Abstract
Background/Objectives
Small dense LDL particles and apolipoprotein (apo) CIII are risk factors for cardiovascular disease (CVD) that can be modulated by diet, but there is little information regarding the effects of dietary saturated fat on their plasma levels. We tested the effects of high vs. low saturated fat intake in the context of a high beef protein diet on levels and composition of LDL subclasses and on apoCIII levels in plasma and LDL.
Subjects/Methods
Following consumption of a baseline diet (50% CHO, 13% protein, 38% total fat, 15% saturated fat) for 3 wk, 14 healthy men were randomly assigned to two reduced carbohydrate high beef protein diets (31% CHO, 31% protein, 38% fat) that differed in saturated fat content (15% vs. 8%) for 3 wk each in a crossover design.
Results
The high saturated fat diet resulted in higher mass concentrations of buoyant LDL I, medium density LDL II and dense LDL III, but not the very dense LDL IV; and significant increases in plasma and LDL apoCIII concentration of 9.4% and 33.5%, respectively. The saturated fat-induced changes in LDL apoCIII were specifically correlated with changes in apoCIII content of LDL IV.
Conclusions
Taken together with previous observations, these findings suggest that, at least in the context of a lower carbohydrate high beef protein diet, high saturated fat intake may increase CVD risk by metabolic processes that involve apoCIII.
Keywords: saturated fat, low-density lipoprotein, apolipoprotein CIII, cardiovascular disease, diet, LDL subfractions
Introduction
It is well established that increased saturated fat intake raises LDL cholesterol (1). However, LDL is comprised of a spectrum of distinct particle subclasses differing in metabolic properties and atherogenic potential (2), and effects of saturated fat on these subclasses have been less extensively evaluated. Lipoprotein profiles characterized by smaller, denser LDL particles are associated with higher CVD risk than those with predominately larger LDL (2–5). Small dense LDL have a number of properties that may contribute to atherosclerosis risk, including lower affinity for LDL receptors, greater binding to arterial proteoglycans and higher oxidative susceptibility than more buoyant LDL (6). It has been reported that very small LDL are particularly strongly associated with coronary atherosclerosis progression (7), a property consistent with the recent finding that a single nucleotide polymorphism (SNP) strongly associated with plasma LDL cholesterol and risk of myocardial infarction is specifically related to plasma levels of very small LDL (8).
Intake of saturated fat, particularly myristic (14:0) and palmitic (16:0) acids, but not stearic acid (18:0), has been found to correlate with levels of large LDL I (9). Moreover, in the setting of a low carbohydrate mixed protein diet, the higher levels of LDL that resulted from increased saturated fat intake were found to be entirely due to increases in larger LDL particles (10). Yet we recently found that consumption of increased saturated fat in conjunction with a high beef protein, low carbohydrate diet resulted in an increase in LDL particles across the full size spectrum, raising the possibility that the effects of saturated fat on LDL particles may depend on dietary context (11). We here describe analyses of lipid and protein composition of LDL subfractions isolated from a subset of participants in that study, with the aim of determining whether higher intake of saturated fat resulted in compositional changes in specific LDL particles. We also examined the effects of higher vs. lower saturated fat on LDL content of apoCIII, an important regulator of lipoprotein metabolism by virtue of its capacity to inhibit lipoprotein lipase (LPL) activity and reduce receptor-mediated clearance of apoB-containing particles (12, 13). The association of apoCIII with apoB-containing lipoproteins has repeatedly been linked with increased CVD risk (14–18), an effect that may be related to the metabolic effects and/or its pro-inflammatory properties of apoCIII (19–21). Finally, we tested for diet-induced changes in LDL content of apoE, a determinant of receptor-mediated plasma lipoprotein clearance (22, 23).
Subjects and methods
Study design and diets
These analyses were performed in a subgroup of 14 healthy male participants in a controlled, randomized, crossover dietary intervention trial aimed at evaluating plasma lipid and lipoprotein responses to high vs. low saturated fat intake in the context of replacement of carbohydrate with protein derived to a large extent from beef (11). All participants consumed a baseline diet for 3 weeks, after which they were assigned in random order to a low carbohydrate high beef protein diet that was either high (15%) or low (8%) in saturated fat for 3 weeks. Each 3 week experimental diet period was separated by a 2 week washout during which participants resumed consumption of their habitual diet. The experimental diets were designed to be low in carbohydrate and high in beef protein, and to differ in saturated fat content (Table 1).
