Abstract
Enterohepatic circulation serves to capture bile acids and other steroid metabolites produced in the liver and secreted to the intestine, for reabsorption back into the circulation and reuptake to the liver. This process is under tight regulation by nuclear receptor signaling. Bile acids, produced from cholesterol, can alter gene expression in the liver and small intestine via activating the nuclear receptors farnesoid X receptor (FXR; NR1H4), pregnane X receptor (PXR; NR1I2), vitamin D receptor (VDR; NR1I1), G protein coupled receptor TGR5, and other cell signaling pathways (JNK1/2, AKT and ERK1/2). Among these controls, FXR is known to be a major bile acid-responsive ligand-activated transcription factor and a crucial control element for maintaining bile acid homeostasis. FXR has a high affinity for several major endogenous bile acids, notably cholic acid, deoxycholic acid, chenodeoxycholic acid, and lithocholic acid. By responding to excess bile acids, FXR is a bridge between the liver and small intestine to control bile acid levels and regulate bile acid synthesis and enterohepatic flow. FXR is highly expressed in the liver and gut, relative to other tissues, and contributes to the maintenance of cholesterol/bile acid homeostasis by regulating a variety of metabolic enzymes and transporters. FXR activation also affects lipid and glucose metabolism, and can influence drug metabolism.
Introduction
In 1995, the farnesoid X receptor (FXR; NR1H4) was identified as an orphan nuclear receptor from mouse [1] and rat [2]. In the early studies, farnesol and related metabolites were proposed as possible ligands for the rat homolog, thus accounting for the original name [2]. However, subsequently, bile acids were found to be the true endogenous ligands for FXR [3–5], so more accurately, this receptor should have been designated the bile acid receptor. To date, more than 80 compounds have been identified as potential FXR ligands with varying degrees of affinity; these include the endogenous bile acids, and synthetic ligands (Table 1). Several structural structurally diverse compounds show high-affinity binding and agonist activity toward FXR, including steroids, aromatics, terpenoids, alkaloids, and fatty acids (Figure 1).
Table 1.
Reported FXR ligands
| Year | Function | Compounds | Structure type | Source | Reference |
|---|---|---|---|---|---|
| 1995 | Agonist | Farnesol Farnesol metabolites |
Terpenoid | Nature | [2] |
| 1999 | Agonist | CDCA DCA LCA TTNPB |
Steroid Aromatics |
Nature Synthesis |
[4] [5] |
| 1999 | Agonist | CDCA CA DCA LCA |
Steroid | Nature | [3] |
| 2000 | Agonist | Forskolin | Steroid | Nature | [7] |
| 2000 | Agonist | GW4064 GW9047 |
Aromatics | Synthesis | [120] |
| 2001 | Agonist | 1,1-Bisphosphonate esters | Aromatics | Synthesis | [121] |
| 2002 | Agonist | 6-ECDCA | Steroid | Semi-synthesis | [10] |
| 2003 | Agonist | fexaramine | Aromatics | Synthesis | [122] |
| 2003 | Antagonist | Guggulsterone | Steroid | Nature | [9] |
| 2003 | Agonist | Fexaramate Fexarene Fexaramine Fexarine fexarchloramide |
Aromatics | Synthesis | [123] |
| 2003 | Agonist Antagonist |
AGN29 AGN 31 AGN 34 |
Aromatics | Synthesis | [124] |
| 2004 | Agonist | UDCA | Steroid | Nature | [125] |
| 2004 | Agonist | CDCA derivatives | Steroid | Semi-synthesis | [126] |
| 2004 | Antagonist | Arachidonic acid Docosahexaenoic acid Linolenic acid |
Fatty acid | Nature | [127] |
| 2005 | Agonist | Xanthohumol | Aromatics | Nature | [128] |
| 2006 | Agonist | 22 (R)-hydroxycholesterol | Steroid | Nature | [129] |
| 2006 | Agonist Antagonist |
Bile alcohols 5β-cyprinol Bile alcohols 5β-bufol Bile alcohols 5α-cyprinol Bile alcohols 5α-bufol |
Steroid | Semi-synthesis | [130] |
| 2006 | Agonist | Androsterone | Steroid | Nature | [6] |
| 2006 | Agonist | Diphenylmethane skeleton | Steroid | Synthesis | [131] |
| 2007 | Antagonist | GW4064 derivatives | Aromatics | Semi-synthesis | [132] |
| 2007 | Antagonist | Stigmasterol | Steroid | Nature | [8] |
| 2007 | Agonist | Cafestol | Terpenoid | Nature | [133] |
| 2008 | Agonist | GSK8062 | Aromatics | Semi-synthesis | [134] |
| 2008 | Agonist | Pyrazolidine-3,5-dione derivatives |
Aromatics | Semi-synthesis | [135] |
| 2008 | Agonis | Coumestrol | Steroid | Nature | [136] |
| 2008 | Agonist | Methyl cholate, Methyl deoxycholate 5β-Cholanic acid, 5β-Cholanic acid-7α,12α-diol NIHS700 marchantinA marchantin E |
Steroid Aromatics |
Semi-synthesis Nature |
[137] |
| 2009 | Agonist | Froglitazone Rosiglitazone Pioglitazone |
Alkaloid | Synthesis | [138] |
| 2009 | Agonist | WAY-362450 | Alkaloid | Synthesis | [139] |
| 2009 | Agonist | Pyrrole [2,3-d]azepines | Alkaloid | Semi-synthesis | [140] |
| 2009 | Agonist | N-oxide pyridine GW4064 | Aromatics | [141] | |
| 2009 | Agonist | Bile alcohols | Steroid | Semi-synthesis | [142] |
| 2010 | Antagonist | Oleanolic acid | Terpenoid | Nature | (20) |
| 2011 | Agonist | GSK2324 | Aromatics | Semi-synthesis | [143] |
| 2011 | Antagonist | Sulfated sterols | Steroid | Semi-synthesis | [144] |
| 2011 | Agonist | 6a-Ethyl-24-norcholanyl-23- amine derivate |
Steroid | Semi-synthesis | [145] |
| 2011 | Antagonist | Tuberatolides | Terpenoid | Nature | [146] |
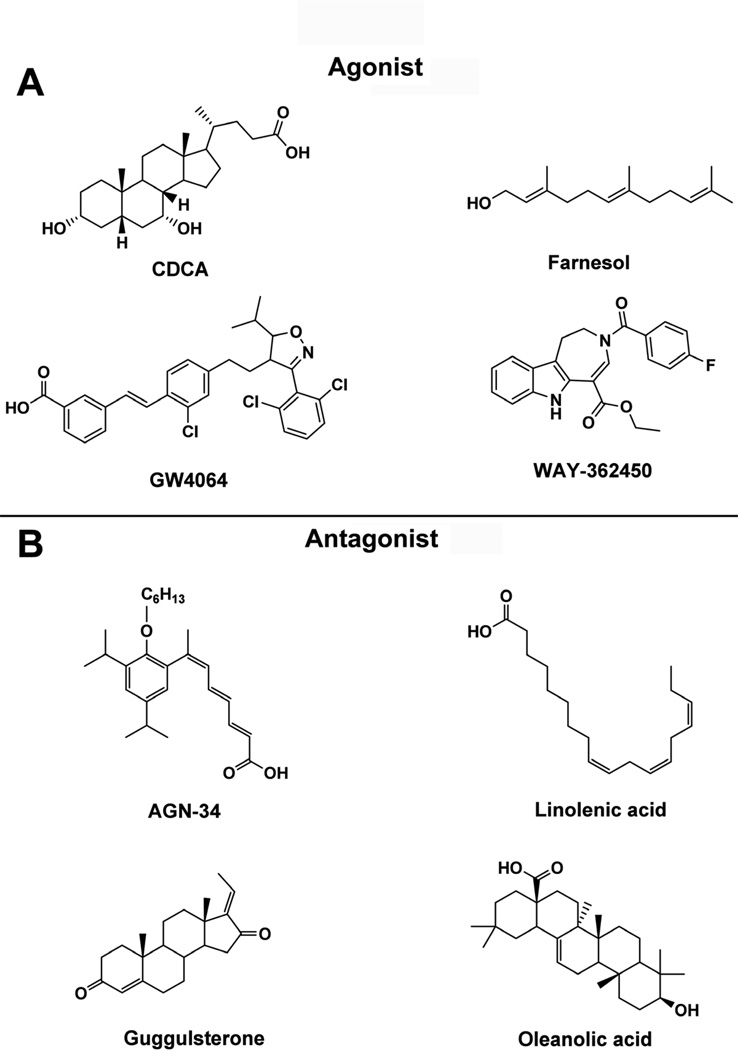
Figure 1. Reported FXR ligands.
(A) FXR agonists, CDCA (steroid), farnesol (terpenoid), GW4064 (aromatics), and WAY-362450 (alkaloid). (B) FXR antagonists, AGN-34 (aromatics), linolenic acid (fatty acid), guggulsterone (steroid), oleanoic acid (terpenoid).
Steroids are the major and most important ligands for FXR. Numerous endogenous compounds and their metabolites with important physiological functions encompass bile acids, cholesterol and hormones. Endogenous bile acids, including the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA), and the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), can activate FXR in vivo and in cultured cells, and bind the receptor in vitro. Bile acids have the general properties of a concave hydrophilic face and a convex hydrophobic face. They combine with the hydrophobic pocket of the FXR ligand-binding domain mainly through the hydrophobic face, while the hydroxyl groups in the hydrophilic phase also can greatly affect the affinity of bile acids with FXR. The potency of bile acids to activate FXR is CDCA>DCA>LCA>CA [5]. Many compounds unrelated to bile acids, can also act as FXR ligands, such as androsterone [6], and the exogenous natural product plant sterols forskolin [7], stigmasterol [8], and guggulsterone [9]. In addition, a series of synthetic bile acid derivatives have been developed as FXR ligands, such as 6α-ethyl-chenodeoxycholic acid (6-ECDCA) and bile alcohols, showing an even higher affinity with FXR than bile acids [10].
