||
| Apgar score at 5 min | ||||||
| > 7 | 1848 | 561 | 1.00 | 0.02 | 1.00 | 0.03 |
| ≤ 7 | 74 | 32 | 1.52 (1.07, 2.17) | 1.49 (1.04, 2.13) | ||
| Child fed colostrum | ||||||
| Yes | 1680 | 524 | 1.00 | 0.99 | ||
| No | 173 | 53 | 1.00 (0.76, 1.33) | |||
| Child received BCG immunization as a newborn | ||||||
| Yes | 1682 | 517 | 1.00 | 0.18 | ||
| No | 171 | 60 | 1.20 (0.92, 1.57) | |||
| Exclusively breastfed5 | ||||||
| No | 25,054 | 477 | 1.00 | 0.01 | 1.00 | 0.05 |
| Yes | 5,866 | 187 | 1.39 (1.07, 1.80) | 1.29 (1.00, 1.67) | ||
| Anemia (Hb <11 g/dL)5 | ||||||
| No | 5,789 | 118 | 1.00 | 0.12 | ||
| Yes | 23,970 | 523 | 1.18 (0.96, 1.44) | |||
| Cotrimoxazole consumption last month5 | ||||||
| 0 | 1,848 | 47 | 1.00 | 0.15 | ||
| 1–20 times | 3,235 | 74 | 0.94 (0.64, 1.37) | |||
| ≥ 21 times | 16,296 | 325 | 0.82 (0.60, 1.14) | |||
| HIV status5 | ||||||
| Negative | 28,533 | 537 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Positive | 2,472 | 126 | 2.65 (2.19, 3.22) | 2.79 (2.29, 3.40) | ||
| Cumulative morbidity episodes5,7 | ||||||
| Diarrhea | ||||||
| 0 | 23,200 | 519 | 1.00 | 0.53 | ||
| 1–2 | 6,930 | 128 | 1.01 (0.82, 1.25) | |||
| ≥ 3 | 909 | 18 | 1.26 (0.78, 2.02) | |||
| Fever | ||||||
| 0 | 17,837 | 422 | 1.00 | |||
| 1–2 | 9,991 | 182 | 0.94 (0.77, 1.15) | 0.83 | ||
| 3–4 | 2,370 | 49 | 1.17 (0.84, 1.62) | |||
| ≥ 5 | 840 | 13 | 0.95 (0.53, 1.70) | |||
| Common cold | ||||||
| 0 | 9,436 | 260 | 1.00 | |||
| 1–2 | 11,646 | 220 | 0.81 (0.66, 0.98) | 0.95 | ||
| 3–4 | 5,599 | 99 | 0.85 (0.64, 1.13) | |||
| ≥ 5 | 4,358 | 87 | 1.05 (0.77, 1.44) | |||
| Vomiting | ||||||
| 0 | 27,975 | 605 | 1.00 | 0.58 | ||
| 1 | 2,493 | 52 | 1.17 (0.87, 1.57) | |||
| ≥ 2 | 571 | 9 | 0.92 (0.47, 1.79) | |||
| Refusal to eat, drink or breastfeed | ||||||
| 0 | 26,962 | 603 | 1.00 | 0.22 | ||
| 1 | 3,025 | 47 | 0.83 (0.611.12) | |||
| ≥ 2 | 1,052 | 16 | 0.84 (0.51, 1.40) | |||
| Cough alone | ||||||
| 0 | 10,660 | 292 | 1.00 | |||
| 1–2 | 9,912 | 178 | 0.77 (0.62, 0.96) | 0.24 | ||
| 3–4 | 5,154 | 113 | 1.00 (0.75, 1.33) | |||
| ≥ 5 | 5,312 | 83 | 0.77 (0.55, 1.06) | |||
| Cough and fever | ||||||
| 0 | 21,808 | 496 | 1.00 | 0.63 | ||
| 1–2 | 7,845 | 140 | 0.96 (0.78, 1.18) | |||
| ≥ 3 | 1,386 | 30 | 1.28 (0.87, 1.89) | |||
| Cough plus difficulty breathing, rapid respiratory rate or refusal to eat | ||||||
| 0 | 26,916 | 582 | 1.00 | 0.27 | ||
| ≥ 1 | 4,123 | 84 | 1.14 (0.90, 1.46) | |||
N represents the number of children for time-fixed predictors, and number of person-months for time-varying predictors
Hazard Ratios (HR) and 95% Confidence Intervals (CI) were obtained from Cox proportional hazards models
Multivariate results additionally adjusted for parent study treatment regimen
The standard first line regimen for mothers was stavudine, lamivudine, and nevirapine
Time-updated variable; N refers to person-months
From a list that includes fan, refrigerator, sofa, radio/television
An episode was defined if the symptom was reported during the 4 weeks prior to the visit
Predictors of wasting
The median age at first episode of wasting among the 705 (32.0%) infants who became wasted was 7.2 months. In the multivariate analysis, maternal education and MUAC were inversely associated with the risk of wasting (Table 3). The risk of wasting was also elevated among infants whose mothers had a more advanced HIV disease stage and were taking ARVs during pregnancy. The effect of ARVs remained when the analyses were restricted to children who were HIV negative at 6 weeks (HR=1.29; 95% CI=1.05, 1.57; p=0.02). The ARV effect also retained significance (HR=1.25; 95% CI=1.03, 1.51; p=0.03) after additionally adjusting for maternal CD4 count during pregnancy.
Table 3.