TABLE 1.
Macronutrient composition of the diets
| Baseline | HSF | LSF | |
|---|---|---|---|
|
| |||
| % of energy | |||
| Carbohydrate | 50 | 31 | 31 |
| Protein | 13 | 31 | 31 |
| Beef protein | 0 | 10 | 11 |
| Total Fat | 38 | 38 | 38 |
| Saturated fat | 15 | 15 | 8 |
| Monounsaturated fat | 15 | 15 | 21 |
| Polyunsaturated fat | 6 | 5 | 6 |
| Cholesterol, mg/d | 468 | 463 | 467 |
Diets were formulated to provide similar amounts of trans fat (1.5% daily E), linoleic acid (0.5% E), linolenic acid (0.5% E), cholesterol (465 mg/3000 kcal), and fiber (25 g/2000 kcal plus 2.5 g/500 kcal above this level), and to have a comparable ratio of sugars to starches (1:1).
E, energy; HSF, high saturated fat; LSF, low saturated fat.
Diets were prepared in the metabolic kitchen of the University of California, San Francisco (UCSF) Clinical and Translational Studies Institute (CTSI). After preparation, entrees were frozen and delivered to participants on a weekly basis. Participants were provided with lunch and dinner meals and were asked to prepare their own breakfasts and snacks according to menus developed in accordance with their assigned diet. Participants were weighed weekly and energy intake was adjusted if weight deviated by more than ± 3%. Dietary adherence was promoted through regular telephone contacts and weekly meetings with the dietitians. The nutrient composition of the diets was assessed using the Nutrition Data System for Research (NDSR) 2007 MN Food and Nutrient Database (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) and confirmed by compositional analysis of daily menus (Covance Inc, Kalamazoo, MI). Daily use of a pedometer allowed monitoring of participants’ physical activity, which was required to remain stable for the duration of the study.
Study participants
Participants were recruited from our extensive recruitment database and the community primarily through internet advertisements. They were healthy, non-smoking men ≥18 years of age, free of chronic disease in the last 5 years, and not taking medications known to affect lipid metabolism. Other eligibility criteria included a BMI between 20–35 kg/m2, total cholesterol and LDL cholesterol less than the 95th percentile for their age and gender, triglyceride < 500 mg/dL, fasting blood glucose < 126 mg/dL, resting blood pressure < 150/90 mmHg and hematocrit ≥ 36%. Participants were asked to refrain from alcohol and dietary supplements for the duration of the study. All participants gave informed consent under a protocol approved by the Institutional Review Board of Children’s Hospital and Research Center Oakland. The study is registered at clinicaltrials.gov (NCT00852267). One participant was eliminated from the present analysis due to a triglyceride level > 2 SD above the mean for all diets (baseline: 270 mg/dL, low saturated fat (LSF): 171 mg/dL and high saturated fat (HSF): 451 mg/dL), since this may affect apoCIII metabolism.
Laboratory measurements
Fasting plasma samples were prepared from venous blood collected in tubes containing Na2EDTA (1.4 g/L) and a preservative cocktail of protease and bacterial inhibitors. Blood and plasma were kept at 4oC throughout processing. Plasma total cholesterol and triglyceride concentrations were determined by enzymatic procedures on an Express 550 Plus analyzer (Ciba Corning, Oberlin, OH). HDL cholesterol was measured after dextran sulfate precipitation of plasma (24), and LDL cholesterol was calculated from the formula of Friedewald et al (25). Measurements were regularly monitored by the standardization program of the Centers for Disease Control-National Heart, Lung and Blood Institute. Plasma apoB concentrations were measured by an immunoturbidometric assay (Bacton Assay Systems, San Marcos, CA, and Express 550 Plus analyzer, Ciba Corning, Oberlin, OH). ApoE and apoCIII were measured in triplicate by sandwich-style ELISA with commercially available goat anti-human apoE and apoCIII, respectively (International Immunology Corp, Murrieta, CA). HDL apoCIII was measured in the supernatant obtained after dextran sulfate precipitation (24) of apoB-containing lipoproteins. The concentration of apoCIII in apoB-containing lipoproteins (non-HDL apoCIII) was calculated by difference (plasma – HDL apoCIII concentration). Glucose concentrations were measured enzymatically (Express 550 Plus Analyzer, Ciba Corning, Oberlin, OH). Insulin concentrations were measured with ELISA (Millipore, Billerica, MA). The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated from glucose and insulin concentrations (26): HOMA-IR = [Insulin (μU/mL) * Glucose (mmol/L)]/22.5.