FXR is the chief sensor of intracellular levels of bile acids, controlling their synthesis and transport. Along with the regulation of bile acid metabolism, FXR is also involved, directly and indirectly, in several important metabolic pathways in vivo, such as modulation of glucose and lipid metabolism. Thus, activation or repression of FXR can have major influences on metabolic homeostasis. In addition, FXR genetic variants are associated with metabolic diseases, including intrahepatic cholestasis of pregnancy [11], and cholesterol cholelithiasis [12]. FXR ligands have been proposed for possible treatment of metabolic diseases, such as cholestasis [13], liver fibrosis [14], inflammatory bowel disease [15], type 2 diabetes [16], atherosclerosis [17], and erectile dysfunction [18]. Ursodeoxycholic acid (UDCA), an FXR agonist [19], was approved by the FDA as a drug for the treatment of primary biliary cirrhosis (PBC), and is widely used for the treatment of a variety of chronic cholestatic diseases. Thus, FXR provides a framework for developing novel therapies for several liver diseases that are due to altered bile acid homeostasis. There are more than 40 target genes for FXR, most of which are positively regulated, although some genes are indirectly down-regulated by FXR [17]. Some of the FXR target genes are involved in hepatic-intestinal bile acid synthesis, transport and homeostasis, while others are functional in other metabolic pathways.
FXR function in small intestine
In the intestine, FXR controls the absorption of bile acids, lipids, vitamins, certain drugs, and other xenobiotics through the regulation of expression of four important transporters, apical sodium dependent transporter (ASBT, also called solute carrier 10A2; SLC10A2), fatty acid-binding protein subclass 6 (FABP6), also known as intestinal bile acid-binding protein (I-BABP), and organic solute transporters α (OSTα) and β (OSTβ), which are responsible for the transport of bile acids from the intestine to the portal system. ASBT, the major bile acid transport system in ileal enterocytes, transports bile acids into the ileal enterocyte brush border (apical) membrane [20]. In humans, ASBT deficiency causes significant bile acid malabsorption [21,22]. FXR is a negative regulator of intestinal ASBT expression in mice but not in rats thus indicating a significant species difference in regulation of the Asbt gene by bile acids [23,24]. In mice, ASBT protein and mRNA are decreased when the animals are fed FXR ligands such as CA and TCA. Mechanistic studies revealed that bile acids exert a negative feedback on ASBT expression by FXR activation of the small heterodimer partner (SHP; NR0B2)-dependent repression of liver related homolog-1 (LRH-1; NR5A2) activity in mice. The negative regulation of ASBT expression was not, however, observed in rats, due to the absence of an LRH-1 responsive element within the rat ASBT promoter [24]. In addition, intestinal ASBT expression is not induced in Fxr-null mice in response to bile acids, thus suggesting that down-regulation of ASBT is associated with ligand-activated FXR. The repression of ASBT is also found in rabbits through a similar FXR/LRH-1/SHP-dependent mechanism [25]. In the human enterocyte-like Caco-2 cells, the mouse Asbt promoter activity is repressed by CDCA, while the rat ASBT promoter was not, indicating that humans respond to bile acids similar to the mouse and rabbit [26]. However, in contrast to the mouse, SHP inhibits positive regulation of the human ASBT gene through interfering with the retinoic acid receptor (RAR;NR2B2)/retinoid X receptor (RXR;NR2B2) heterodimer complex, in contrast to mice in which SHP interferes with LRH-1 [24]. In humans, the ASBT gene is activated by retinoic acid, a finding that has implications for the treatment of patients with cholestasis or chronic diseases of the gastrointestinal system with vitamin A and retinoic acid-based drugs [26]. However, in contrast to mice that offer a model for pharmacological and genetic manipulation, the precise mechanism of bile acids suppression of ASBT in humans is difficult to determine. The biological significance of the species differences in ASBT suppression is also not completely understood, in particular the roles of the positive regulators LRH-1 and RAR/RXR. Finally, other studies have revealed that the membrane protein β-Klotho, involved in fibroblast growth factor (FGF) 15 (FGF-19 in humans) signaling, suppresses basal ASBT activity through the LRH-1 cis-element, presumably affecting the FXR/SHP pathway [27]. However, the signaling pathway linking β-Klotho and FXR is not known.
FABP6 also known as I-BABP, is expressed in the ilium and shuttles bile acids from the apical to basolateral membrane in the enterocyte [28]. It was suggested that FABP6 plays an important role in enterohepatic circulation through the regulation of bile acid trafficking [29]. In Caco-2 cells, bile acid-activated FXR can induce FABP6 gene expression through binding to the promoter [30]. In vivo studies in mice further demonstrated that cholestyramine treatment dramatically decreased FABP6 mRNA levels, whereas TCA treatment increased mRNA levels. Furthermore FABP6 mRNA levels are significantly decreased in Fxr-null mice, thus suggesting that FXR positively regulates the Fabp6 gene in mice [31]. Finally, the heteromeric organic solute transporters OSTα and OSTβ move bile salts to blood vessels, in accordance with their location at the basolateral membrane [32]. OSTα and OSTβ are expressed not only in the ileum, but also in the liver and kidney [32]. Ileum expression of both the genes is induced in wild-type mice after CA exposure; induction was not observed in Fxr-null mice [33]. Treatment with GW4064, a synthetic FXR ligand, also induces OSTα and OSTβ mRNAs in the intestine. Another study identified FXR regulatory elements (FXRE) in the promoters of the genes encoding OSTα and OSTβ in both humans and mice [34]. Introduction of human OSTα and OSTβ into HepG2 cells facilitates the uptake of conjugated-CDCA and the activation of FXR target genes. Taken together, these studies demonstrated that FXR controls transport of bile acids from the intestine to blood vessel to liver.
Intestinal FXR activation also affects hepatic events. Fibroblast growth factor 19 (FGF19 in humans, and its mouse homolog FGF15, referred to as FGF15/19) is highly expressed in the small intestine [35,36]. FGF15/19, secreted from the intestine, circulates to the liver and suppresses bile acid through binding and activation of the FGF receptor 4 (FGFR4) complexed with β-Klotho located on the surface of hepatocytes and other epithelial cells [37,38]. Activation of the FGFR4/β-Klotho complex stimulates the c-Jun N-terminal kinase (JNK) pathway, eventually suppressing the gene encoding CYP7A1, the cholesterol 7α-hydroxylase and the rate-limiting bile acid synthetic enzyme [39,40]; these effects were not observed in Shp-null mice thus suggesting that SHP is required for the suppressive effects of FGF15/19 [40]. Extracellular signal-regulated kinase (ERK) was markedly elevated by FGF15 administration to mice and deficiency of both JNK and ERK pathways prevented FGF15-mediated suppression of Cyp7a1 and Cyp8b1 expression; deficiency of either pathway alone and minimal effect on FGF15 suppression of these genes [41].
FXR is a major regulator of the gene encoding FGF15/19 in the intestine and thus bile acid-activated intestinal FXR can down-regulate CYP7A1 expression through direct activation of intestinal FGF15/19. In addition, FGF15/19 was reported to work as a hormone for gallbladder filling via its interaction with FGFR3, a receptor that is highly expressed in the gallbladder [42]. These studies indicate that FXR-FGF15/19 signaling contributes to the control of intestinal bile acid levels. FGF15/19 was also recently reported to activate hepatic glycogen synthesis by activating glycogen synthase kinase 3 (GSK3) [43], and to inhibit hepatic gluconeogenesis [44] by inhibiting the cAMP regulatory element-binding protein (CREB)-peroxisome proliferators-activated receptor γ coactivator protein-1α (PGC-1α) pathway. Stimulation of hepatic glycogen synthesis and inhibition of glucose metabolism is mediated via extracellular signal-regulated protein (E) activated by the FGF15/19-stimulated FGFR4/β-Klotho complex, independent of insulin signaling. Thus, bile acids are involved in maintaining hepatic glucose homeostasis though FGF15/19, independent of FXR, and that FGF15/19 is postprandial regulator of hepatic carbohydrate homeostasis [44].
FXR is also expressed in pancreatic β-cells and regulates insulin signaling. In βTC-6 cells, an insulin-secreting cell line derived from transgenic mice expressing the large T-antigen of simian virus 40 (SV40) in pancreatic β-cells cells, FXR induces expression of the glucose regulated transcription factor KLF11 [45]. KLF11 may account of the effect of FXR on glucose-induced insulin gene transcription. In addition FXR regulates insulin secretion by non-genomic effects though increasing Akt phosphorylation and GLUT2 translocation at the plasma membrane, resulting in increasing the glucose uptake by these cells. The mechanisms for these effects are not known. Recent studies also revealed that bile acids can affect insulin secretion in pancreatic β-cells by altering cytosolic calcium concentrations [46]. Treatment of primary β-cell cultures with the FXR ligands taurochenodeoxycholate (TCDC) and the synthetic FXR agonist GW4064, stimulated the electrical activity and increased calcium concentration through FXR inhibition of K(ATP) channel activity; these effects were not found in Fxr-null mouse β-cells. The mechanism by which FXR effects K(ATP) channel activity is not through altering gene expression and thus represent a novel non-genomic mechanism whereby FXR affects insulin secretion. The role of bile acids and FXR in regulating insulin secretion and glucose transport in the intact pancreas has not been determined.