Maternal, socioeconomic and child risk factors of time to wasting
| N1 | Events | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI)2 | p | HR (95% CI)2,3 | p | |||
|
Maternal Characteristics
| ||||||
| Age at delivery (years) | ||||||
| <20 | 72 | 25 | 1.00 | 0.99 | ||
| 20–24 | 488 | 150 | 0.87 (0.57, 1.33) | |||
| ≥ 25 | 1513 | 497 | 0.90 (0.61, 1.35) | |||
| Marital status | ||||||
| Single/Widowed/Divorced/Other | 275 | 92 | 1.00 | 0.50 | ||
| Married | 1858 | 606 | 0.93 (0.74, 1.15) | |||
| Number of prior pregnancies | ||||||
| 0 | 472 | 148 | 1.00 | 0.88 | ||
| 1–3 | 1487 | 492 | 1.03 (0.85, 1.23) | |||
| ≥ 4 | 175 | 57 | 1.01 (0.74, 1.37) | |||
| Formal education (years) | ||||||
| 0 | 143 | 54 | 1.00 | <0.0001 | 1.00 | 0.005 |
| 1–7 | 1539 | 539 | 0.98 (0.74, 1.29) | 0.99 (0.75, 1.32) | ||
| ≥ 8 | 455 | 105 | 0.59 (0.43, 0.83) | 0.70 (0.50, 0.98) | ||
| Occupation | ||||||
| Housewife without income | 1393 | 469 | 1.00 | |||
| Housewife with income | 181 | 61 | 0.99 (0.76, 1.29) | 0.77 | ||
| Business woman | 123 | 27 | 0.62 (0.42, 0.92) | |||
| Other | 383 | 131 | 1.04 (0.86, 1.26) | |||
| Mid-Upper Arm Circumference during pregnancy (cm) | ||||||
| < 22.0 | 143 | 68 | 1.00 | <0.0001 | 1.00 | 0.006 |
| ≥ 22.0 | 1989 | 630 | 0.60 (0.47, 0.77) | 0.70 (0.54, 0.90) | ||
| Height (cm) | ||||||
| < 155.0 | 1076 | 360 | 1.00 | 0.39 | ||
| ≥ 155.0 | 1077 | 344 | 0.94 (0.81, 1.09) | |||
| WHO Stage | ||||||
| 1 | 975 | 292 | 1.00 | <0.0001 | 1.00 | 0.003 |
| 2 | 194 | 78 | 1.42 (1.11, 1.82) | 1.35 (1.05, 1.75) | ||
| ≥ 3 | 158 | 67 | 1.61 (1.23, 2.10) | 1.40 (1.07, 1.84) | ||
| Received ARVs during pregnancy4 | ||||||
| No | 1561 | 495 | 1.00 | 0.003 | 1.00 | 0.008 |
| Yes | 393 | 157 | 1.31 (1.10, 1.57) | 1.28 (1.07, 1.55) | ||
| Vital status5 | ||||||
| Alive | 3415 | 703 | 1.00 | 0.97 | ||
| Dead | 116 | 2 | 0.97 (0.24, 3.90) | |||
|
| ||||||
|
Socioeconomic Characteristics
| ||||||
| Household food expenditure (TShs/person/day) | ||||||
| < 500 | 269 | 103 | 1.00 | 0.18 | ||
| ≥ 500 | 1759 | 564 | 0.87 (0.70, 1.07) | |||
| Household possessions6 | ||||||
| 0 | 390 | 151 | 1.00 | <0.0001 | 1.00 | 0.004 |
| 1–2 | 1021 | 350 | 0.88 (0.71, 1.08) | 0.92 (0.75, 1.14) | ||
| 3–4 | 723 | 197 | 0.66 (0.54, 0.84) | 0.75 (0.60, 0.94) | ||
|
| ||||||
|
Child Characteristics
| ||||||
| Sex | ||||||
| Female | 986 | 284 | 1.00 | 0.0002 | 1.00 | <0.0001 |
| Male | 1168 | 421 | 1.33 (1.15, 1.55) | 1.40 (1.20, 1.63) | ||
| Birth weight (g) | ||||||
| ≥ 2,500 | 1941 | 616 | 1.00 | <0.0001 | 1.00 | 0.004 |
| < 2,500 | 129 | 61 | 1.78 (1.37, 2.31) | 1.49 (1.20, 1.63) | ||
| Gestational age at birth (weeks) | ||||||
| ≥ 37 | 1816 | 572 | 1.00 | 0.002 | 1.00 | 0.02 |
| < 37 | 308 | 122 | 1.37 (1.13, 1.67) | 1.28 (1.05, 1.57) | ||
| Place of birth | ||||||
| Home | 40 | 10 | 1.00 | 0.42 | ||
| Hospital/clinic | 2022 | 667 | 1.30 (0.69, 2.42) | |||
| Child fed colostrum | ||||||
| Yes | 1686 | 543 | 1.00 | 0.94 | ||
| No | 170 | 55 | 0.99 (0.75, 1.31) | |||
| Child received BCG immunization as a newborn | ||||||
| Yes | 1685 | 540 | 1.00 | 0.77 | ||
| No | 171 | 58 | 1.04 (0.79, 1.37) | |||
| Exclusively breastfed5 | ||||||
| No | 24,432 | 516 | 1.00 | 0.76 | ||
| Yes | 6,021 | 185 | 1.04 (0.82, 1.31) | |||