Isolation of LDL and LDL subfractions
LDL were isolated from the serum of each participant by ultracentrifugation under standard conditions (27). The LDL fractions were grouped into four major LDL subclasses, based on density, designated LDL I (d < 1.028), LDL II (1.028–1.034), LDL III (1.034–1.044) and LDL IV (1.044–1.060) (6).
Chemical composition of LDL subfractions
Fractions were analyzed for concentrations of total cholesterol (TC) (Siemens Medical Solutions Diagnostics, Tarrytown, NY), triglyceride (Sigma-Aldrich, St. Louis, MO), free cholesterol (FC) (Wako Chemicals USA, Inc., Richmond, VA) and phospholipids (PL) (Wako Chemicals USA, Inc., Richmond, VA) using commercially available enzymatic kits. Cholesterol ester (CE) concentrations were calculated as [(TC − FC) × 1.68]. Protein concentrations were determined by a modification of the method of Lowry et al. using BSA as the standard (28). The lipoproteins were read on an ELx 808 Ultra Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT). Assays were carried out in duplicate with standards, controls, and unknowns on the same 96-well plate, and with coefficients of variation within 10%. On average over 90% of the plasma apolipoproteins (B, CIII and E) in LDL subclasses were recovered from density gradient ultracentrifugation.
Statistical analysis
JMP statistical software (version 7.0; SAS Institute, Cary, NC) was used for statistical analysis. Data are presented as means ± SD. Shapiro-Wilk test was used to test the skewness of the distributions. Non-normally distributed variables were log-transformed before analysis. Paired t-tests were used to evaluate differences in lipids, lipoproteins and apolipoproteins between the diets and across LDL subclasses. Spearman’s correlation coefficients were used to evaluate the relationship between diet-induced changes in lipids, lipoproteins and apolipoproteins. P values < 0.05 were considered statistically significant.
Results
Participant characteristics at baseline
The mean (± SD) age of the men was 44.5 ± 14.4 y (range: 24 to 67 years), weight was 78.6 ± 10.8 kg, BMI was 25.0 ± 2.4 kg/m2 and body fat was 20.9 ± 6.3%. The participants had normal fasting blood glucose (89.3 ± 8.0 mg/dL), plasma insulin concentrations (5.1 ± 2.4 μU/mL) and HOMA-IR (1.1 ± 0.6).
Plasma lipid and lipoprotein response to dietary intervention
In the context of moderate carbohydrate intake, consumption of LSF vs. HSF intake resulted in an overall more favorable lipid profile with lower plasma TC (P = 0.007), LDL cholesterol (P = 0.007), TC/HDL cholesterol (P = 0.0004), apoB (P = 0.06) and apoCIII concentrations (P = 0.03) (Table 2).
TABLE 2.
Plasma measurements following consumption of baseline (B), high saturated fat (HSF) and low saturated fat (LSF) diets
| 50% CHO, 13% protein
|
31% CHO, 31% protein
|
Δ SF | ||
|---|---|---|---|---|
| B | HSF | LSF | ||
| Triglyceride (mg/dL) | 97.7 ± 47.9 | 85.4 ± 33.4 | 82.5 ± 33.8 | −2.7 ± 14.9 |
| Total cholesterol (TC) (mg/dL) | 177.6 ± 32.0 | 170.4 ± 30.0 | 156.7 ± 23.3 | −13.6 ± 15.8** |
| LDL cholesterol (mg/dL) | 108.9 ± 29.1 | 106.2 ± 31.2 | 93.3 ± 22.0 | −12.9 ± 15.3** |
| HDL cholesterol (mg/dL) | 49.2 ± 10.9 | 47.2 ± 12.8 | 46.9 ± 13.4 | −0.3 ± 2.7 |
| TC/HDL cholesterol (mg/dL) | 3.8 ± 1.2 | 3.9 ± 1.4 | 3.6 ± 1.1 | −0.3 ± 0.3** |
| ApoA-I (mg/dL) | 109.1 ± 13.1 | 103.1 ± 10.6 | 103.2 ± 14.6 | 0.1 ± 8.6 |
| ApoB (mg/dL) | 70.5 ± 17.7 | 69.5 ± 15.3 | 64.1 ± 15.2 | −5.0 ± 9.4 |
| ApoCIII (mg/dL) | 8.8 ± 2.0 | 8.7 ± 2.0 | 8.0 ± 2.3 | −0.8 ± 1.2* |
| HDL apoCIII (mg/dL) | 5.9 ± 1.0 | 5.8 ± 1.2# | 5.5 ± 1.2# | −0.1 ± 0.9 |
| Non-HDL apoCIII (mg/dL) | 2.9 ± 1.4 | 2.9 ± 1.2# | 2.3 ± 1.4# | −0.6 ± 1.2 |
| Ratio of HDL to non-HDL apoCIII | 2.5 ± 1.2 | 2.3 ± 1.2# | 3.1 ± 1.9# | 0.9 ± 2.3 |
Values are means ± SD. n=14.
