Abstract
Aquatic stability and impact of titanium dioxide nanoparticles (TiO2 NPs, 10-30 nm) was investigated using Artemia salina. Acute exposure was conducted on nauplii (larvae) and adults in seawater in a concentration range from 10 to 100 mg/L TiO2 NPs for 24 h and 96 h. Rapid aggregation occurred in all suspensions of TiO2 NPs to form micrometer size particles. Yet, both nauplii and adults accumulated the aggregates significantly. Average TiO2 content in nauplii ranged from 0.47 to 3.19 mg/g and from 1.29 to 4.43 mg/g in 24 h and 96 h, respectively. Accumulation in adults was higher ranging from 2.30 to 4.19 mg/g and from 4.38 to 6.20 mg/g in 24 h and 96 h, respectively. Phase contrast microscopy images revealed that Artemia were unable to excrete the particles. Thus, the TiO2 aggregates filled inside the guts. No significant mortality or toxicity occurred within 24 h at any dose. Lipid peroxidation levels characterized with malondialdehyde (MDA) concentrations were not statistically different from those of the controls (p>0.05). These results suggested that suspensions of the TiO2 NPs were nontoxic to Artemia, most likely due to the formation of benign TiO2 aggregates in water. In contrast, both mortality and lipid peroxidation increased in extended exposure to 96 h. Highest mortality occurred in 100 mg/L TiO2 NP suspensions; 18% for nauplii and 14% for adults (LC50 > 100 mg/L). These effects were attributed to the particle loading inside the guts leading to oxidative stress as a result of impaired food uptake for a long period of time.
Keywords: TiO2 nanoparticle, Artemia salina, Aggregation, Accumulation, Toxicity
Introduction
Nanotechnology is the new frontier worldwide and is predicted to become a trillion US dollar industry in the near future (Schmidt 2009). As new nanomaterials and products containing nano-scale particles are manufactured, as many will inevitably reach environmental repositories. The release of nanomaterials from commercial products into the aquatic environment has already been reported (Benn and Westerhoff 2008; Geranio et al. 2009). Nevertheless, the effects on human and environmental health are poorly understood because of the complexity of factors that affect chemical and toxicological properties of nanomaterials (Chatterjee 2008; Choi et al. 2009).
Titanium dioxide nanoparticles (TiO2 NPs) exhibit photocatalytic and antibacterial properties and thus have been used in various consumer products and environmental applications, including paint, sunscreens, surface coatings and water disinfection (Bahnemann et al. 2002; Schulz et al. 2002; Mills et al. 2004; Zeynalov and Allen 2006; Choi et al. 2006). The release of products containing TiO2 NPs into fresh and coastal waters (e.g., estuaries) is concerning as it may impact the aquatic species and marine food chain, particularly algae and zooplankton (Moore 2006; Farre et al. 2009). A number of groups have evaluated the ecotoxicity of TiO2 NPs on freshwater models, such as Daphnia magna (Hund-Rinke and Simon 2006; Lovern and Klaper 2006; Warheit et al. 2007; Handy et al. 2008; Farkas et al. 2010; Kim et al. 2010; Zhu et al. 2010). Nevertheless, our understanding about their fate and toxic effects is still in its infancy because of the controversies among the findings associated with the differences in test models, experimental conditions and surface properties of TiO2 NPs. For instance, Wiench et al. (2009) found little acute toxicity from nano-scale and microscale TiO2 on Daphnia magna using different test media and several NP formulations (EC50 > 100 mg/L). A similar result was reported for zebrafish (Danio rerio) embryos for which no significant toxicity was observed from TiO2 NPs at concentrations as high as 500 mg/L (Zhu et al. 2008). Conversely, TiO2 NPs induced lethal effects in a long-term exposure study in that 40% of D. magna died when exposed to 20 mg/L levels (Adams et al. 2006). Relatively high toxicity was reported from Zhu et al. (2010) for uncoated TiO2 NPs that induced 13% mortality on D. magna within 72 h at 0.1 mg/L level.
Artemia salina (brine shrimp) are zooplankton that are used to feed larval fish in cultures like copepods and daphnids (Sorgeloos 1980). They play an important role in the energy flow of the food chain in marine environment. In addition, they are used as a laboratory bioassay organism to develop standard toxicology assays (Vanhaecke et al. 1981; Sanchez et al. 1997; Nunes et al. 2006; Kanwar 2007). Artemia are hypo/hyper-osmotic regulators that are able to maintain hemolymph ion concentrations within narrow limits over an external salinity range from 0.26% NaCl to supersaturated brines. With this capability, Artemia appear to be suitable model species to investigate the fate and ecotoxicity of nanomaterials in marine ecosystems through laboratory experiments.
In this study, we conducted exposure studies on Artemia, both nauplii (larvae) and adults, in aqueous suspensions of uncoated TiO2 NPs to elucidate the effects of TiO2 NPs on the marine ecosystems. Total TiO2 content (accumulation) and toxic effects (mortality and lipid peroxidation) were determined under acute exposure for 24 h and 96 h. Colloidal stability of the NPs in water was also examined to understand the influences on NP accumulation and toxicity.