In addition to being a major regulator of bile acid homeostasis, FXR plays an important role in the intestinal defense against inflammation, interacting with nuclear factor-kappaB (NF-kB) signaling. Exposure of LPS-activated macrophages to an FXR ligand leads to the reciprocal regulation of NF-kB-dependent genes such as TNFα and IL-1β [47]. Intestinal FXR activation, responding to bile acids, controls bacterial growth and maintains mucosal integrity, regulating the expression of a variety of genes involved in defense against inflammation and mucosa protection. Therefore, FXR might be a critical factor regulating intestinal innate immunity and homeostasis.
Role of hepatic FXR in bile acid homeostasis
FXR functions as the chief sensor of intracellular bile acid levels. Hepatic bile acid levels are maintained by the control of uptake, synthesis, metabolism and export. Na+-taurocholate cotransporting polypeptide (NTCP, also termed solute carrier 10A1; SLC10A1) and organic anion-transporting peptides (OATPs, also named SLCO family) are the major bile acid transporters in the hepatocellular basolateral membrane for the uptake of bile acids and organic solutes from portal vein to liver [48,49]. NTCP is responsible for the uptake of conjugated bile acids, whereas the OATPs are largely involved in the uptake of unconjugated bile acids. In the cholestasis mouse model of liver disease, the expression of NTCP and OATPs are significantly reduced to avoid excess accumulation of bile acids in the liver [50]. Hepatic NTCP expression is regulated by SHP that is induced by FXR upon bile acid activation [51]. The glucocorticoid receptor (GR; NR3C1) is also a transcriptional activator of the NTCP gene in humans [52]. GR induction of endogenous NTCP expression is suppressed by CDCA or GW4046, through FXR by its induction of SHP. Hepatocyte nuclear factor 4α (HNF4α) also binds the NTCP gene promoter in the rat at a cite overlapping with the retinoic acid receptor (RAR)/RXR response element, and HNF4α-mediated NTCP expression was also reported to be inhibited by SHP [53]. However, there are marked species differences in constitutive control of the gene encoding NTCP. HNF4α and HNF1α constitutively regulate the rat NTCP gene promoter and not the mouse or human promoters, while the mouse and human genes are controlled by the CCAAT/enhancer binding protein β (C/EBP β) [53]. Thus, regulation of NTCP is accomplished by several transcription factors, both positively and negatively, although species differences exist.
Certain members of the OATP family facilitate Na+-independent transmembrane transport of various endogenous and xenobiotic compounds, such as bile acids, bilirubin, steroid hormone conjugates, thyroid hormones, prostaglandins, clinically used drugs, and toxins [54]. Altered FXR function can directly affect hepatic expression of the OATPs family members. CA treatment significantly decreases OATP1 (SLCO1A1) expression and increases OATP2 (SLCO1A4) expression in wild-type mouse liver; OATP1, OATP2, and OATP3 (SLCO1A5) mRNA levels in the livers of Fxr-null mice were similar thus indicating the involvement of FXR [55]. In human liver, bile acids repress expression of the OATP1B1 gene but the mechanistic role of FXR was not determined in this study [56]. Taken together, FXR can negatively regulate bile acid uptake in a feedback fashion through SHP-alteration of hepatic GR, RAR, HNF4α, and HNF1α activity.
In addition to the negative regulation of bile acid transport, FXR also represses some important cytochromes P450 (CYP) that are involved in bile acid synthesis. This was firmly established in vivo by analysis of Fxr-null [31] and intestine-specific and liver-specific Fxr-null mice [57]. Notably, CYP7A1 and CYP8B1 are two hepatic enzymes catalyzing formation of the primary bile acids CA and CDCA from cholesterol [58]. CYP7A1 expression is negatively regulated via the hepatic FXR-SHP and intestinal FXR-FGF15/19 pathways. SHP inhibits the CYP7A1 gene by interaction with the positive regulator LRH-1 resulting in a non-productive transcription factor [59]. Study of the CYP7A1 gene revealed a negative bile acid response element in the promoter that can bind LRH-1 and HNF4α [60,61]. LRH-1-responsive elements are also located in the regulatory regions of SHP gene, and overexpression of LRH-1 can activate the SHP promoter in mice and tissue culture cells [62,63]. SHP-mediated inhibition of LRH1-dependent suppression of transcription is due in part to recruitment of SIRT1 histone deacetylase protein; LRH-1 activation of CYP7A1 and SHP gene transcription was significantly repressed by both SHP and SIRT1 while the inhibition of SIRT1 activity by inhibitors or a dominant negative SIRT1, or direct knockdown of SHP, released the inhibitory effect [64]. Others found that Brahma chromatin remodeling protein, Sin3a scaffold co-repressor, and histone deacetylase-1, increased the occupancy of SHP at the CYP7A1 promoter [65]. Finally, SHP can be modified by ligand binding as revealed by use of a retinoid-like compound, 4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamic acid (3Cl-AHPC), that was found to bind to SHP and increase its interaction with LRH-1 [65]. Along with SHP and FGF15/19, HNF4α can suppress CYP7A1 expression; chromatin immunoprecipitation (ChIP) assays suggest that bile acids can suppress transcription of the CYP7A1 through blocking the association of HNF4α with the coactivators PGC-1α and CREB-binding protein (CBP) [66]. Similarly, CYP8B1 is regulated by FXR via its target gene SHP. The CYP8B1 promoter also contains a negative bile acid response element harboring overlapping binding sites for HNF4α [67] and LRH-1 [68]. Induction of SHP by FXR can in turn inhibit CYP8B1 expression by negative interference of the positive transcriptional activity of HNF4α and LRH-1. However, another study indicated that there was no significant difference in repression of the CYP8B1 gene by bile acids between the Fxr-null mice and wild-type mice, thus suggesting that feedback inhibition of CYP8B1 is FXR-independent [31]. In addition, CYP27A1, catalyzing the acidic pathway of bile acid biosynthesis, also can be suppressed by bile acids as revealed in a human cell line [69]. A negative bile acid response element in CYP27A1 promoter can bind to HNF4α, suggesting a similar negative regulation of CYP27A1 via the FXR-SHP pathway as found with the CYP7A1 and CYP8B1 genes [69,70]. CYP17A1, and enzyme involved in the 17α-hydroxylation of C21 steroids such as progesterone, is also suppressed by bile acids through a mechanism that likely involved FXR/SHP/LRH-1 pathway [71]. 17-Hydroxy steroid metabolites are involved in liver injury and thus suppression of CYP17A1 by high hepatic bile acids would be a protective mechanism.
Members of the CYP3A family of cytochromes P450 expressed in liver and intestine, are also involved in bile acid metabolism, by catalyzing hydroxylation of bile acids such as CDCA, LCA and DCA at different positions on the molecules [72,73]. Human hepatic CYP3A4. the dominant CYP3A in human liver, carries out the metabolism of xenobiotic compounds including many clinically-used drugs [74]. This enzyme is also highly expressed in the intestine where it plays an important role in first-pass metabolism of many orally dosed drugs [75]. FXR was shown to regulate the expression CYP3A4. For example, in HepG2 cells, mRNA encoding CYP3A4, was found to be elevated 24 hours after exposure to 100 μM CDCA and 1 μM GW4064 [76]. When wild-type, Fxr-null and Pxr-null mice were treated with GW4064, a significant induction of CYP3A11, the mouse equivalent of human CYP3A4, expression was observed in wild-type and Pxr-null mice, but not in Fxr-null mice [76]. In accordance with this result, secondary bile acids might activate transcription of the mouse Cyp3a11, the putative mouse homolog of CYP3A4, through FXR, although the presence of FXR regulatory elements have not been observed in the Cyp3a11 gene. A recent study revealed that CDCA can induce CYP3A4 expression in human liver, not human ileum [77]. These results provide evidence that FXR is involved in bile acid-induced CYP3A expression, although the participation of other receptors such as PXR, constitutive androstane receptor (CAR; NR1I3) and vitamin D receptor (VDR; NR1I1), activated by bile acids, that also regulate CYP3A expression, cannot be excluded. In addition to CYP3A, FXR was reported to up-regulate transcription of the human drug conjugating enzyme uridine 5’-diphosphate-glucuronosyltransferase 2B4 (UGT2B4) [78] that catalyzes production glucuronidated 6α-hydroxylated bile acids such as hyodeoxycholic acid [79], and sulfotransferase 2A1 (SULT2A1) [80] that catalyzes sulfate conjugation of many hydroxysteroid substrates, such as bile acids, pregnenolone, and estrogens [81]. Treatment of hepatocytes and HepG2 cells with the FXR agonists CDCA and GW4064 led to an increase in endogenous UGT2B4 expression and activity. Potential FXR induction of SULT2A1 expression was assessed using a gene reporter system and endogenous SULT2A1 expression was decreased in HepG2 cells treated with CDCA or GW4064 [82]. FXR also regulates two enzymes involved in bile acid conjugation with taurine and glycine, bile acid-CoA synthetase (BACS, also called SLC27A5) and bile acid-CoA:aminoacid N-acetyltransferase (BAAT). The level of BACS and BAAT expression was activated in hepatocytes and Fisher rats treated by CDCA and GW 4064 [83]. Functional response elements were found in the proximal promoter of BACS and in the intronic region between exons 1 and 2 of the BAAT gene. Further mutational analysis confirmed that the inverted repeat-1 (IR-1) element of BACS and BAAT genes binds the FXR-RXR heterodimer.