n=13.
CHO, carbohydrate; Δ SF, LSF – HSF.
P < 0.05,
P < 0.01, LSF vs. HSF (paired t-test).
Compositional characteristics of LDL and LDL subclasses at baseline
Analyses of lipid and apolipoprotein content were performed on total LDL and LDL subfractions (Table 3). The baseline compositional trends of LDL subclasses for percent free cholesterol, cholesterol esters, phospholipids and protein across LDL subclasses were consistent with previous findings (6, 27, 29). Percent triglyceride at baseline was greater in LDL I versus LDL II–IV.
TABLE 3.
Composition and apolipoprotein content of LDL subclasses at baseline
| total mass mg/dL | TG | FC | CE | PL | protein | apoB | apoCIII | apoE | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| % composition | mg/dL plasma | ||||||||
| LDL total | 251.6 ± 67.5 | 3.7 ± 0.9 | 8.0 ± 1.2 | 41.0 ± 2.4 | 21.1 ± 1.2 | 26.2 ± 1.4 | 54.47 ± 21.57 | 0.59 ± 0.31 | 0.47 ± 0.17 |
| LDL I | 26.5 ± 9.1a | 7.7 ± 2.2a | 9.6 ± 1.3a | 39.5 ± 2.9a | 21.8 ± 0.6a | 21.4 ± 1.8a | 4.51 ± 1.62a | 0.09 ± 0.09ab | 0.06 ± 0.05a |
| LDL II | 46.6 ± 14.9b | 3.7 ± 1.4b | 8.8 ± 1.3a | 43.8 ± 3.2b | 21.6 ± 1.1a | 22.1 ± 1.6a | 9.30 ± 2.99b | 0.06 ± 0.08a | 0.02 ± 0.02b |
| LDL III | 117.6 ± 55.1c | 2.8 ± 0.8c | 8.8 ± 2.5ab | 40.4 ± 11.4abc | 21.7 ± 4.5ab | 26.2 ± 5.3b | 27.38 ± 12.45c | 0.09 ± 0.07b | 0.07 ± 0.04a |
| LDL IV | 28.5 ± 18.5ab | 3.3 ± 1.7bc | 7.7 ± 0.4b | 33.8 ± 3.1c | 20.0 ± 1.2b | 35.2 ± 2.6c | 6.29 ± 4.23ab | 0.18 ± 0.09c | 0.13 ± 0.07c |
Values are means ± SD. n=10. Means in a column without a common letter differ, P < 0.05. Paired t-test across LDL subclasses.
FC, free cholesterol; CE, cholesterol ester; PL, phospholipid.
LDL I and LDL IV particles were significantly lower in number (as measured by apoB) and total mass concentration compared to LDL II and III, with LDL III being the most abundant subfraction (Table 3). ApoCIII and apoE levels were highest in LDL IV (Table 3). We also calculated the relative enrichment of apoCIII and apoE per apoB molecule of total LDL and across LDL subclasses (Table 4). ApoCIII/apoB and apoE/apoB ratios were significantly higher in LDL I and IV than in LDL II and III with no significant difference between LDL I and IV.
TABLE 4.
Apolipoprotein ratios of LDL and LDL subfractions at baseline
| apoCIII/apoB | apoE/apoB | |
|---|---|---|
| LDL total | 0.012 ± 0.005 | 0.009 ± 0.004 |
| LDL I | 0.019 ± 0.014a | 0.013 ± 0.006a |
| LDL II | 0.006 ± 0.007b | 0.003 ± 0.002b |
| LDL III | 0.003 ± 0.001b | 0.003 ± 0.002b |
| LDL IV | 0.034 ± 0.018a | 0.026 ± 0.013a |
Values are means ± SD. n=10. Means in a column without a common letter differ, P < 0.005. Paired t-test across LDL subclasses.