| Anemia (Hb <11 g/dL)5 | ||||||
| No | 5,423 | 132 | 1.00 | 0.79 | ||
| Yes | 23,769 | 543 | 1.03 (0.85, 1.25) | |||
| Cotrimoxazole consumption last month5 | ||||||
| 0 | 1,822 | 45 | 1.00 | 0.23 | ||
| 1–20 times | 3,025 | 68 | 0.88 (0.59, 1.29) | |||
| ≥ 21 times | 15,952 | 334 | 0.82 (0.59, 1.14) | |||
| HIV status5 | ||||||
| Negative | 27,975 | 573 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Positive | 2,521 | 128 | 2.39 (1.98, 2.90) | 2.26 (1.86, 2.74) | ||
| Cumulative morbidity episodes5,7 | ||||||
| Diarrhea | ||||||
| 0 | 23,117 | 601 | 1.00 | 0.03 | 1.00 | 0.04 |
| 1–2 | 6,596 | 94 | 0.76 (0.60, 0.96) | 0.78 (0.61, 0.98) | ||
| ≥ 3 | 818 | 10 | 0.82 (0.43, 1.55) | 0.80 (0.42, 1.52) | ||
| Fever | ||||||
| 0 | 17,950 | 480 | 1.00 | |||
| 1–2 | 9,645 | 184 | 1.03 (0.85, 1.25) | 0.93 | ||
| 3–4 | 2,241 | 37 | 1.21 (0.84, 1.75) | |||
| ≥ 5 | 694 | 4 | 0.49 (0.18, 1.35) | |||
| Common Cold | ||||||
| 0 | 9,667 | 273 | 1.00 | |||
| 1–2 | 11,400 | 298 | 1.11 (0.92, 1.32) | 0.61 | ||
| 3–4 | 5,304 | 87 | 0.95 (0.72, 1.27) | |||
| ≥ 5 | 4,159 | 47 | 0.86 (0.59, 1.25) | |||
| Vomiting | ||||||
| 0 | 27,821 | 664 | 1.00 | 0.58 | ||
| 1 | 2,235 | 37 | 1.01 (0.72, 1.42) | |||
| ≥ 2 | 473 | 4 | 0.63 (0.23, 1.58) | |||
| Refusal to eat, drink or breastfeed | ||||||
| 0 | 26,725 | 656 | 1.00 | 0.12 | ||
| 1 | 2,874 | 42 | 0.90 (0.65, 1.25) | |||
| ≥ 2 | 931 | 7 | 0.54 (0.25, 1.15) | |||
| Cough and fever | ||||||
| 0 | 21,725 | 551 | 1.00 | 0.52 | ||
| 1–2 | 7,581 | 136 | 1.05 (0.86, 1.29) | |||
| ≥ 3 | 1,224 | 18 | 1.14 (0.70, 1.86) | |||
| Cough plus difficulty breathing, rapid respiratory rate or refusal to eat | ||||||
| 0 | 26,590 | 641 | 1.00 | 0.84 | ||
| ≥ 1 | 3,941 | 64 | 0.97 (0.74, 1.27) | |||
N represents the number of children for time-fixed predictors, and number of person-months for time-varying
Hazard Ratios (HR) and 95% Confidence Intervals (CI) were obtained from Cox proportional hazards models
Multivariate results additionally adjusted for parent study treatment regimen
The standard first line regimen for mothers was stavudine, lamivudine, and nevirapine
Time-updated variable; N refers to person-months
From a list that includes fan, refrigerator, sofa, radio/television
An episode was defined if the symptom was reported during the 4 weeks prior to the visit
The risk of wasting was lower among children born to families owning more household possessions. Child sex, birth weight, gestational age at birth, and current HIV status were all significantly independently associated with the risk of wasting. The HR of wasting among males vs. females, infants with a birth weight < 2500 g vs. ≥ 2500 g, and those born at < 37 weeks vs. ≥ 37 weeks gestational age were 1.40 (95% CI = 1.20, 1.63; p<0.0001), 1.49 (95% CI=1.20, 1.63; p=0.003), and 1.28 (95% CI=1.05, 1.57; p=0.02), respectively. HIV-infected infants were more than twice as likely to become wasted than HIV-uninfected infants, and those with more diarrhea episodes were slightly less likely to become wasted.
Predictors of underweight