FXR has a critical role in the elimination of hepatic bile acids through the regulation of the ATP-binding cassette (ABC) transporters. Bile salt exporting pump (BSEP, also termed ABCB11), is a major efflux transporter of bile acids from liver to gallbladder. BSEP deficiencies are associated with progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis type 2 (BRIC2), and several acquired forms of cholestasis [84]. Fxr-null mice fed CA-supplement diet showed intrahepatic cholestasis, similar with that of the human BSEP deficiency. Reporter gene assays showed that the BSEP promoter was positively controlled by FXR and bile acids [85]. Mutation of the FXR regulatory element strongly represses the FXR-dependent induction of BSEP expression. Consistent with a role for FXR in BSEP regulation, an IR-1 element in the BSEP promoter specifically binds FXR/RXRα heterodimers [86]. In Fxr-null mice, BSEP expression was significantly reduced and the FXR agonist GW4064 cannot induce the expression Bsep gene thus confirming that FXR controls BSEP expression [87]. Expression of another ABC transporter ABCB4 (also called MDR3 in human and MDR2 in rodents), is significantly reduced in Fxr-null mice, where GW4064-induced Abcb4 expression is not observed [87]. ABCB4 is a critical transporter of phospholipids, across canalicular membranes of hepatocytes [88]. After the phospholipids are transported to gallbladder from liver via ABCB4, the formation of mixed micelles containing cholesterol, bile acids, and phospholipids will increase their solubility and reduce their toxicity to the bile duct. Similar to BSEP, ABCB4 deficiency in humans can cause PFIC3 [89,90]. FXR regulates the ABCB4 gene through binding a highly conserved IR-1 element at the distal promoter [91]. In primary human hepatocytes, ABCB4 mRNA is induced by CDCA and GW4064 in a time- and dose-dependent fashion. In rats, GW4064 treatment increases the expression of mouse ABCB4 gene in the liver [13]. Thus, FXR can be a critical factor for bile acid homeostasis through regulating hepatic BSEP and MDR2/3 expression.
Role of FXR in the metabolism and transport of xenobiotics
In addition to bile acid homeostasis, FXR can contribute to the metabolism and elimination of the xenobiotics, through regulation of the phase I and II drug-metabolizing enzymes and drug transporters (Table 2). As noted above, FXR regulates several important enzymes involved in drug metabolism, such as CYPs, UGTs and SULTs, converting the hydrophobic compounds to more hydrophilic and less toxic conjugated derivatives that can more easily be eliminated from the body. A recent study reported that activation of FXR protects mice from acetaminophen (APAP)-induced heptotoxicity [92]. Under normal therapeutic dosing, APAP is metabolized in the liver mainly through direct conjugation by UGTs and SULTs. However, excessive APAP will saturate both glucuronidation and sulfation pathways, leading to accumulation of the toxic NAPQI metabolite produced by CYPs, notably CYP2E1 [93]. NAPQI is also subject to conjugation by glutathione S-transferase (GST) but under conditions of high doses of APAP, the amount of NAPQI exceeds the conjugation capacity and the cosubstrate for GST, glutathione, is depleted. The liver toxicity induced by high dose APAP could be attenuated by FXR up-regulation of several phase II enzymes. To identify which drug metabolizing enzymes might be regulated by FXR, three models were employed, a constitutively-active form of FXR (FXR-VP16), native FXR, and treating wild-type and Fxr-null mice with an FXR agonist. The expression levels of several GSTs (GSTα3, GSTα4, GSTμ1, GSTμ3), SULTS (SULT1A1 and SULT1A2), and UGTs (UGT1A1), were induced by FXR activation [92]. FXR response elements were found in some of these gene promoters by use of ChIP-Seq genome-wide binding site analysis. Others have also reported multiple binding sites for FXR in untreated [94], GW4064 ligand treated [95] and in high-fat diet-induced obese mice [96], thus revealing a surprisingly large number of genes with FXR binding sites, notably the characterized indirect repeat 1 (IR-1) element. While these genes were involved in multiple pathways, the functional relevance of the FXR binding for most of the genes has not been determined. Among the other notable findings by the in vivo binding analysis was the large degree of tissue-specific FXR binding; only 11% of total sites were shared between liver and intestine, the main sited of FXR activity [95]. The state of obesity also resulted in differential FXR chromatin binding thus suggesting a broader role of FXR in metabolism beyond the control of bile acid synthesis and transport [96].
Table 2.
FXR involved in the regulation of bile acid and xenobiotics metabolism
| Metabolism | Gene | Substrate | Regulation | Manner | Reference |
|---|---|---|---|---|---|
| phase 0 | NTCP | Bile acids | Suppression | Indirect effect | [51] |
| OATP1B1 | Bile acid, xenobiotics | Suprression | Indirect effect | [147] | |
| OATP1B3 | Bile acid, xenobiotics | Activation | Direct effect | [148] | |
| Phase I | CYP3A4 | Bile acid, xenobiotics | Activation | Direct and indirect effect | [77,149] |
| CYP7A1 | Cholesterol | Suprression | Indirect effect | [59] | |
| CYP8B1 | Cholesterol | Suprression | Indirect effect | [68] | |
| CYP27A1 | Cholesterol | Suprression | Indirect effect | [69] | |
| Phase II | UGT1A1 | Bile acid, xenobiotics | Activation | Direct effect | [92] |
| UGT2B4 | Bile acid, xenobiotics | Activation | Direct effect | [78] | |
| UGT2B7 | Bile acid, xenobiotics | Suppression | Direct effect | [150] | |
| SULT1a1 | Bile acid, xenobiotics | Activation | Direct effect | [92] | |
| SULT1a2 | Bile acid, xenobiotics | Activation | Direct effect | [92] | |
| SULT2A1 | Bile acid, xenobiotics | Activation | Direct effect | [80] | |
| GSTα3 | xenobiotics | Activation | Direct effect | [92] | |
| GSTα4 | xenobiotics | Activation | Direct effect | [92] | |
| GSTμ1 | xenobiotics | Activation | Direct effect | [92] | |
| GSTμ3 | xenobiotics | Activation | Direct effect | [92] | |
| BACS | Bile acid | Activation | Direct effect | [151] | |
| BAT | Bile acid | Activation | Direct and indirect effect | [151,152] | |
| Phase III | BSEP | Bile acid | Activation | Direct effect | [86] |
| MRP2 | Bile acid, xenobiotics | Activation | Direct and indirect effect | [153] | |
| MDR3 | PC | Activation | Direct effect | [91] | |
| OSTα | Bile acid | Activation | Direct effect | [33,34] | |
| OSTβ | Bile acid | Activation | Direct effect | [33,34] | |
| FABP6 | Bile acid | Activation | Direct effect | [30] | |
| ASTB | Bile acid | Suppression | Indirect effect | [24] |
Role of FXR in Cancer and Hepatotoxicity
While a critical role for FXR in bile acid homeostasis was established using mice with targeted disruption of FXR [31], FXR deficiency was also found to increase the development of liver and intestine cancer. A high incidence of hepatocellular adenoma, carcinoma, and hepatocholangiocellular carcinoma were detected in in 12-month-old male and female Fxr-null mice [97,98]. This was associated with an upregulation of genes involved in inflammation and cell cycle control in Fxr-null mice. Another study also indicated that FXR deficiency can promote cell proliferation, inflammation, and tumorigenesis in the intestine [99,100]. These results suggest that activation of FXR by its ligands may protect against liver and intestinal carcinogenesis. FXR expression was down-regulated in human hepatocellular carcinoma compared normal liver tissues and this was associated with increased expression of proinflammatory cytokines led to the hypothesis that decreased FXR expression through inhibition of FXR gene promoter promoted liver cancer [101]. However, others reported that FXR expression is downregulated by the microRNA miR-421 through inhibition of translation that could account for the decreased levels of FXR in liver tumors [102]. However, the precise nature of the FXR target genes involved in protection against liver cancer is not known. The possibility exist that the protection is the indirect result of alteration in bile acids and/or suppression of inflammation.
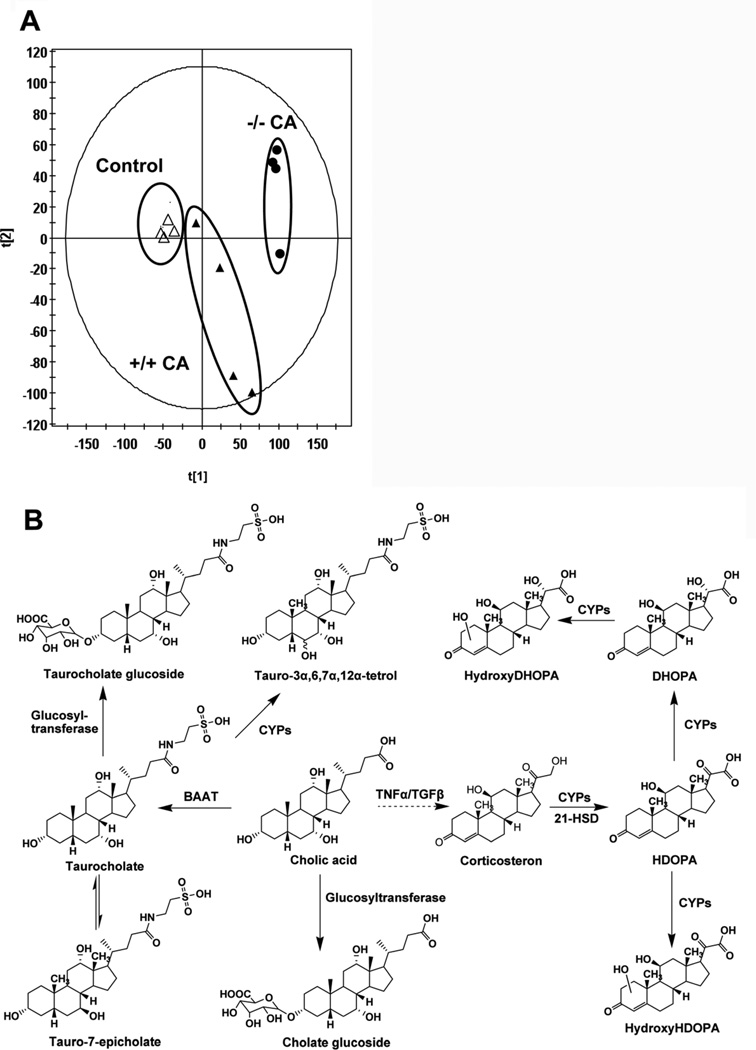
Previous studies indicated that wild-type mice exposed to 1% CA diet led to slightly decrease body weight as compared to control mice without CA, whereas there were no significant differences in alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels in the CA-treated group and control group [103]. Although the CA diet does not cause toxicity in wild-type mice, a challenge of CA diet to Fxr-null mice results in marked liver toxicity and cholestasis [104]. Fxr-null mice fed a CA-diet were further analyzed using metabolomics, revealing activation of adaptive metabolic pathways upon bile acid challenge (Figure 2). Urine of Fxr-null mice fed a 1% CA diet had increased p-cresol, corticosterone and several other metabolites, as compared to wild-type mice [103]. Among all metabolites, taurine-conjugated tetrahydroxy bile acids, likely generated through induction of CYP3A11, were highly increased in Fxr-null mice. In LCA-induced cholestasis, the excretion of the similar taurine-conjugated tetrahydroxy bile acid was also greatly increased in urine [103]. The excreted tetrahydroxy bile acid in LCA-treated Fxr-null mice that are resistant to LCA-induced intrahepatic cholestasis was greater than in LCA-treated wild-type mice, thus suggesting that hydroxylation of bile acids contributes to the detoxification of cholestatic bile acids in Fxr-null mice. These results also demonstrate that tetrahydroxyl bile acids are potential biomarkers for hepatotoxicity and cholestasis. In addition, the enhanced serum corticosterone in cholestatic animal models and humans also was observed in CA-treated Fxr-null mice. The urinary biomarker of abnormal corticosterone metabolism in CA-treated Fxr-null mice, characterized by the increased excretion of corticosterone metabolites HDOPA, DHOPA, and their hydroxylation metabolites. Future studies will be required to further determine the molecular mechanism linking liver injury (cholestasis, hepatitis) with hepatic and adrenal steroid metabolism.