Effects of dietary saturated fat on compositional characteristics of LDL and LDL subclasses
In the setting of moderate carbohydrate intake, HSF vs. LSF intake significantly increased LDL total mass concentration by 15.2% as well as LDL apoCIII content by 33.5% (Table 5). The change in LDL mass was attributable to significant increases in the mass concentrations of LDL subclasses I, II and III by 19.0, 20.1 and 16.5%, respectively, but was significantly correlated only with changes in LDL III (r = 0.65, P = 0.01) (data not shown). The relative enrichment of apoCIII per apoB in total LDL tended to increase with HSF vs. LSF intake (P = 0.07) (data not shown). Saturated fat-induced increases in plasma and LDL apoCIII were positively and significantly correlated with changes in LDL IV apoCIII (Table 6 and Figure 1), but not LDL I–III apoCIII. No significant changes in apoE content were observed in total LDL or LDL subclasses with HSF vs. LSF intake (Table 5).
TABLE 5.
Effects of dietary saturated fat on composition and apolipoprotein content of LDL and LDL subclasses
| total mass mg/dL | TG | FC | CE | PL | protein | apoB | apoCIII | apoE | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| % composition | mg/dL plasma | ||||||||
| LDL total | |||||||||
| HSF | 259.6 ± 60.2 | 3.9 ± 1.0 | 7.6 ± 1.1 | 41.5 ± 5.2 | 19.7 ± 2.0 | 27.3 ± 5.7 | 51.13 ± 17.55 | 0.73 ± 0.36 | 0.40 ± 0.18 |
| LSF | 225.3 ± 48.8** | 4.1 ± 0.9 | 8.2 ± 0.9 | 39.9 ± 2.3 | 21.0 ± 1.2* | 26.8 ± 2.2 | 47.08 ± 14.84 | 0.55 ± 0.20* | 0.39 ± 0.24 |
| LDL I | |||||||||
| HSF | 35.2 ± 12.7 | 7.2 ± 2.1 | 9.1 ± 1.2 | 39.2 ± 4.3 | 20.2 ± 1.8 | 24.3 ± 5.9 | 5.19 ± 1.44 | 0.13 ± 0.12 | 0.06 ± 0.04 |
| LSF | 29.6 ± 9.8* | 7.8 ± 1.5 | 9.6 ± 1.4 | 39.0 ± 2.5 | 21.8 ± 1.2* | 21.8 ± 1.8 | 4.75 ± 1.73 | 0.08 ± 0.07 | 0.06 ± 0.04 |
| LDL II | |||||||||
| HSF | 47.9 ± 16.2 | 4.1 ± 1.6 | 9.3 ± 0.9 | 44.0 ± 5.0 | 20.8 ± 1.9 | 21.9 ± 3.5 | 8.62 ± 3.28 | 0.04 ± 0.02 | 0.02 ± 0.01 |
| LSF | 39.8 ± 13.2** | 4.3 ± 1.4 | 9.5 ± 1.1 | 41.7 ± 2.9 | 22.2 ± 1.5 | 22.3 ± 2.7 | 6.90 ± 3.24 | 0.03 ± 0.03 | 0.02 ± 0.02 |
| LDL III | |||||||||
| HSF | 121.9 ± 44.9 | 2.9 ± 0.9 | 8.6 ± 0.9 | 41.3 ± 3.7 | 20.1 ± 1.6 | 27.0 ± 4.9 | 25.56 ± 10.81 | 0.12 ± 0.10 | 0.06 ± 0.02 |
| LSF | 104.6 ± 34.6** | 3.0 ± 0.8 | 9.1 ± 0.7 | 41.1 ± 2.0 | 21.5 ± 0.9* | 25.4 ± 1.9 | 22.72 ± 10.00 | 0.08 ± 0.05 | 0.06 ± 0.03 |
| LDL IV | |||||||||
| HSF | 27.6 ± 14.2 | 2.7 ± 1.4 | 7.1 ± 1.2 | 35.8 ± 5.1 | 19.5 ± 2.2 | 34.8 ± 7.1 | 5.60 ± 2.61 | 0.18 ± 0.09 | 0.10 ± 0.03 |
| LSF | 25.2 ± 11.8 | 2.9 ± 1.1 | 7.7 ± 0.5 | 34.5 ± 3.1 | 20.9 ± 2.1 | 34.0 ± 3.2 | 5.40 ± 3.54 | 0.16 ± 0.07 | 0.10 ± 0.05 |
Values are means ± SD. n=14.