The median age at which the first episode of underweight occurred among the 649 (29.9%) infants who became underweight was 7.0 months. As with stunting, the risk of underweight was lower in the multivariate analysis among infants born to mothers with more education and whose height was above the median value (Table 4). Ownership of more household assets was protective against underweight. Male sex, low birthweight, preterm birth, and HIV-infection were associated with a significant independent increased risk of underweight.
Table 4.
Maternal, socioeconomic and child risk factors of time to underweight
| N1 | Events | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI)2 | p | HR (95% CI)2,3 | p | |||
|
Maternal Characteristics
| ||||||
| Age at delivery (years) | ||||||
| <20 | 71 | 24 | 1.00 | 0.12 | ||
| 20–24 | 498 | 156 | 0.93 (0.61, 1.43) | |||
| ≥ 25 | 1476 | 438 | 0.82 (0.55, 1.24) | |||
| Marital status | ||||||
| Single/Widowed/Divorced/Other | 274 | 82 | 1.00 | 0.93 | ||
| Married | 1834 | 562 | 0.99 (0.79, 1.25) | |||
| Number of prior pregnancies | ||||||
| 0 | 477 | 132 | 1.00 | 0.62 | ||
| 1–3 | 1467 | 461 | 1.09 (0.90, 1.32) | |||
| ≥ 4 | 165 | 49 | 1.03 (0.74, 1.42) | |||
| Formal education (years) | ||||||
| 0 | 141 | 55 | 1.00 | <0.0001 | 1.00 | 0.02 |
| 1–7 | 1519 | 494 | 0.86 (0.65, 1.14) | 0.94 (0.71, 1.25) | ||
| ≥ 8 | 452 | 95 | 0.52 (0.37, 0.72) | 0.72 (0.51, 1.01) | ||
| Occupation | ||||||
| Housewife without income | 1370 | 437 | 1.00 | |||
| Housewife with income | 175 | 49 | 0.85 (0.64, 1.15) | 0.33 | ||
| Business woman | 118 | 24 | 0.61 (0.40, 0.92) | |||
| Other | 393 | 121 | 0.97 (0.79, 1.19) | |||
| Mid-Upper Arm Circumference during pregnancy (cm) | ||||||
| < 22.0 | 132 | 58 | 1.00 | <0.0001 | 1.00 | 0.07 |
| ≥ 22.0 | 1976 | 587 | 0.64 (0.49, 0.84) | 0.77 (0.59, 1.02) | ||
| Height (cm) | ||||||
| < 155.0 | 1038 | 371 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| ≥ 155.0 | 1091 | 278 | 0.65 (0.55, 0.75) | 0.67 (0.58, 0.79) | ||
| WHO HIV disease stage | ||||||
| 1 | 955 | 268 | 1.00 | 0.10 | ||
| 2 | 193 | 56 | 1.07 (0.80, 1.42) | |||
| ≥ 3 | 147 | 51 | 1.30 (0.97, 1.76) | |||
| Received ARVs during pregnancy4 | ||||||
| No | 1552 | 473 | 1.00 | 0.57 | ||
| Yes | 380 | 119 | 1.06 (0.87, 1.30) | |||
| Vital status5 | ||||||
| Alive | 31459 | 648 | 1.00 | 0.59 | ||
| Dead | 104 | 1 | 0.58 (0.08, 4.12) | |||
|
| ||||||
|
Socioeconomic Characteristics
| ||||||
| Household food expenditure (TShs/person/day) | ||||||
| < 500 | 271 | 96 | 1.00 | 0.19 | ||
| ≥ 500 | 1729 | 521 | 0.87 (0.70, 1.08) | |||
| Household possessions6 | ||||||
| 0 | 296 | 116 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1–2 | 967 | 321 | 0.82 (0.67, 1.02) | 0.86 (0.69, 1.07) | ||
| ≥ 3 | 846 | 206 | 0.57 (0.45, 0.72) | 0.62 (0.49, 0.79) | ||
|
| ||||||
|
Child Characteristics
| ||||||
| Sex | ||||||
| Female | 985 | 280 | 1.00 | 0.03 | 1.00 | 0.003 |
| Male | 1145 | 369 | 1.18 (1.01, 1.38) | 1.28 (1.09, 1.50) | ||
| Birth weight (g) | ||||||
| ≥ 2,500 | 1977 | 577 | 1.00 | <0.0001 | 1.00 | 0.0006 |
| < 2,500 | 73 | 37 | 2.16 (1.55, 3.02) | 1.81 (1.29, 2.55) | ||
| Gestational age at birth (weeks) | ||||||
| ≥ 37 | 1825 | 535 | 1.00 | 1.00 | ||
| < 37 | 275 | 106 | 1.42 (1.15, 1.75) | 0.001 | 1.30 (1.05, 1.61) | 0.02 |
| Place of birth | ||||||
| Home | 43 | 16 | 1.00 | 0.10 | ||
| Hospital/clinic | 1997 | 600 | 0.66 (0.40, 1.08) | |||