Figure 2. Metabolomic analysis of CA-induced and untreated wild-type (+/+) and Fxr-null (−/−) mice by ultraperformance™ liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry.
(A) Scores scatter plot of principal components analysis (PCA) model of urine from the control and CA-treated groups in both wild-type (+/+) and Fxrnull (−/−) mice. (B) The adaptive metabolic pathways of Fxr-null mice upon CA challenge. Under the effect of bile acid-CoA:amino acid N-acyltransferase (BAAT) and UDP-glucuronosyltransferase (UGT), cholic acid (CA) is converted to taurocholate and cholate glucoside. Taurocholate can be further metabolized into taurocholate glucoside and tauro-3α,6,7α,12α-tetrol under the effect of glucosyl-transferase and cytochromes P450, respectively. The level of inflammatory cytokines TNFα and TGFβ increase in Fxr-null mice upon CA challenge, which enhances the level of corticosterone in vivo. Under the cytochromes P450 catalysis and 21-hydroxysteroid dehydrogenase (HSD), corticosterone is transformed to HDOPA. HDOPA can further be converted to DHOPA and hydroxy-HDOPA under by cytochrome P450. Hydroxy-DHOPA is generated from DHOPA.
The potency of FXR ligand as clinic therapeutic agents
Since FXR is a modulator of the metabolism and transport of bile acids and xenobiotics in liver and intestine, and FXR deficiency impairs bile acid and lipid homeostasis [31], therapeutic activation of FXR could be used to protect against intra- and extra-cholestasis [13]. Inflammatory bowel disease [15], and type 2 diabetes [16] have also been proposed as therapeutic targets for FXR ligand-based drugs. By use of animal models, GW4064, an aromatic ligand for FXR, shows effects on several types of diseases through activation of FXR. GW4064 protects against cholestatic liver damage in a rat model of intrahepatic and extrahepatic cholestasis. The naphthylisothiocyanate and bile duct-ligation (BDL) models are represent intrahepatic and extrahepatic cholestasis, respectively. Both cholestasis models treated with GW4064 result in significant reductions in serum ALT, aspartate aminotransferase, and lactate dehydrogenase, as well as other markers of liver damage [13]. GW4064-treated cholestatic rats also had decreased expression of the bile acid biosynthetic enzymes such as CYP7A1 and CYP8B1, and increased expression of genes encoding the bile acid transporters BSEP and MRP2. Thus, compounds with FXR agonist activity and favorable bioavailability such as GW4064 could have potential in the prevention of cholesterol gallstone disease that has a high prevalence in the United States. In the C57BL6/J mouse model susceptible to cholesterol-induced gallstones, GW4064 treatment can reduce cholesterol precipitation and gallstone formation through induction of Abcb11 and Abcb4 and the resultant increased biliary concentrations of bile salts and phospholipids [87]. In CYP7A1-overexpressing mice with high biliary and fecal cholesterol, GW4064 treatment induces hepatic ABCG5/G8 expression through FXR activation, thus suggesting that GW4064 could reduce gallstone formation by increasing the transport of cholesterol in the liver [105]. In addition, GW4064 may prevent epithelial deterioration and bacterial translocation in patients with impaired bile flow. In the BDL model in mice, populations of a number of aerobic and anaerobic bacteria in the ileum and cecum are increased. After administration of GW4064 to BDL mice, bacterial overgrowth in the ileum and cecum is completely blocked [106]. Finally, GW4064 holds promise for the treatment of type 2 diabetes mellitus. Treatment with GW4064 can significantly decrease the level of plasma glucose, triglycerides, and cholesterol in wild-type and genetically obese, diabetic, leptin receptor-deficient db/db mice, which is dependent on FXR activation [16].
6-ECDCA, also known as INT-747, is another efficacious FXR agonist under testing for various diseases associated with bile acid dysfunction, such as liver fibrosis [14] and inflammatory bowel disease [15]. The FDA and the EMEA have granted this agent orphan drug status for the treatment of PBC [107]. 6-ECDCA has been evaluated in phase I clinical trials in healthy volunteers, and phase II clinical trials in patients with type 2 diabetes mellitus, non-alcoholic fatty liver disease (NAFLD) and PBC. 6-ECDCA shows antifibrotic activity in three liver fibrosis models through activation FXR. In the porcine serum-induced rat liver fibrosis model, 12-week administration of 6-ECDCA can reduce expression of α1(I) collagen, transforming growth factor-β1 (TGF-β1), and α-smooth muscle actin in liver [14]. In the BDL rat model, 6-ECDCA can reduce liver fibrosis and α1(I) collagen, TGF-β1, α-SMA as well as tissue metalloproteinase inhibitor (TIMP)-1 and 2 mRNA by 70%–80% [14]. In the CCl4 liver toxicity model, 6-ECDCA administration results in induction of SHP, prevents up-regulation of TIMP-1 mRNA, and accelerate collagen elimination. 6-ECDCA also shows anticholeretic activity on two cholestasis models [108]. In LCA-induced cholestasis, 6-ECDCA treatment can fully reverse the reduced bile flow and transient protect against the liver injury [10]. In estrone-induced cholestasis, administration of 6-ECDCA reduces serum ALP activity and improves the cholestatic changes caused by estrogen and partially abrogates the reduction of bile acid output through increased MCA and TCDCA secretion [109]. Additionally, 6-ECDCA treatment can decrease the level of glucose, free fatty acid and HDL in plasma, and the triglyceride, free fatty acid, cholesterol, and glycogen content in the liver via FXR activation [110], thus suggesting that 6-ECDCA is a potential therapy for non-alcoholic steatohepatitis (NASH) or non-alcoholic fatty liver disease (NAFLD). In clinical trials, 6-ECDCA can protect against a broad range of chronic liver diseases [111]. 6-ECDCA treatment of patients with type 2 diabetes mellitus and NAFLD can increase glucose disposal rates and reduce body weight [112]. FXR ligands could provide therapeutic benefit for bile acid-related human diseases in the clinic via its regulation of FXR targets. By combination with increasing bile acid export from liver through elevation of BSEP expression and suppression of bile acid uptake from blood through down-regulated NTCP and OATPs, FXR agonists can decrease bile acid levels largely through activation of hepatic FXR-SHP and intestinal FXR-FGF15/19 pathways, potentially contributing to the alleviation of cholestasis-induced liver injury. Cholesterol-induced gallstones could also be inhibited by FXR agonists through increased biliary concentration of bile salts and phospholipids following the induction of BSEP and ABCB4 [87]. In addition, FXR agonists can inhibit inflammation and preserve the intestinal barrier function in inflammatory bowel disease through the suppression of key proinflammatory cytokines expression, such as tumor necrosis factor α (TNF-α) [15]. Similarly, the antagonists of FXR can lower serum low density lipoprotein cholesterol and triglyceride levels and increased high density lipoprotein levels through its regulation of a subset of FXR targets, including BSEP [113]. Lack of FXR results in reduces obstructive cholestasis in a cholestasis model via the regulation of the hepatic and intestinal bile acid transporters, such as BSEP and IBABP [114], thus suggesting that FXR antagonists might perform the function through their effects on bile acid transporters.
