Abstract
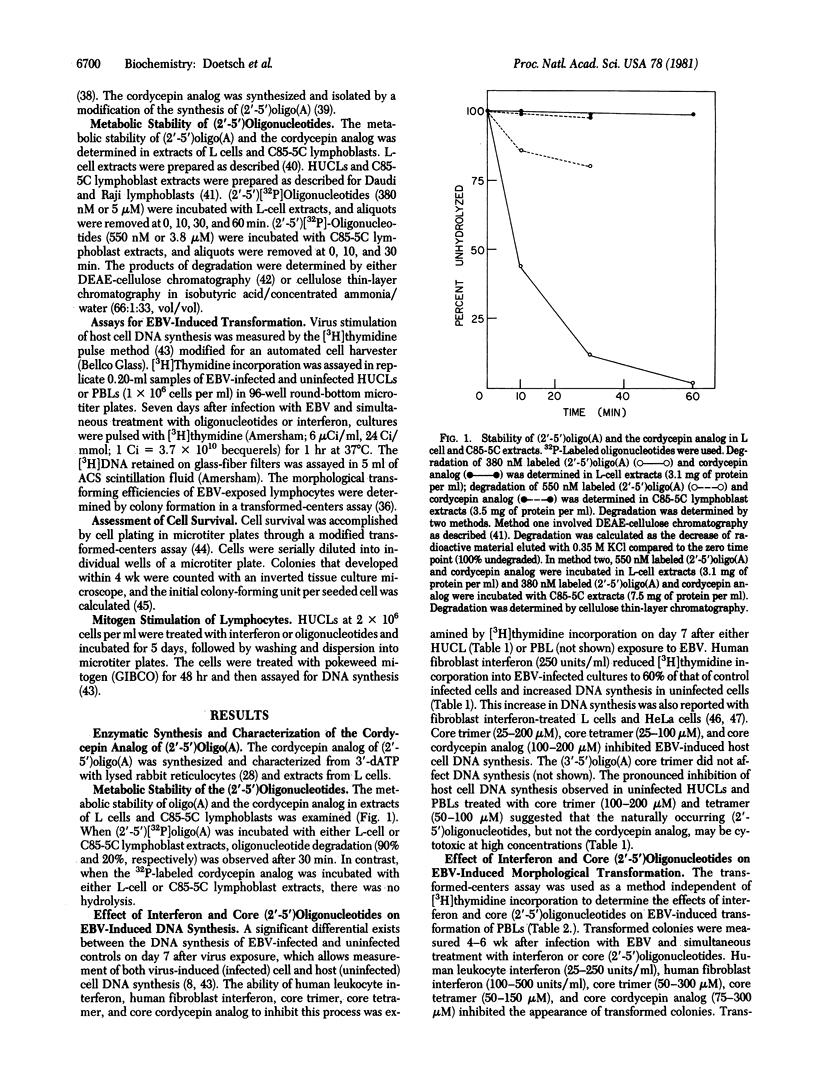
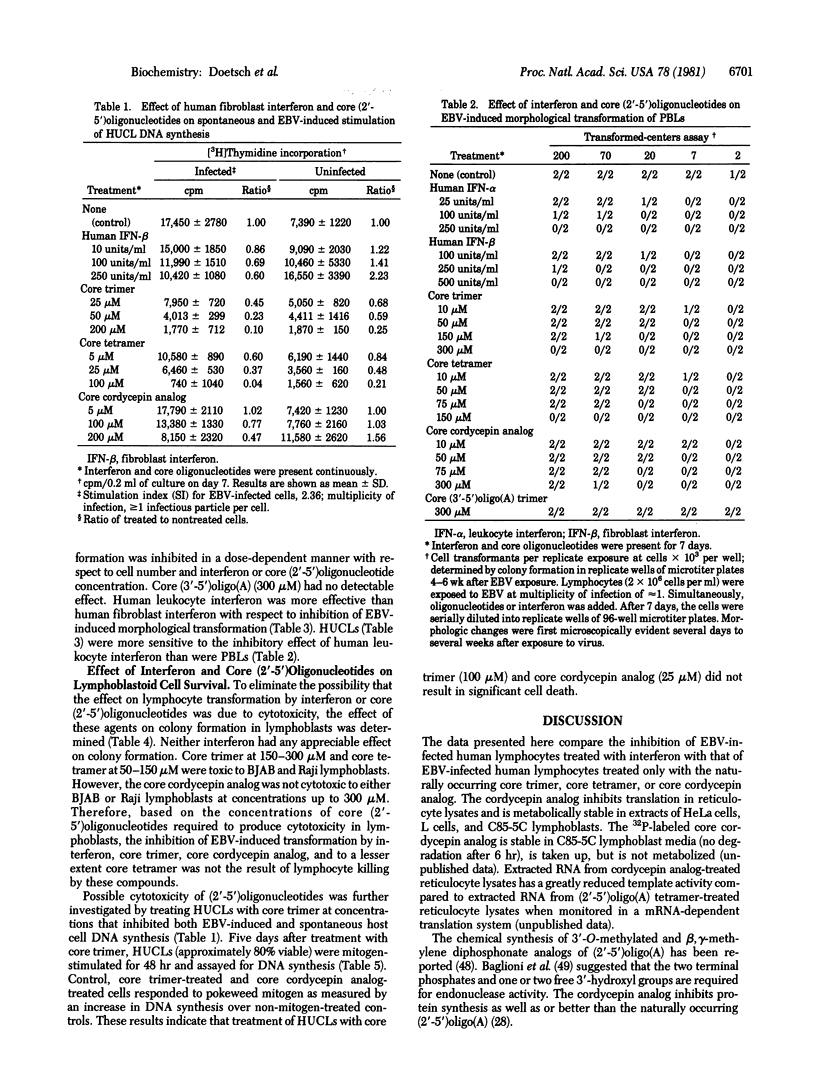
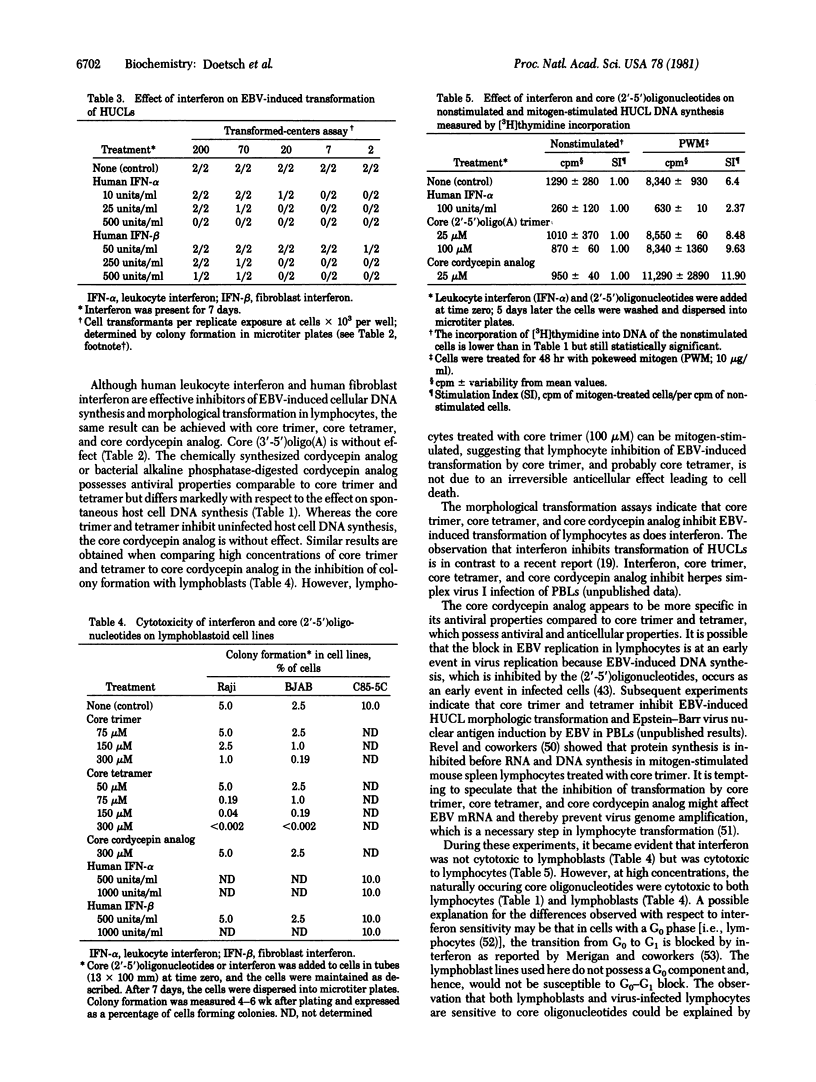
The 3'-deoxyadenosine (cordycepin) analog of (2'-5')oligo(A) [(2'-5')oligoadenylate with a triphosphate at the 5' end], synthesized enzymatically from cordycepin 5'-triphosphate in lysed rabbit reticulocytes or L-cell extracts was (i) inhibitory to translation in lysed rabbit reticulocytes and (ii) metabolically stable in extracts of either L cells or C85-5C lymphoblasts. The 5' dephosphorylated (core) (2'-5')oligo(A) and the core cordycepin analog can replace human fibroblast interferon in preventing the transformation of human lymphocytes after infection with Epstein--Barr virus B95-8 (EBV) as determined by the decreased incorporation of [3H]thymidine into cellular DNA and the inhibition of morphological transformation of EBV-infected lymphocytes. Whereas the naturally occurring core (2'-5')oligo(A) was cytotoxic to uninfected lymphocytes and proliferating lymphoblasts, the core cordycepin analog was not. Human leukocyte interferon was more effective than human fibroblast interferon in the inhibition of EBV-induced transformation of human umbilical cord lymphocytes and adult peripheral blood lymphocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiman W. A. The Epstein-Barr virus and EB virus infections in childhood. J Pediatr. 1979 Aug;95(2):171–182. doi: 10.1016/s0022-3476(79)80646-0. [DOI] [PubMed] [Google Scholar]
- Baglioni C., D'Alessandro S. B., Nilsen T. W., den Hartog J. A., Crea R., van Boom J. H. Analogs of (2'-5')oligo(A). Endonuclease activation and inhibition of protein synthesis in intact cells. J Biol Chem. 1981 Apr 10;256(7):3253–3257. [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Siegel P. J., Baer J. Effects of adenine arabinoside on lymphocytes infected with Epstein-Barr virus. J Virol. 1978 Sep;27(3):475–482. doi: 10.1128/jvi.27.3.475-482.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantell K. Towards the clinical use of interferon. Endeavour. 1978;2(1):27–30. doi: 10.1016/0160-9327(78)90030-3. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory J. G., Suhadolnik R. J., Resnick B., Rich M. A. Incorporation of cordycepin (3'-deoxyadenosine) into ribonucleic acid and deoxyribonucleic acid of human tumor cells. Biochim Biophys Acta. 1965 Aug 10;103(4):646–653. doi: 10.1016/0005-2787(65)90085-7. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Bartholomew J. C., Merigan T. C. Role of G0-G1 arrest in the inhibition of tumor cell growth by interferon. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1471–1475. doi: 10.1073/pnas.77.3.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P., Wu J. M., Sawada Y., Suhadolnik R. J. Synthesis and characterization of (2'-5')ppp3'dA(p3'dA)n, an analogue of (2'-5')pppA(pA)n. Nature. 1981 May 28;291(5813):355–358. doi: 10.1038/291355a0. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Samanta H., Farrell P. J., Lengyel P. Interferon, double-stranded RNA, and RNA degradation. Isolation of homogeneous pppA(2'p5'A)n-1 synthetase from Ehrlich ascites tumor cells. J Biol Chem. 1980 May 10;255(9):3813–3816. [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinan F. M., Bishop J., Lee S. H., Rozee K. R. The characteristics of enhanced DNA synthesis in L cells treated with interferon and arginine-deprived. Exp Cell Res. 1977 Dec;110(2):283–288. doi: 10.1016/0014-4827(77)90294-4. [DOI] [PubMed] [Google Scholar]
- Henderson E. E., Long W. K., Ribecky R. Effects of nucleoside analogs on Epstein-Barr virus-induced transformation of human umbilical cord leukocytes and Epstein-Barr virus expression in transformed cells. Antimicrob Agents Chemother. 1979 Jan;15(1):101–110. doi: 10.1128/aac.15.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Miller G., Robinson J., Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977 Jan;76(1):152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Swartz M. N. Drug therapy: antiviral agents (second of two parts). N Engl J Med. 1980 Apr 24;302(17):949–953. doi: 10.1056/NEJM198004243021705. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J. N. Anticellular and antiviral effects of pppA(2'p5'A)n. Virology. 1980 Feb;101(1):81–90. doi: 10.1016/0042-6822(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Regulation of lymphocyte mitogenesis by (2'--5') oligo-isoadenylate. Nature. 