FC, free cholesterol; CE, cholesterol ester; PL, phospholipid; HSF, high saturated fat; LSF, low saturated fat.
Significantly different from HSF:
P < 0.05,
P < 0.01 (paired t-test).
TABLE 6.
Spearman’s correlations between saturated fat-induced changes in plasma & LDL apoCIII and changes in apoCIII in LDL subclasses
| Δ apoCIII in LDL subclasses | Δ plasma apoCIII | Δ LDL apoCIII |
|---|---|---|
| LDL I | 0.34 | 0.17 |
| LDL II | 0.17 | 0.10 |
| LDL III | 0.07 | 0.42 |
| LDL IV | 0.54* | 0.66* |
low saturated fat – high saturated fat.
P < 0.05.
FIGURE 1.
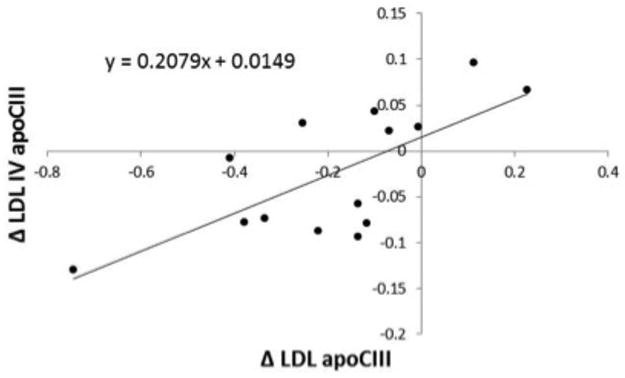
Spearman’s correlation between saturated fat-induced changes in total LDL apoCIII (mg/dL) and LDL IV apoCIII (mg/dL) in men. Δ, low saturated fat – high saturated fat. N = 14, ρ = 0.66, P = 0.01.
Discussion
Our results show that in the context of a reduced carbohydrate, high beef protein diet, the increase in plasma and LDL cholesterol with high vs. low saturated fat is associated with concurrent increases in the concentration of plasma and LDL apoCIII and the mass of large, medium and small LDL particles. The change in total LDL apoCIII was correlated with changes in apoCIII in the LDL IV fraction (ρ = 0.66). Consistent with previous reports, the concentration of apoCIII was significantly higher in LDL IV compared to other LDL subfractions (Table 3) (30, 31). Increased LDL apoCIII has been shown to be an important marker of CVD risk that is independent of standard lipid risk factors and in fact much of the risk ordinarily attributed to LDL appears to be due to LDL particles that contain apoCIII (18, 32). The increased apoCIII content of small dense LDL and greater affinity of these particles for arterial proteoglycans (31) may increase interaction with the arterial wall and facilitate oxidative modification. Furthermore, clinical observations suggest that apoCIII may play a direct role in the development of atherosclerosis by activating inflammatory responses through monocyte-endothelial interactions (33).
Consistent with the results of the larger study, we found that saturated fat increased the mass of LDL I–III with no change in LDL IV (Table 5). This contrasts with results from an earlier study, where high (15%) vs. low (9%) saturated fat intake in the context of a diet high in protein from mixed sources increased LDL cholesterol primarily through elevations in mass of larger LDL without changes in levels of small LDL particles (10). While the basis for the differing findings is unclear, one explanation may be related to differences in dietary protein source between the two studies.
To our knowledge, this study is the first to show significant increases in plasma and LDL apoCIII with saturated vs. monounsaturated fat feeding, with all other macronutrients held constant. Others (34–37) have reported diet-induced increases in plasma apoCIII and concurrent elevations in plasma triglycerides, mainly as a result of increased dietary carbohydrate intake. In contrast to the current study, the saturated fat-mediated changes in plasma and LDL apoCIII were independent of changes in triglycerides. Studies in cynomolgus monkeys have shown significantly higher hepatic apoCIII mRNA levels with saturated vs. monounsaturated fat intake (38). Consistent with our observations, the increase in hepatic apoCIII in the monkey studies was not paralleled by an increase in plasma triglycerides. Thus, the saturated fat induced rise in plasma and LDL apoCIII in our study may be due to the increase in production of apoCIII at the transcriptional level.