| Child fed colostrum | ||||||
| Yes | 1667 | 495 | 1.00 | 0.93 | ||
| No | 179 | 51 | 0.99 (0.74, 1.32) | |||
| Child received BCG immunization as a newborn | ||||||
| Yes | 1665 | 484 | 1.00 | 0.08 | 1.00 | 0.78 |
| No | 179 | 62 | 1.26 (0.97, 1.65) | 1.04 (0.79, 1.37) | ||
| Exclusively breastfed5 | ||||||
| No | 25,463 | 477 | 1.00 | 0.04 | 1.00 | 0.30 |
| Yes | 6,003 | 171 | 1.29 (1.01, 1.64) | 1.14 (0.89, 1.44) | ||
| Anemia (Hb <11 g/dL)5 | ||||||
| No | 5,674 | 117 | 1.00 | 0.40 | ||
| Yes | 24,553 | 499 | 1.09 (0.89, 1.34) | |||
| Cotrimoxazole consumption last month5 | ||||||
| 0 | 1,788 | 37 | 1.00 | 0.91 | ||
| 1–20 times | 3,211 | 75 | 1.06 (0.71, 1.58) | |||
| ≥ 21 times | 16,759 | 324 | 0.88 (0.62, 1.26) | |||
| HIV status5 | ||||||
| Negative | 29,065 | 509 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Positive | 2,463 | 138 | 3.13 (2.59, 3.78) | 3.21 (2.65, 3.89) | ||
| Cumulative morbidity episodes5,7 | ||||||
| Diarrhea | ||||||
| 0 | 23,580 | 536 | 1.00 | 0.06 | 1.00 | 0.42 |
| 1–2 | 7,011 | 105 | 0.87 (0.69, 1.09) | 0.98 (0.78, 1.24) | ||
| ≥ 3 | 971 | 8 | 0.55 (0.27, 1.12) | 0.65 (0.31, 1.34) | ||
| Fever | ||||||
| 0 | 18,281 | 435 | 1.00 | |||
| 1–2 | 10,019 | 165 | 0.95 (0.78, 1.17) | 0.75 | ||
| 3–4 | 2,395 | 43 | 1.25 (0.88, 1.78) | |||
| ≥ 5 | 866 | 6 | 0.52 (0.22, 1.18) | |||
| Common Cold | ||||||
| 0 | 9,702 | 238 | 1.00 | |||
| 1–2 | 11,603 | 272 | 1.12 (0.92, 1.35) | 0.54 | ||
| 3–4 | 5,695 | 84 | 0.94 (0.70, 1.27) | |||
| ≥ 5 | 4,562 | 55 | 0.86 (0.60, 1.24) | |||
| Vomiting | ||||||
| 0 | 28,552 | 609 | 1.00 | 0.14 | ||
| 1 | 2,431 | 37 | 0.95 (0.68, 1.34) | |||
| ≥ 2 | 610 | 3 | 0.35 (0.11, 1.08) | |||
| Refusal to eat, drink or breastfeed | ||||||
| 0 | 27,487 | 609 | 1.00 | 0.003 | 1.00 | 0.07 |
| 1 | 2,939 | 33 | 0.69 (0.48, 0.99) | 0.83 (0.56, 1.23) | ||
| ≥ 2 | 1,137 | 7 | 0.40 (0.19, 0.86) | 0.51 (0.23, 1.13) | ||
| Cough alone | ||||||
| 0 | 10,905 | 273 | 1.00 | |||
| 1–2 | 9,967 | 231 | 1.14 (0.93, 1.38) | |||
| 3–4 | 5,263 | 83 | 1.01 (0.74, 1.37) | 0.47 | ||
| ≥ 5 | 5,426 | 62 | 0.81 (0.56, 1.17) | |||
| Cough and fever | ||||||
| 0 | 22,315 | 499 | 1.00 | 0.75 | ||
| 1–2 | 7,854 | 131 | 1.05 (0.85, 1.30) | |||
| ≥ 3 | 1,394 | 19 | 1.01 (0.62, 1.64) | |||
| Cough plus difficulty breathing, rapid respiratory rate or refusal to eat | ||||||
| 0 | 27,363 | 599 | 1.00 | 0.03 | 1.00 | 0.19 |
| ≥ 1 | 4,199 | 50 | 0.72 (0.53, 0.97) | 0.80 (0.32, 1.97) | ||
N represents the number of children for time-fixed predictors, and number of person-months for time-varying
Hazard Ratios (HR) and 95% Confidence Intervals (CI) were obtained from Cox proportional hazards models
Multivariate results additionally adjusted for parent study treatment regimen
The standard first line regimen for mothers was stavudine, lamivudine, and nevirapine
Time-updated variable; N refers to person-months
From a list that includes fan, refrigerator, sofa, radio/television
An episode was defined if the symptom was reported during the 4 weeks prior to the visit
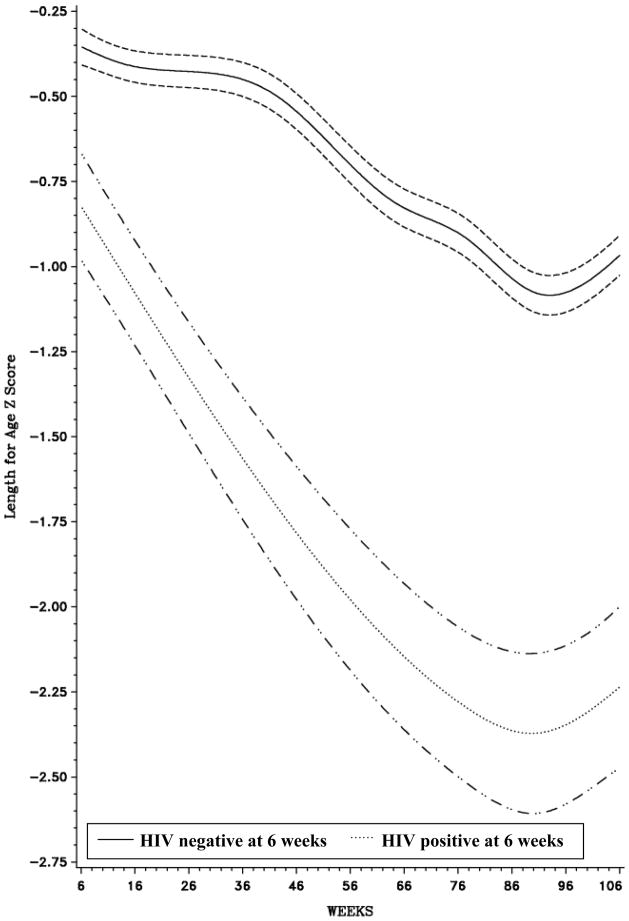
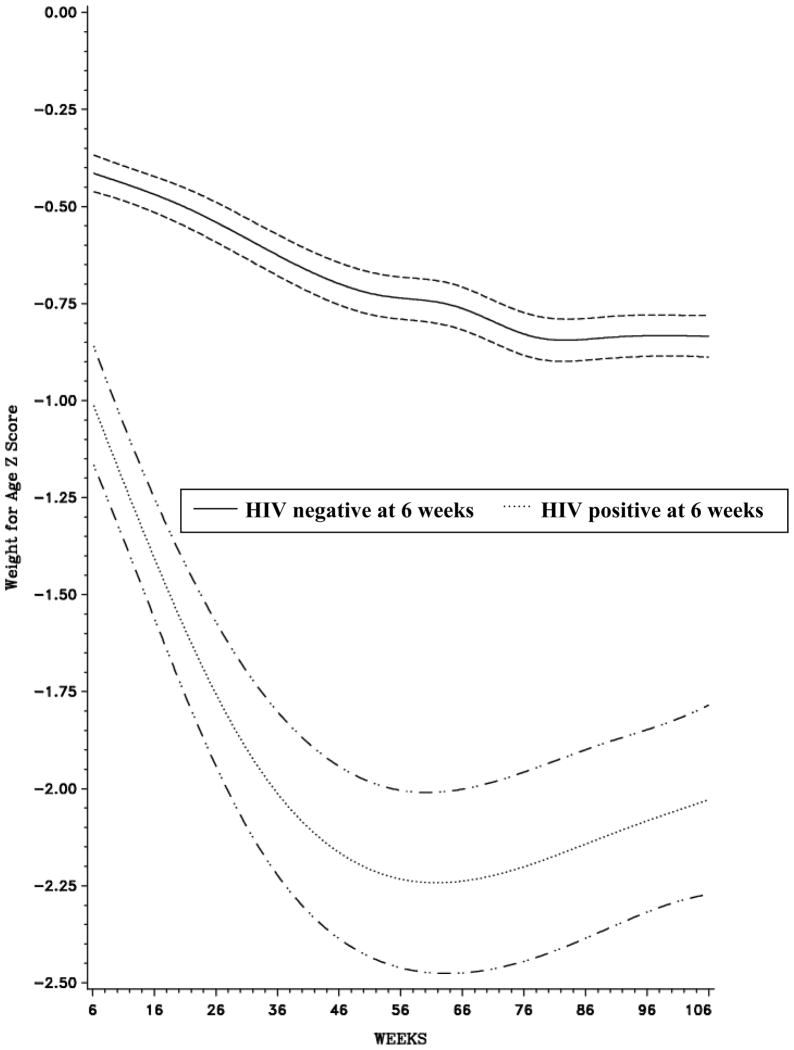
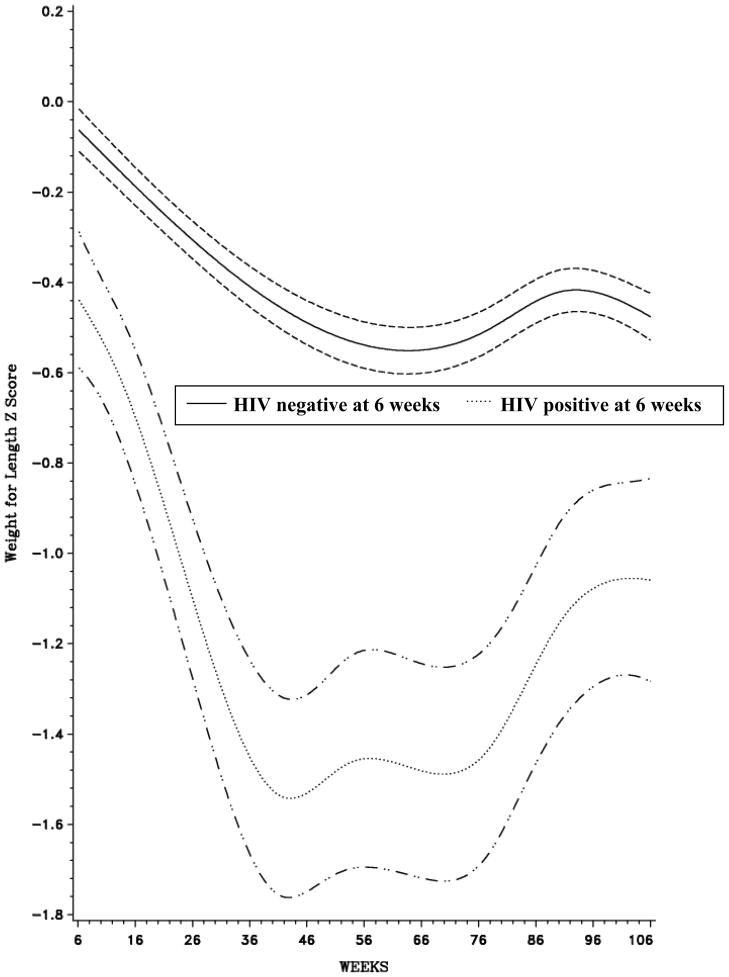