Conclusion
Intake endogenous chemicals, toxicants and xenobiotic compounds go though the small intestine and liver, and diffuse into whole body. In these two sites, exposed to high concentrations of bile acids, FXR plays an important role in endogenous chemical homeostasis and protection from potential toxicity (Figure 3). Recent discoveries suggest that alteration of hepatic and intestinal FXR signal transduction is involved in multiple diseases. Further understanding of FXR signaling in enterohepatic system can contribute to development of clinical agents for use in the therapy of metabolic diseases such as liver cholestasis, type 2 diabetes and atherosclerosis [17]. However, potent agonist for FXR would likely have unfavorable side effects such as lowering HDL levels and thus ligands will need to be carefully selected for the desired effects. One possibility is to develop selective FXR ligands or modulators similar to those under development for the estrogen receptor [115]. Another approach is to develope a gut-specific FXR activator that might have utility in the treatment of cholestasis by induction of FGF19 and reducing hepatic bile acid synthesis [116]. This would avoid any side effects from activation of the liver FXR. For example, a ligand for PXR, rifaximin, is gut specific and under trials for the treatment in intestinal inflammatory disorders [117,118]. However, it should be noted that FGF19-transgenic mice have increased hepatocytes proliferation and develop hepatocellular carcinoma [119], and thus chronic long-term treatment with and FXR agonist need to account for this possible side effect. In addition to gut-liver selective FXR modulators, perhaps, gene- and metabolic pathway-specific FXR modulators would be also important to consider as possible therapeutic strategies for treatment of cholestatic and metabolic diseases.
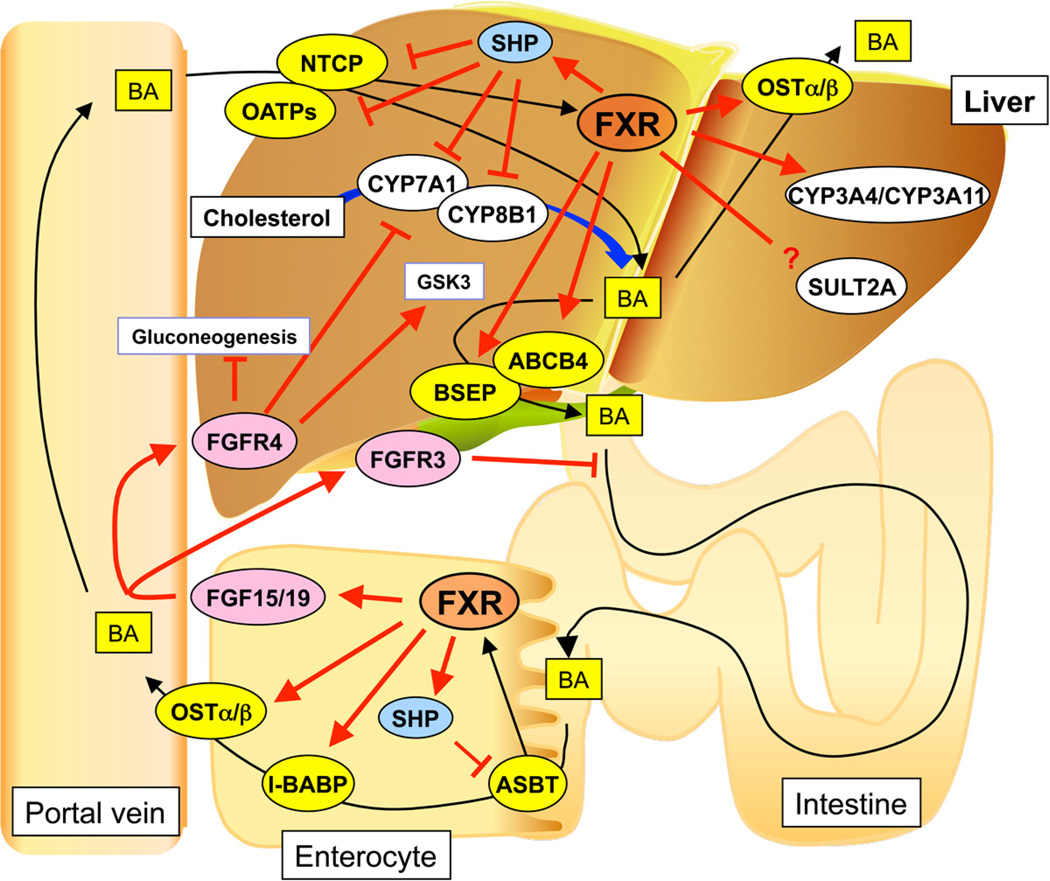
Figure 3.
Major roles of FXR signaling in the enterohepatic system. FXR accelerates bile acid export from liver through induction of BSEP and ABCB4 expression and decreasing bile acid uptake to liver by the suppression of NTCP and OATPs expression via hepatic FXR-SHP signaling, decreases bile acid absorption at the intestine through suppression of ASBT via FXR-SHP signaling, and attenuates cholesterol metabolism/bile acid synthesis by suppression of CYP7A1 and CYP8B1 expression via the hepatic FXR-SHP and intestinal FGF15/19 pathways. Thus, FXR action leads to decreased bile acid pool size. Intestinal FXR-FGF15/19 signaling decreases hepatic glucose metabolism through FGFR4 and induces gallbladder filling through FGFR3.
HIGHLIGHTS.
Bile acids are critical for many hepatic and intestinal and metabolic functions
Enterohepatic bile acid circulation is tightly regulated by farnesoid X receptor
Farnesoid X receptor controls bile synthesis and transport in liver and intestine
FGF15/19 hormone production in the intestine is regulated by farnesoid X receptor
FGF15/19 from intestine circulates to liver and regulates hepatic bile acid synthesis
Abbreviations
- ASBT
apical sodium dependent transporter
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CREB
cAMP regulatory element-binding protein
- DCA
deoxycholic acid
- LCA
lithocholic acid
- I-BABP
intestinal bile acid binding protein
- ERK1
extracellular signal-regulated protein
- FGF
fibroblast growth factor
- FXR
farnesoid X receptor
- FGF1
fibroblast growth factor
- GSK3
glycogen synthase kinase 3
- IR-1
indirect repeat 1
- IL-1β
interleukin-1β
- NASH
non-alcoholic steatohepatitis
- NAFLD
non-alcoholic steatohepatitis
- NTCP
Na+-taurocholate cotransporting polypeptide
- OATP
organic anion-transporting peptides
- PPAR
peroxisome proliferator-activated receptor
- PGC-1α
peroxisome proliferators-activated receptor γ coactivator protein-1α
- PXR
pregnane X receptor
- PBC
primary biliary cirrhosis
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- TNFα
tumor necrosis factor α
- SHP
small heterodimer partner
- TCDC
taurochenodeoxycholate
- UDCA
ursodeoxycholic acid
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 2.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 5.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology. 2006;147:4025–4033. doi: 10.1210/en.2005-1485. [DOI] [PubMed] [Google Scholar]
- 7.Howard WR, Pospisil JA, Njolito E, Noonan DJ. Catabolites of cholesterol synthesis pathways and forskolin as activators of the farnesoid X-activated nuclear receptor. Toxicol Appl Pharmacol. 2000;163:195–202. doi: 10.1006/taap.1999.8869. [DOI] [PubMed] [Google Scholar]
- 8.Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–306. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 9.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 10.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 11.Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, Nervi F, Mendez-Sanchez N, Uribe M, Bock HH, Schirin-Sokhan R, Stumvoll M, Mossner J, Lammert F, Wittenburg H. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–124. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79–92. doi: 10.1111/j.1582-4934.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morelli A, Vignozzi L, Maggi M, Adorini L. Farnesoid X receptor activation improves erectile dysfunction in models of metabolic syndrome and diabetes. Biochim Biophys Acta. 2011;1812:859–866. doi: 10.1016/j.bbadis.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Campana G, Pasini P, Roda A, Spampinato S. Regulation of ileal bile acid-binding protein expression in Caco-2 cells by ursodeoxycholic acid: Role of the farnesoid X receptor. Biochemical Pharmacology. 2005;69:1755–1763. doi: 10.1016/j.bcp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr. 2001;32:407–417. doi: 10.1097/00005176-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Heubi JE, Balistreri WF, Fondacaro JD, Partin JC, Schubert WK. Primary bile acid malabsorption: defective in vitro ileal active bile acid transport. Gastroenterology. 1982;83:804–811. [PubMed] [Google Scholar]
- 22.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081–1087. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Chen F, Shang Q, Pan LX, Shneider BL, Chiang JYL, Forman BM, Ananthanarayanan M, Tint GS, Salen G, Xu GR. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;288:G60–G66. doi: 10.1152/ajpgi.00170.2004. [DOI] [PubMed] [Google Scholar]
- 26.Neimark E, Chen F, Li XP, Shneider BL. Bile acid-induced negative feedback regulation of the human lleal bile acid transporter. Hepatology. 2004;40:149–156. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- 27.Sinha J, Chen F, Miloh T, Burns RC, Yu ZS, Shneider BL. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295:G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppola CP, Gosche JR, Arrese M, Ancowitz B, Madsen J, Vanderhoof J, Shneider BL. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology. 1998;115:1172–1178. doi: 10.1016/s0016-5085(98)70088-5. [DOI] [PubMed] [Google Scholar]
- 29.Kramer W, Girbig F, Gutjahr U, Kowalewski S, Jouvenal K, Muller G, Tripier D, Wess G. Intestinal bile acid absorption. Na(+)-dependent bile acid transport activity in rabbit small intestine correlates with the coexpression of an integral 93-kDa and a peripheral 14-kDa bile acid-binding membrane protein along the duodenum-ileum axis. J Biol Chem. 1993;268:18035–18046. [PubMed] [Google Scholar]
- 30.Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM, Ono T, Besnard P. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 31.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 32.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm. 2008;5:42–48. doi: 10.1021/mp700105z. [DOI] [PubMed] [Google Scholar]
- 36.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in Liver Metabolism. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 37.Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, Foster J, Liang J, Brush J, Gu Q, Hillan K, Goddard A, Gurney AL. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11:729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 38.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 39.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012 doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 43.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Dufer M, Horth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, Oberwinkler J, Lukowski R, Gonzalez FJ, Krippeit-Drews P, Drews G. Bile Acids Acutely Stimulate Insulin Secretion of Mouse beta-Cells via Farnesoid X Receptor Activation and ATP-Sensitive Potassium Channel Inhibition. Diabetes. 2012 doi: 10.2337/db11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 48.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stieger B, Hagenbuch B, Landmann L, Hochli M, Schroeder A, Meier PJ. In situ localization of the hepatocytic Na+/Taurocholate cotransporting polypeptide in rat liver. Gastroenterology. 1994;107:1781–1787. doi: 10.1016/0016-5085(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 50.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H, Zatloukal K, Trauner M. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 51.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 52.Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol. 2006;20:65–79. doi: 10.1210/me.2005-0159. [DOI] [PubMed] [Google Scholar]
- 53.Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol. 2004;286:G752–G761. doi: 10.1152/ajpgi.00456.2003. [DOI] [PubMed] [Google Scholar]