ApoCIII enriched apoB-containing lipoproteins may be particularly atherogenic by virtue of their decreased affinity for the LDL receptor (12) and increased binding to extracellular vascular proteoglycans (39). LDL with apoCIII strongly predicts coronary events in diabetic patients independent of other lipids (18). Notably, in plasma isolated from patients with metabolic syndrome or type 2 diabetes mellitus, the content of apoCIII in small dense LDL has been shown to be significantly correlated with an enhanced affinity of these particles for arterial proteoglycans (31). A recent report in 141 healthy middle-aged men showed a significant positive association between plasma apoCIII concentration and levels of the smallest of LDL subclasses (LDL IV) independent of plasma triglycerides and concluded that enrichment of apoCIII in small dense LDL may contribute to the metabolic and pathologic properties of these particles (40). A recent kinetic study (41) showed accelerated hepatic lipase-mediated conversion of light LDL to dense LDL when precursor particles were enriched with apoCIII. It is possible that in vivo, apoCIII positively modulates hepatic lipase to increase dense LDL particle concentrations. Based on the results from the total set of individuals participating in the current dietary intervention (n = 40) (11), higher saturated fat intake combined with high beef intake tended to increase the activity of hepatic lipase activity (P = 0.08) supporting the role of hepatic lipase in promoting the formation of small dense LDL.
Limitations of the current study include a small sample size, providing limited power for multivariable statistical adjustments, and the potential for type I error as a result of multiple testing. Nonetheless, the present findings suggest that increased levels of small, apoCIII-containing LDL may contribute to the adverse effects of a diet high in beef and saturated fat on CVD risk.
Acknowledgments
This study was supported by the Beef Checkoff, through the National Cattlemen’s Beef Association and in part by NIH/NCRR UCSF-CTSI grant number UL1 RR024131. We thank the study participants and Robin S. Rawlings for subject recruitment and clinical assistance; Casey Geaney, Vanessa Kreger, Joe Orr, Jeff Payumo, Bahareh Sahami, and Katie Wojnoonski for laboratory support; and Harriett S. Fernstrom and Cewin Chao for assistance in the dietary intervention.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
RERFERENCES
- 1.Denke MA. Dietary fats, fatty acids, and their effects on lipoproteins. Curr Atheroscler Rep. 2006;8(6):466–71. doi: 10.1007/s11883-006-0021-0. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21(4):305–11. doi: 10.1097/MOL.0b013e32833b7756. [DOI] [PubMed] [Google Scholar]
- 3.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192(1):211–7. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 4.St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–9. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- 5.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–80. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43(9):1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, et al. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol. 2003;23(2):314–21. doi: 10.1161/01.atv.0000053385.64132.2d. [DOI] [PubMed] [Google Scholar]
- 8.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–9. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. The American journal of clinical nutrition. 1998;67(5):828–36. doi: 10.1093/ajcn/67.5.828. [DOI] [PubMed] [Google Scholar]
- 10.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. The American journal of clinical nutrition. 2006;83(5):1025–31. doi: 10.1093/ajcn/83.5.1025. quiz 205. [DOI] [PubMed] [Google Scholar]
- 11.Mangravite LM, Chiu S, Wojnoonski K, Rawlings RS, Bergeron N, Krauss RM. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J Nutr. 2011;141(12):2180–5. doi: 10.3945/jn.111.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol. 1995;15(7):963–71. doi: 10.1161/01.atv.15.7.963. [DOI] [PubMed] [Google Scholar]
- 13.Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985;75(2):384–90. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102(16):1886–92. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 15.Gervaise N, Garrigue MA, Lasfargues G, Lecomte P. Triglycerides, apo C3 and Lp B:C3 and cardiovascular risk in type II diabetes. Diabetologia. 2000;43(6):703–8. doi: 10.1007/s001250051366. [DOI] [PubMed] [Google Scholar]