Figures 1–3 all highlight a deterioration in nutritional status over time; however, the magnitude of the decline is noticeably greater among HIV-infected infants. In all three curves, the association between age and Z-score among HIV-infected and HIV-uninfected children was non-linear (p<0.0001). There was a steep decline in LAZ immediately after enrollment among HIV-infected children and mean LAZ reached its lowest level at approximately 92 weeks in both groups of children (Figure 1). Mean WLZ decreased after 6 weeks of age among HIV-infected and HIV-uninfected children; however, the rate of decline was more precipitous among HIV-infected children (Figure 2). Mean WAZ declined gradually over the course of follow-up among HIV-uninfected children, reaching its lowest level at approximately 60 weeks of age (Figure 3).
Figure 1.
Length-for-Age Z-Scores, by age (weeks), stratified by HIV status at 6 weeks1
1 Bands represent 95% Confidence Intervals (CI)
Figure 3.
Weight-for-Age Z-Score, by age (weeks), stratified by baseline HIV status1
1 Bands represent 95% Confidence Intervals (CI)
Figure 2.
Weight-for-Length Z-Scores, by age (weeks), stratified by HIV status at 6 weeks1
1Bands represent 95% Confidence Intervals (CI)
Discussion
We assessed predictors of stunting, wasting, and underweight in a cohort of 2,387 Tanzanian infants born to HIV-infected women who were followed from 6 weeks of age for 24 months. Our study population is the largest reported cohort of young children born to HIV-infected women in sub-Saharan Africa with longitudinal anthropometric measurements to-date. Our ability to perform repeated anthropometric measurements at frequent, monthly intervals enabled precise time-to-event analysis and the construction of curves that plotted nutritional status over time. We not only identified several important biological and socioeconomic correlates that were independently associated with the risk of child undernutrition, but also quantified the magnitude of the risk that was associated with each characteristic. A particular novel finding was the increased risk of stunting, wasting, and underweight experienced by male vs. female children.
The strength and consistency of the association between maternal education and each form of child undernutrition deserves special note. Our findings are consistent with studies of similar populations in sub-Saharan Africa. Villamor et al. (14) reported that a low level of maternal education predicted linear growth retardation among infants under 12 months of age who were born to HIV-infected women in Tanzania. Similarly, Webb et al. (15) found that greater maternal schooling significantly reduced the deterioration in LAZ and WLZ scores from birth to 24 months, and McGrath et al. found higher maternal education to decrease the risk of stunting and underweight among HIV-exposed children (16). In an analysis of Demographic and Health Survey data from six countries in sub-Saharan Africa, maternal secondary education was positively associated with WAZ in Ghana, Nigeria, and Tanzania (17). Mothers with higher levels of formal education may possess greater knowledge of proper hygiene practices and optimal child caring and feeding practices, which could be particularly important in the context of HIV.