- 54.Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab. 2011;12:139–153. doi: 10.2174/138920011795016863. [DOI] [PubMed] [Google Scholar]
- 55.Maeda T, Miyata M, Yotsumoto T, Kobayashi D, Nozawa T, Toyama K, Gonzalez FJ, Yamazoe Y, Tamai I. Regulation of drug transporters by the farnesoid X receptor in mice. Mol Pharm. 2004;1:281–289. doi: 10.1021/mp0499656. [DOI] [PubMed] [Google Scholar]
- 56.Jung D, Elferink MG, Stellaard F, Groothuis GM. Analysis of bile acid-induced regulation of FXR target genes in human liver slices. Liver Int. 2007;27:137–144. doi: 10.1111/j.1478-3231.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 60.Del Castillo-Olivares A, Gil G. Role of FXR and FTF in bile acid-mediated suppression of cholesterol 7alpha-hydroxylase transcription. Nucleic Acids Res. 2000;28:3587–3593. doi: 10.1093/nar/28.18.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Fabiani E, Mitro N, Anzulovich AC, Pinelli A, Galli G, Crestani M. The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem. 2001;276:30708–30716. doi: 10.1074/jbc.M103270200. [DOI] [PubMed] [Google Scholar]
- 62.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 63.Chen W, Owsley E, Yang Y, Stroup D, Chiang JY. Nuclear receptor-mediated repression of human cholesterol 7alpha-hydroxylase gene transcription by bile acids. J Lipid Res. 2001;42:1402–1412. [PubMed] [Google Scholar]
- 64.Chanda D, Xie YB, Choi HS. Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation. Nucleic Acids Research. 2010;38:4607–4619. doi: 10.1093/nar/gkq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao J, Choi SE, Seok SM, Yang L, Zuercher WJ, Xu Y, Willson TM, Xu HE, Kemper JK. Ligand-Dependent Regulation of the Activity of the Orphan Nuclear Receptor, Small Heterodimer Partner (SHP), in the Repression of Bile Acid Biosynthetic CYP7A1 and CYP8B1 Genes. Molecular Endocrinology. 2011;25:1159–1169. doi: 10.1210/me.2011-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278:39124–39132. doi: 10.1074/jbc.M305079200. [DOI] [PubMed] [Google Scholar]
- 67.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690–41699. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 68.Del Castillo-Olivares A, Gil G. Alpha 1-fetoprotein transcription factor is required for the expression of sterol 12alpha -hydroxylase, the specific enzyme for cholic acid synthesis. Potential role in the bile acid-mediated regulation of gene transcription. J Biol Chem. 2000;275:17793–17799. doi: 10.1074/jbc.M000996200. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Chiang JY. Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4alpha (HNF4alpha) Gene. 2003;313:71–82. doi: 10.1016/s0378-1119(03)00631-0. [DOI] [PubMed] [Google Scholar]
- 70.Garuti R, Croce MA, Piccinini L, Tiozzo R, Bertolini S, Calandra S. Functional analysis of the promoter of human sterol 27-hydroxylase gene in HepG2 cells. Gene. 2002;283:133–143. doi: 10.1016/s0378-1119(01)00874-5. [DOI] [PubMed] [Google Scholar]
- 71.Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. Journal of Clinical Investigation. 2011;121:86–95. doi: 10.1172/JCI42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Araya Z, Wikvall K. 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta. 1999;1438:47–54. doi: 10.1016/s1388-1981(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 73.Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687:84–93. doi: 10.1016/j.bbalip.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Bu HZ. A literature review of enzyme kinetic parameters for CYP3A4-mediated metabolic reactions of 113 drugs in human liver microsomes: structure-kinetics relationship assessment. Curr Drug Metab. 2006;7:231–249. doi: 10.2174/138920006776359329. [DOI] [PubMed] [Google Scholar]
- 75.Kato M. Intestinal first-pass metabolism of CYP3A4 substrates. Drug Metab Pharmacokinet. 2008;23:87–94. doi: 10.2133/dmpk.23.87. [DOI] [PubMed] [Google Scholar]
- 76.Gnerre C, Blattler S, Kaufmann MR, Looser R, Meyer UA. Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics. 2004;14:635–645. doi: 10.1097/00008571-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Khan AA, Chow EC, van Loenen-Weemaes AM, Porte RJ, Pang KS, Groothuis GM. Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur J Pharm Sci. 2009;37:115–125. doi: 10.1016/j.ejps.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Barbier O, Torra IP, Sirvent A, Claudel T, Blanquart C, Duran-Sandoval D, Kuipers F, Kosykh V, Fruchart JC, Staels B. FXR induces the UGT2B4 enzyme in hepatocytes: a potential mechanism of negative feedback control of FXR activity. Gastroenterology. 2003;124:1926–1940. doi: 10.1016/s0016-5085(03)00388-3. [DOI] [PubMed] [Google Scholar]
- 79.Pillot T, Ouzzine M, Fournel-Gigleux S, Lafaurie C, Radominska A, Burchell B, Siest G, Magdalou J. Glucuronidation of hyodeoxycholic acid in human liver. Evidence for a selective role of UDP-glucuronosyltransferase 2B4. J Biol Chem. 1993;268:25636–25642. [PubMed] [Google Scholar]
- 80.Song CS, Echchgadda I, Baek BS, Ahn SC, Oh T, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid X receptor. J Biol Chem. 2001;276:42549–42556. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- 81.Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB. Sulfation and sulfotransferases 1: Sulfotransferase molecular biology: cDNAs and genes. FASEB J. 1997;11:3–14. [PubMed] [Google Scholar]
- 82.Miyata M, Matsuda Y, Tsuchiya H, Kitada H, Akase T, Shimada M, Nagata K, Gonzalez FJ, Yamazoe Y. Chenodeoxycholic acid-mediated activation of the farnesoid X receptor negatively regulates hydroxysteroid sulfotransferase. Drug Metab Pharmacokinet. 2006;21:315–323. doi: 10.2133/dmpk.21.315. [DOI] [PubMed] [Google Scholar]
- 83.Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278:27703–27711. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- 84.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- 85.Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, Muller M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35:589–596. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- 86.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 87.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- 88.Van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 89.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Nemeth A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 90.De Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Royo I, Blevins RA, Pelaez F, Wright SD, Cui J. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem. 2003;278:51085–51090. doi: 10.1074/jbc.M308321200. [DOI] [PubMed] [Google Scholar]
- 92.Lee FY, de Aguiar Vallim TQ, Chong HK, Zhang Y, Liu Y, Jones SA, Osborne TF, Edwards PA. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- 94.Chong HK, Infante AM, Seo YK, Jeon TI, Zhang YQ, Edwards PA, Xie XH, Osborne TF. Genome-wide interrogation of hepatic FXR reveals an asymmetric IR-1 motif and synergy with LRH-1. Nucleic Acids Research. 2010;38:6007–6017. doi: 10.1093/nar/gkq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas AM, Hart SN, Kong B, Fang JW, Zhong XB, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J, Seok SM, Yu P, Kim K, Smith Z, Rivas-Astroza M, Zhong S, Kemper JS. Genomic analysis of hepatic farnesoid X receptor (FXR) binding sites reveals altered binding in obesity and direct gene repression by FXR. Hepatology. 2012 doi: 10.1002/hep.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 99.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 100.Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ, Guo GL. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–477. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu N, Meng Z, Lou G, Zhou W, Wang X, Zhang Y, Zhang L, Liu X, Yen Y, Lai L, Forman BM, Xu Z, Xu R, Huang W. Hepatocarcinogenesis in FXR−/− Mice Mimics Human HCC Progression That Operates through HNF1alpha Regulation of FXR Expression. Mol Endocrinol. 2012 doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Gong W, Dai S, Huang G, Shen X, Gao M, Xu Z, Zeng Y, He F. Downregulation of Human Farnesoid X Receptor by miR-421 Promotes Proliferation and Migration of Hepatocellular Carcinoma Cells. Mol Cancer Res. 2012 doi: 10.1158/1541-7786.MCR-11-0473. [DOI] [PubMed] [Google Scholar]
- 103.Cho JY, Matsubara T, Kang DW, Ahn SH, Krausz KW, Idle JR, Luecke H, Gonzalez FJ. Urinary metabolomics in Fxr-null mice reveals activated adaptive metabolic pathways upon bile acid challenge. J Lipid Res. 2010;51:1063–1074. doi: 10.1194/jlr.M002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyata M, Tozawa A, Otsuka H, Nakamura T, Nagata K, Gonzalez FJ, Yamazoe Y. Role of farnesoid X receptor in the enhancement of canalicular bile acid output and excretion of unconjugated bile acids: a mechanism for protection against cholic acid-induced liver toxicity. J Pharmacol Exp Ther. 2005;312:759–766. doi: 10.1124/jpet.104.076158. [DOI] [PubMed] [Google Scholar]
- 105.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JY. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Intercept Pharmaceuticals Inc Company World Wide Web Site. 2010 Jun 18; [Google Scholar]
- 108.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–595. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 109.Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- 110.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Intercept Pharmaceuticals Inc press release. 2010 Jul 28; [Google Scholar]