1979 Dec 20;282(5741):849–851. doi: 10.1038/282849a0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Waters H. L., Buchanan J. K. Survival of human lymphoblastoid cells after DNA damage measured by growth in microtiter wells. Mutat Res. 1980 Sep;72(2):285–294. doi: 10.1016/0027-5107(80)90043-3. [DOI] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. Increased levels of (2'-5')oligo(A) polymerase activity in human lymphoblastoid cells treated with glucocorticoids. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6506–6510. doi: 10.1073/pnas.77.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. R., Chatterjee G. E., Maroney P. A., Baglioni C. Phosphorylated sugars stimulate protein synthesis and Met-tRNAf binding activity in extracts of mammalian cells. Biochemistry. 1978 Jan 10;17(1):80–87. doi: 10.1021/bi00594a011. [DOI] [PubMed] [Google Scholar]
- Menezes J., Patel P., Dussault H., Joncas J., Leibold W. Effect of interferon on lymphocyte transformation and nuclear antigen production by Epstein-Barr virus. Nature. 1976 Apr 1;260(5550):430–432. doi: 10.1038/260430a0. [DOI] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Synthesis of 2'5'-oligo(A) in extracts of interferon-treated HeLa cells. J Biol Chem. 1979 Jun 25;254(12):5058–5064. [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972 Nov;17(2):233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Niederman J. C., Evans A. S., Subrahmanyan L., McCollum R. W. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970 Feb 12;282(7):361–365. doi: 10.1056/NEJM197002122820704. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A. Effects of arabinofuranosylthymine on Epstein-Barr virus replication. Virology. 1980 Jul 15;104(1):219–223. doi: 10.1016/0042-6822(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Huang C. H., Huang Y. T. Epstein-Barr virus genome in infectious mononucleosis. Nature. 1976 Oct 28;263(5580):787–789. doi: 10.1038/263787a0. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Staal S. P. Effect of interferon on murine leukemia virus infection. III. Effect of interferon on MSV-induced transformation. Virology. 1978 Oct 1;90(1):151–155. doi: 10.1016/0042-6822(78)90343-4. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Ward D. C. Nucleoside analogs with antiviral activity. Biochem Pharmacol. 1976 Jun 1;25(11):1233–1239. doi: 10.1016/0006-2952(76)90083-6. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., DeFlorio D., Jr, Hutt L. M., Bhawan J., Yang J. P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med. 1977 Nov 17;297(20):1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Brown N., Andiman W., Halliday K., Francke U., Robert M. F., Andersson-Anvret M., Horstmann D., Miller G. Diffuse polyclonal B-cell lymphoma during primary infection with Epstein-Barr virus. N Engl J Med. 1980 Jun 5;302(23):1293–1297. doi: 10.1056/NEJM198006053022306. [DOI] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Smith D. Infection of human B lymphocytes with high multiplicities of Epstein-Barr virus: kinetics of EBNA expression, cellular DNA synthesis, and mitosis. Virology. 1981 Mar;109(2):336–343. doi: 10.1016/0042-6822(81)90504-3. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Chernajovsky Y., Shulman L., Federman P., Berissi H., Revel M. An interferon-induced phosphodiesterase degrading (2'-5') oligoisoadenylate and the C-C-A terminus of tRNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4788–4792. doi: 10.1073/pnas.76.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Todaro G. J., Baron S. The role of interferon in the inhibition of SV40 transformation of mouse cell line 3T3. Proc Natl Acad Sci U S A. 1965 Sep;54(3):752–756. doi: 10.1073/pnas.54.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht A., Treuner J., Flehmig B., Joester K. E., Niethammer D. Interferon-neutralizing antibodies in a patient treated with human fibroblast interferon. Nature. 1981 Feb 5;289(5797):496–497. doi: 10.1038/289496a0. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Kerr I. M. Activation of a nuclease by pppA2'p5'A2'p5'A in intact cells. FEBS Lett. 1979 Sep 1;105(1):47–52. doi: 10.1016/0014-5793(79)80885-6. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M. Inhibition of protein synthesis by 2'-5' linked adenine oligonucleotides in intact cells. Nature. 1978 Nov 2;276(5683):88–90. doi: 10.1038/276088a0. [DOI] [PubMed] [Google Scholar]
- den Hartog J. A., Wijnands R. A., van Boom J. H., Crea R. Chemical synthesis of an effective inhibitor of protein synthesis in eukaryotic cells: pppA2'-5'A2'-5'A and some analogues. Nucleic Acids Symp Ser. 1980;(7):157–166. [PubMed] [Google Scholar]