Our findings also highlight the importance of maternal nutritional status in predicting the risk of undernutrition in her offspring, which is in agreement with results from several other studies (4, 16–20). While the influence of maternal height on the risk of child stunting may partially reflect genetic potential, MUAC may act as a proxy of many factors that could indirectly affect the risk of child wasting. A mother with a low MUAC may have more advanced HIV disease stage or be co-infected with other diseases which could lower her nutritional status and overall health, consequently impairing her ability to properly care for her child (21, 22). A low maternal MUAC could also reflect household food insecurity, which might adversely affect complementary feeding the child receives (23). We also found that the risk of wasting was elevated among children whose mothers had a more advanced HIV disease stage and who took ARVs during pregnancy. However, the effect of ARVs must be interpreted with caution, since a standardized protocol for ARV therapy was implemented only part way through the study.
We found a consistent association between child sex and each nutritional outcome. Male children were 28%, 40%, and 28% more likely to become stunted, wasted, and underweight, respectively, in comparison to females. Similar observations were made in a study from Andhra Pradesh, India, which reported that male children were twice and 50% more likely than females to be wasted and underweight, after controlling for multiple other risk factors in the respective analyses (24). Moreover, in a meta-analysis from 10 countries in sub-Saharan Africa, Wamani et al. reported a higher prevalence of stunting in males vs. females (25). To our knowledge, our study is the first to identify an increased risk or stunting, wasting, and underweight among male children born to HIV-infected women. Although the mechanism behind this association has not been fully explored, possible explanations include preferential treatment towards girls in terms of healthcare-seeking behavior or dietary consumption.
The magnitude of the effect of the infant’s HIV status on stunting, wasting, and underweight was also striking (Figures 1–3). This observation has been made in several other studies including the European Collaborative Study of children born to HIV-infected women in Europe, which reported that although there were no differences in length or weight between HIV-infected and HIV-uninfected infants at birth, between 6 and 12 months of age, uninfected infants grew an estimated 1.6% faster in height and 6.2% faster in weight, and between 8 and 10 years age, these figures reached 16% and 44%, respectively (3). Such findings emphasize the importance of early diagnosis of HIV infection and timely delivery of supportive interventions to this vulnerable group. We found that low birth weight infants were more likely to become stunted, wasted, and underweight, which is consistent with previous reports (9, 15, 17, 18, 20, 27, 28), and underscores the importance of optimal prenatal care (29).
Although the link between infection and nutrition is well-established (11), epidemiological analyses that have assessed the effect of various morbidities on the subsequent risk of stunting, wasting, and underweight have produced mixed results. In a study of HIV-infected and uninfected children, Villamor et al. found no association between the incidence of diarrhea overall and attained weight after 1 year, or episodes of respiratory infection and height or weight gain (14). Webb et al. found that episodes of diarrhea or respiratory infections were related to lower WLZ, but not LAZ in a HIV-exposed children (15). On the other hand, Bailey et al. reported that prolonged diarrhea was risk factor for stunting among a population of HIV-uninfected, -exposed, and -infected children in the Democratic Republic of the Congo (4) and McGrath et al. found a positive association between diarrhea morbidity and risk of wasting among HIV-exposed infants in Kenya (16). In the current study, we were aware of the potential for reverse causation between morbidity and undernutrition (i.e. the risk of morbidity may increase with declining nutritional status) and attempted to capture the effect of chronic morbidity by ending the updating of the child’s morbidity history 8 weeks prior to the end of each time interval. Using this approach, diarrhea was inversely associated with the risk of wasting, but not stunting or underweight.
Our study has both strengths and limitations. We were able to obtain repeated anthropometric measures at monthly intervals on a large sample of infants born to HIV-infected women. We also collected data on a comprehensive set of predictors including demographic, socioeconomic, anemia, and morbidity indicators. We used statistical techniques to account for the time-varying nature of certain predictors. Although our analysis does not include a comparison group of HIV-uninfected infants born to HIV-uninfected women, WHO Z scores provide this comparison. Although we considered a comprehensive set of variables in our analyses, residual confounding from unmeasured covariates cannot be ruled out. Finally, a standardized ARV therapy protocol for mothers was implemented part way through the study, which makes it difficult to interpret the true effect of maternal ARV therapy on the risk of child undernutrition.
In conclusion, maternal education and nutritional status, as well as child HIV status, sex, and birth weight were significant, independent predictors of stunting, wasting, and underweight among infants born to HIV-infected women in Tanzania. Strategies to promote female education, improve maternal nutritional status, increase socioeconomic status, reduce low birth weight, and lower the transmission of mother-to-child transmission of HIV could all help to lower the burden of child undernutrion among HIV-exposed infants.
Acknowledgments
Sources of support: NICHD R01 HD043688-01 and K24HD058795
Footnotes
clinicaltrials.gov identifier: NCT00197730
Conflict of Interest Statement
None of the authors had a personal or financial conflict of interest. The opinions and statements in this article are those of the authors and may not reflect official UNICEF policies.