- 112.Mason A, Luketic V, Lindor K, Hirschfield G, Gordon S, Mayo M, Kowdley K, Parés A, Trauner M, Castelloe E, Sciacca C, Jones TB, Böhm O, Shapiro D. Farnesoid-X receptor agonists: a new class of drugs for the treatment of PBC? An international study evaluating the addition of INT-747 to ursodeoxycholicacid. J. Hepatol. 2010;52:S1–S2. [Google Scholar]
- 113.Cui J, Huang L, Zhao A, Lew JL, Yu JH, Sahoo S, Meinke PT, Royo I, Pelaez F, Wright SD. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. Journal of Biological Chemistry. 2003;278:10214–10220. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- 114.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Komm BS, Chines AA. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2011 doi: 10.1016/j.maturitas.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Velazquez JA, Carrillo-Cordova LD, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Nuclear receptors in nonalcoholic Fatty liver disease. J Lipids. 2012;2012:139875. doi: 10.1155/2012/139875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010;335:32–41. doi: 10.1124/jpet.110.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guslandi M. Rifaximin for inflammatory bowel disease. Dig Dis Sci. 2010;55:1805. doi: 10.1007/s10620-010-1245-y. [DOI] [PubMed] [Google Scholar]
- 119.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM. A mouse model of hepatocellular carcinoma - Ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. American Journal of Pathology. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 121.Niesor EJ, Flach J, Lopes-Antoni I, Perez A, Bentzen CL. The nuclear receptors FXR and LXRalpha: potential targets for the development of drugs affecting lipid metabolism and neoplastic diseases. Curr Pharm Des. 2001;7:231–259. doi: 10.2174/1381612013398185. [DOI] [PubMed] [Google Scholar]
- 122.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JG, Noel JP, Nicolaou KC, Evans RM. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nicolaou KC, Evans RM, Roecker AJ, Hughes R, Downes M, Pfefferkorn JA. Discovery and optimization of non-steroidal FXR agonists from natural product-like libraries. Org Biomol Chem. 2003;1:908–920. [PubMed] [Google Scholar]
- 124.Dussault I, Beard R, Lin M, Hollister K, Chen J, Xiao JH, Chandraratna R, Forman BM. Identification of gene-selective modulators of the bile acid receptor FXR. J Biol Chem. 2003;278:7027–7033. doi: 10.1074/jbc.M209863200. [DOI] [PubMed] [Google Scholar]
- 125.Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Pelaez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856–8861. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- 126.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 127.Zhao A, Yu J, Lew JL, Huang L, Wright SD, Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]
- 128.Nozawa H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem Biophys Res Commun. 2005;336:754–761. doi: 10.1016/j.bbrc.2005.08.159. [DOI] [PubMed] [Google Scholar]
- 129.Deng R, Yang D, Yang J, Yan B. Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver X receptor. J Pharmacol Exp Ther. 2006;317:317–325. doi: 10.1124/jpet.105.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishimaki-Mogami T, Kawahara Y, Tamehiro N, Yoshida T, Inoue K, Ohno Y, Nagao T, Une M. 5Alpha-bile alcohols function as farnesoid X receptor antagonists. Biochem Biophys Res Commun. 2006;339:386–391. doi: 10.1016/j.bbrc.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 131.Kainuma M, Kasuga J, Hosoda S, Wakabayashi K, Tanatani A, Nagasawa K, Miyachi H, Makishima M, Hashimoto Y. Diphenylmethane skeleton as a multi-template for nuclear receptor ligands: preparation of FXR and PPAR ligands. Bioorg Med Chem Lett. 2006;16:3213–3218. doi: 10.1016/j.bmcl.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 132.Kainuma M, Makishima M, Hashimoto Y, Miyachi H. Design, synthesis, and evaluation of non-steroidal farnesoid X receptor (FXR) antagonist. Bioorg Med Chem. 2007;15:2587–2600. doi: 10.1016/j.bmc.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 133.Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Muller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 134.Akwabi-Ameyaw A, Bass JY, Caldwell RD, Caravella JA, Chen L, Creech KL, Deaton DN, Jones SA, Kaldor I, Liu Y, Madauss KP, Marr HB, McFadyen RB, Miller AB, Iii FN, Parks DJ, Spearing PK, Todd D, Williams SP, Wisely GB. Conformationally constrained farnesoid X receptor (FXR) agonists: Naphthoic acid-based analogs of GW 4064. Bioorg Med Chem Lett. 2008;18:4339–4343. doi: 10.1016/j.bmcl.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 135.Deng G, Li W, Shen J, Jiang H, Chen K, Liu H. Pyrazolidine-3,5-dione derivatives as potent non-steroidal agonists of farnesoid X receptor: virtual screening, synthesis, and biological evaluation. Bioorg Med Chem Lett. 2008;18:5497–5502. doi: 10.1016/j.bmcl.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 136.Takahashi M, Kanayama T, Yashiro T, Kondo H, Murase T, Hase T, Tokimitsu I, Nishikawa J, Sato R. Effects of coumestrol on lipid and glucose metabolism as a farnesoid X receptor ligand. Biochem Biophys Res Commun. 2008;372:395–399. doi: 10.1016/j.bbrc.2008.04.136. [DOI] [PubMed] [Google Scholar]
- 137.Suzuki T, Tamehiro N, Sato Y, Kobayashi T, Ishii-Watabe A, Shinozaki Y, Nishimaki-Mogami T, Hashimoto T, Asakawa Y, Inoue K, Ohno Y, Yamaguchi T, Kawanishi T. The novel compounds that activate farnesoid X receptor: the diversity of their effects on gene expression. J Pharmacol Sci. 2008;107:285–294. doi: 10.1254/jphs.08006fp. [DOI] [PubMed] [Google Scholar]
- 138.Kaimal R, Song X, Yan B, King R, Deng R. Differential modulation of farnesoid X receptor signaling pathway by the thiazolidinediones. J Pharmacol Exp Ther. 2009;330:125–134. doi: 10.1124/jpet.109.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flatt B, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH, Foster P, Li J, Pircher P, Petrowski M, Schulman I, Westin S, Wrobel J, Yan G, Bischoff E, Daige C, Mohan R. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR) J Med Chem. 2009;52:904–907. doi: 10.1021/jm8014124. [DOI] [PubMed] [Google Scholar]
- 140.Mehlmann JF, Crawley ML, Lundquist JTt, Unwalla RJ, Harnish DC, Evans MJ, Kim CY, Wrobel JE, Mahaney PE. Pyrrole[2,3-d]azepino compounds as agonists of the farnesoid X receptor (FXR) Bioorg Med Chem Lett. 2009;19:5289–5292. doi: 10.1016/j.bmcl.2009.07.148. [DOI] [PubMed] [Google Scholar]
- 141.Feng S, Yang M, Zhang Z, Wang Z, Hong D, Richter H, Benson GM, Bleicher K, Grether U, Martin RE, Plancher JM, Kuhn B, Rudolph MG, Chen L. Identification of an N-oxide pyridine GW4064 analog as a potent FXR agonist. Bioorg Med Chem Lett. 2009;19:2595–2598. doi: 10.1016/j.bmcl.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 142.Iguchi Y, Yamaguchi M, Sato H, Kihira K, Nishimaki-Mogami T, Une M. Bile alcohols function as the ligands of membrane-type bile acid-activated G protein-coupled receptor. J Lipid Res. 2010;51:1432–1441. doi: 10.1194/jlr.M004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bass JY, Caravella JA, Chen L, Creech KL, Deaton DN, Madauss KP, Marr HB, McFadyen RB, Miller AB, Mills WY, Navas F, 3rd, Parks DJ, Smalley TL, Jr, Spearing PK, Todd D, Williams SP, Wisely GB. Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg Med Chem Lett. 2011;21:1206–1213. doi: 10.1016/j.bmcl.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 144.Sepe V, Bifulco G, Renga B, D'Amore C, Fiorucci S, Zampella A. Discovery of sulfated sterols from marine invertebrates as a new class of marine natural antagonists of farnesoid-X-receptor. J Med Chem. 2011;54:1314–1320. doi: 10.1021/jm101336m. [DOI] [PubMed] [Google Scholar]
- 145.Gioiello A, Macchiarulo A, Carotti A, Filipponi P, Costantino G, Rizzo G, Adorini L, Pellicciari R. Extending SAR of bile acids as FXR ligands: discovery of 23-N-(carbocinnamyloxy)-3alpha,7alpha-dihydroxy-6alpha-ethyl-24-nor-5beta- cholan-23-amine. Bioorg Med Chem. 2011;19:2650–2658. doi: 10.1016/j.bmc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 146.Choi H, Hwang H, Chin J, Kim E, Lee J, Nam SJ, Lee BC, Rho BJ, Kang H. Tuberatolides, potent FXR antagonists from the Korean marine tunicate Botryllus tuberatus. J Nat Prod. 2011;74:90–94. doi: 10.1021/np100489u. [DOI] [PubMed] [Google Scholar]
- 147.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 148.Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology. 2002;122:1954–1966. doi: 10.1053/gast.2002.33583. [DOI] [PubMed] [Google Scholar]
- 149.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 150.Lu Y, Heydel JM, Li X, Bratton S, Lindblom T, Radominska-Pandya A. Lithocholic acid decreases expression of UGT2B7 in Caco-2 cells: a potential role for a negative farnesoid X receptor response element. Drug Metab Dispos. 2005;33:937–946. doi: 10.1124/dmd.104.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vessey DA, Crissey MH, Zakim D. Kinetic studies on the enzymes conjugating bile acids with taurine and glycine in bovine liver. Biochem J. 1977;163:181–183. doi: 10.1042/bj1630181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem. 2004;279:2480–2489. doi: 10.1074/jbc.M311015200. [DOI] [PubMed] [Google Scholar]
- 153.Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, Trauner M. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]