- 16.Chivot L, Mainard F, Bigot E, Bard JM, Auget JL, Madec Y, et al. Logistic discriminant analysis of lipids and apolipoproteins in a population of coronary bypass patients and the significance of apolipoproteins C-III and E. Atherosclerosis. 1990;82(3):205–11. doi: 10.1016/0021-9150(90)90042-h. [DOI] [PubMed] [Google Scholar]
- 17.Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol. 1997;17(4):715–22. doi: 10.1161/01.atv.17.4.715. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Campos H, Moye LA, Sacks FM. LDL containing apolipoprotein CIII is an independent risk factor for coronary events in diabetic patients. Arterioscler Thromb Vasc Biol. 2003;23(5):853–8. doi: 10.1161/01.ATV.0000066131.01313.EB. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113(5):691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27(1):219–25. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114(7):681–7. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 22.Choi SY, Komaromy MC, Chen J, Fong LG, Cooper AD. Acceleration of uptake of LDL but not chylomicrons or chylomicron remnants by cells that secrete apoE and hepatic lipase. J Lipid Res. 1994;35(5):848–59. [PubMed] [Google Scholar]
- 23.Pitas RE, Innerarity TL, Mahley RW. Cell surface receptor binding of phospholipid. protein complexes containing different ratios of receptor-active and -inactive E apoprotein. J Biol Chem. 1980;255(11):5454–60. [PubMed] [Google Scholar]
- 24.Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31(2):217–22. [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Shen MM, Krauss RM, Lindgren FT, Forte TM. Heterogeneity of serum low density lipoproteins in normal human subjects. J Lipid Res. 1981;22(2):236–44. [PubMed] [Google Scholar]
- 28.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87(1):206–10. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 29.La Belle M, Blanche PJ, Krauss RM. Charge properties of low density lipoprotein subclasses. J Lipid Res. 1997;38(4):690–700. [PubMed] [Google Scholar]
- 30.Lee DM, Alaupovic P. Apolipoproteins B, C-III and E in two major subpopulations of low-density lipoproteins. Biochim Biophys Acta. 1986;879(2):126–33. doi: 10.1016/0005-2760(86)90094-9. [DOI] [PubMed] [Google Scholar]
- 31.Davidsson P, Hulthe J, Fagerberg B, Olsson BM, Hallberg C, Dahllof B, et al. A proteomic study of the apolipoproteins in LDL subclasses in patients with the metabolic syndrome and type 2 diabetes. J Lipid Res. 2005;46(9):1999–2006. doi: 10.1194/jlr.M500161-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124(19):2065–72. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16(1):6–11. doi: 10.5551/jat.e607. [DOI] [PubMed] [Google Scholar]
- 34.Archer WR, Desroches S, Lamarche B, Deriaz O, Landry N, Fontaine-Bisson B, et al. Variations in plasma apolipoprotein C-III levels are strong correlates of the triglyceride response to a high-monounsaturated fatty acid diet and a high-carbohydrate diet. Metabolism. 2005;54(10):1390–7. doi: 10.1016/j.metabol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Desroches S, Ruel IL, Deshaies Y, Paradis ME, Archer WR, Couture P, et al. Kinetics of plasma apolipoprotein C-III as a determinant of diet-induced changes in plasma triglyceride levels. Eur J Clin Nutr. 2008;62(1):10–7. doi: 10.1038/sj.ejcn.1602673. [DOI] [PubMed] [Google Scholar]
- 36.Shin MJ, Blanche PJ, Rawlings RS, Fernstrom HS, Krauss RM. Increased plasma concentrations of lipoprotein(a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C-III bound to apolipoprotein B-containing lipoproteins. Am J Clin Nutr. 2007;85(6):1527–32. doi: 10.1093/ajcn/85.6.1527. [DOI] [PubMed] [Google Scholar]
- 37.Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ, et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87(6):1623–30. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brousseau ME, Ordovas JM, Osada J, Fasulo J, Robins SJ, Nicolosi RJ, et al. Dietary monounsaturated and polyunsaturated fatty acids are comparable in their effects on hepatic apolipoprotein mRNA abundance and liver lipid concentrations when substituted for saturated fatty acids in cynomolgus monkeys. J Nutr. 1995;125(3):425–36. doi: 10.1093/jn/125.3.425. [DOI] [PubMed] [Google Scholar]
- 39.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PH, Wight TN, et al. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res. 2002;43(11):1969–77. doi: 10.1194/jlr.m200322-jlr200. [DOI] [PubMed] [Google Scholar]
- 40.Shin MJ, Krauss RM. Apolipoprotein CIII bound to apoB-containing lipoproteins is associated with small, dense LDL independent of plasma triglyceride levels in healthy men. Atherosclerosis. 2010;211(1):337–41. doi: 10.1016/j.atherosclerosis.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30(2):239–45. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]