References
- 1.Arpadi SM. Growth failure in children with HIV infection. J Acquir Immune Defic Syndr. 2000;25(Suppl 1):S37–42. doi: 10.1097/00042560-200010001-00006. Epub 2000/12/29. [DOI] [PubMed] [Google Scholar]
- 2.Bobat R, Coovadia H, Moodley D, Coutsoudis A, Gouws E. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21(3):203–10. doi: 10.1080/02724930120077772. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Borja MC, Peckham C. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111(1):e52–60. doi: 10.1542/peds.111.1.e52. Epub 2003/01/02. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 1999;28(3):532–40. doi: 10.1093/ije/28.3.532. [DOI] [PubMed] [Google Scholar]
- 5.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr Rev. 2009;67(6):343–59. doi: 10.1111/j.1753-4887.2009.00207.x. Epub 2009/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggan C, Fawzi W. Micronutrients and child health: studies in international nutrition and HIV infection. Nutrition Reviews. 2001;59(11):358–69. doi: 10.1111/j.1753-4887.2001.tb06963.x. [DOI] [PubMed] [Google Scholar]
- 7.Periquet BA, Jammes NM, Lambert WE, Tricoire J, Moussa MM, Garcia J, et al. Micronutrient levels in HIV-1-infected children. Aids. 1995;9(8):887–93. doi: 10.1097/00002030-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Omene JA, Easington CR, Glew RH, Prosper M, Ledlie S. Serum beta-carotene deficiency in HIV-infected children. J Natl Med Assoc. 1996;88(12):789–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Sunguya BF, Poudel KC, Otsuka K, Yasuoka J, Mlunde LB, Urassa DP, et al. Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC Public Health. 2011;11:869. doi: 10.1186/1471-2458-11-869. Epub 2011/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. The WHO Child Growth Standards. [cited 2011 October 13]; Available from: http://www.who.int/childgrowth/standards/en/
- 11.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. American Journal of Clinical Nutrition. 1997;66(2):464S–77S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 12.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The Comparison of Alternative Smoothing Methods for Fitting Non-Linear Exposure-Response Relationships with Cox Models in a Simulation Study. Int J Biostat. 2009;5(1):Article2. doi: 10.2202/1557-4679.1104. Epub 2009/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villamor E, Fataki MR, Bosch RJ, Mbise RL, Fawzi WW. Human immunodeficiency virus infection, diarrheal disease and sociodemographic predictors of child growth. Acta Paediatr. 2004;93(3):372–9. [PubMed] [Google Scholar]
- 15.Webb AL, Manji K, Fawzi WW, Villamor E. Time-independent maternal and infant factors and time-dependent infant morbidities including HIV infection, contribute to infant growth faltering during the first 2 years of life. J Trop Pediatr. 2009;55(2):83–90. doi: 10.1093/tropej/fmn068. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath CJ, Nduati R, Richardson BA, Kristal AR, Mbori-Ngacha D, Farquhar C, et al. The Prevalence of Stunting Is High in HIV-1-Exposed Uninfected Infants in Kenya. J Nutr. 2012;142(4):757–63. doi: 10.3945/jn.111.148874. Epub 2012/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madise NJ, Matthews Z, Margetts B. Heterogeneity of Child Nutritional Status between Households: A Comparison of Six Sub-Sharan African Countries. Population Studies. 1999;53(3):331–43. [Google Scholar]
- 18.Rahman A, Chowdhury S. Determinants of chronic malnutrition among preschool children in Bangladesh. J Biosoc Sci. 2007;39(2):161–73. doi: 10.1017/S0021932006001295. Epub 2006/03/29. [DOI] [PubMed] [Google Scholar]
- 19.Pryer JA, Rogers S, Rahman A. The epidemiology of good nutritional status among children from a population with a high prevalence of malnutrition. Public Health Nutr. 2003;7(2):311–7. doi: 10.1079/PHN2003530. [DOI] [PubMed] [Google Scholar]
- 20.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell ML. Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS. 2010;24(3):437–45. doi: 10.1097/QAD.0b013e3283345f91. Epub 2009/11/17. [DOI] [PubMed] [Google Scholar]
- 21.Grinspoon S, Corcoran C, Miller K, Biller BM, Askari H, Wang E, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997;82(5):1332–7. doi: 10.1210/jcem.82.5.3907. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 22.Lartey A. Maternal and child nutrition in Sub-Saharan Africa: challenges and interventions. Proc Nutr Soc. 2008;67(1):105–8. doi: 10.1017/S0029665108006083. Epub 2008/02/01. [DOI] [PubMed] [Google Scholar]
- 23.Villamor E, Msamanga G, Spiegelman D, Coley J, Hunter DJ, Peterson KE, et al. HIV status and sociodemographic correlates of maternal body size and wasting during pregnancy. Eur J Clin Nutr. 2002;56(5):415–24. doi: 10.1038/sj.ejcn.1601328. Epub 2002/05/10. [DOI] [PubMed] [Google Scholar]
- 24.Meshram II, Laxmaiah A, Gal Reddy C, Ravindranath M, Venkaiah K, Brahmam GN. Prevalence of under-nutrition and its correlates among under 3 year-old children in rural areas of Andhra Pradesh, India. Ann Hum Biol. 2011;38(1):93–101. doi: 10.3109/03014460.2010.498387. Epub 2010/09/04. [DOI] [PubMed] [Google Scholar]
- 25.Wamani H, Astrom AN, Peterson S, Tumwine JK, Tylleskar T. Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007;7:17. doi: 10.1186/1471-2431-7-17. Epub 2007/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Msamanga G, Manji K, Villamor E, Bosch RJ, Hertzmark E, et al. Sex differences in the effects of maternal vitamin supplements on mortality and morbidity among children born to HIV-infected women in Tanzania. Br J Nutr. 2010;103(12):1784–91. doi: 10.1017/S0007114509993862. Epub 2010/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maleta K, Virtanen SM, Espo M, Kulmala T, Ashorn P. Childhood malnutrition and its predictors in rural Malawi. Paediatr Perinat Epidemiol. 2003;17(4):384–90. doi: 10.1046/j.1365-3016.2003.00519.x. Epub 2003/11/25. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh KK, Lurie MN, Triche EW, De Bruyn G, Harwell JI, McGarvey ST, et al. Growth of infants born to HIV-infected women in South Africa according to maternal and infant characteristics. Trop Med Int Health. 2010;15(11):1364–74. doi: 10.1111/j.1365-3156.2010.02634.x. Epub 2010/10/20. [DOI] [PubMed] [Google Scholar]
- 29.Manji KP, Massawe AW, Mgone JM. Birthweight and neonatal outcome at the Muhimbili Medical Centre, Dar es Salaam, Tanzania. East African Medical Journal. 1998;75(7):382–7. [PubMed] [Google Scholar]