Materials and methods
Reagents and chemicals
Titanium dioxide nanoparticles (TiO2 NPs, 99.5% rutile polymorph) were purchased, as uncoated nanomaterials, from Skyspring Nanomaterials Inc., in Houston, TX USA. The NPs were spherical with an average particle size (D50) between 10 and 30 nm and approximate surface area of 50 m2/g. The morphology of the NPs was rutile with pale yellow color, which is most widely found in polymorph of TiO2 in nature.
Artemia cysts (The Great Salt Lake, Utah harvest) were purchased from Artemia International LLC, Houston TX, and were kept at 4°C temperature moisture-free container in a refrigerator. Deionized water produced by Barnstead E-pure system with resistivity of 18 MΩ cm was used to prepare the exposure medium and experimental solutions. Trace metal grade nitric acid (HNO3, Fisher Scientific) and hydrofluoric acid (HF, Sigma Aldrich) were used for digestion of the Artemia collected at the end of the exposure to determine the total TiO2 contents. The use of HF was necessary for effective solubilization of TiO2 to Ti ions in solution for ICP-MS measurements. Stock titanium standard solution (1000 μg/mL) was purchased from SCP Science (Champlain, NY) and used for preparation of ICP-MS standards in 5% HNO3. Carbon coated Cu TEM grids (300 mesh) were used to measure the size of NPs. The grids were purchased from Electron Microscopy Sciences (EMS), Hatfield, PA.
Preparation of test organism
Artemia cysts were hatched in seawater (30‰ m/v). The seawater was prepared by dissolving appropriate amount of Instant Ocean® salt in deionized water, stirred for 24 h under aeration and then filtered through 30-μm Millipore cellulose filters. Artemia were hatched by using the procedure described by Persoone et al. (1989). Briefly, encysted Artemia were first hydrated in distilled water at 4 °C for 12 h and then washed to separate the floating cysts from those that sink. The sinking cysts were collected on a Buchner funnel and washed with cold deionized water. Approximately 3 g of the pre-cleaned cysts were incubated in 1.5 L seawater in a conical plastic contained with graduations at 30 ± 1 °C. A 1500 lux day-light was provided continuously by a fluorescent lamp. Aeration was maintained by a small line extending to the bottom of the hatching device from an aquarium air-pump. Under these conditions, Artemia hatched within 24 h.
Counting hatched Artemia
The rate of hatching was variable; therefore, it was important to determine the number of Artemia nauplii and adults as accurately as possible prior to the start of exposure. The counting was performed according to the procedure described by Sorgeloos (1980). Briefly, 100 mL solution containing the hatched Artemia nauplii was taken into a clean beaker. Under continuous stirring, 1 mL of this stock was diluted to 100 mL with seawater (100-fold dilution). Next, 0.1 mL of the diluted solution was taken under stirring and placed in petridish. The number of nauplii was determined by counting visually in this volume (0.1 mL).
Preparation of aqueous suspensions of TiO2 NPs
Preliminary exposure studies were conducted with up to 5 mg/L TiO2 NPs on Artemia nauplii to estimate the exposure concentration. No significant immobilization or mortality occurred within 24 h; therefore, the experimental NP suspensions were prepared between 10 and 100 mg/L to achieve measurable effects.
A stock suspension of 20% (m/v) was prepared by dispersing appropriate amount of TiO2 NP powder in deionized water. This solution was vortexed for 20 s, and then sonicated in an ultrasonic bath for about 10 min for maximum dispersion. Appropriate volumes of the stock suspension were then immediately transferred into the exposure tanks containing Artemia nauplii or adults in the seawater.
Size distribution of TiO2 NP suspensions
Morphology and size distribution of the TiO2 NPs were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). For stock TiO2 NP suspensions in deionized, measurements were made immediately after the preparation of the suspension. A drop of the colloidal solution of TiO2 NPs was placed onto 50 Å thick carbon-coated copper grids and allowed to dry for TEM measurements. The images were recorded by JEOL-1011 TEM instrument providing a resolution of JEM-1011 is 0.2 nm lattice with magnification of 50 to 1×106 under the accelerating voltage of 40 to 100 kV. TEM images were then analyzed by using ImageJ software package. Particle size distribution was collocated for a group of 100 particles in random fields of view. DLS measurements were conducted by DynaPro DLS instrument (Wavelength: 826nm, Power: 58mW, at 100% usage). A portion of the stock suspension solution from stock TiO2 suspension was placed in a clean cuvette and measured five times successively. Particle size measurements from exposure medium (e.g., salt water) were made similarly by both TEM and DLS. In this case, measurements were conducted 12 h after the start of the exposure to verify possible aggregation and changes in particle size.
Exposure studies
Acute exposure was conducted on both Artemia nauplii and adults for 24 h and 96 h according to Organization for Economic Cooperation and Development, OECD 202 testing guidelines (OECD 2004). Three different test concentrations (10, 50 and 100 mg/L) of TiO2 NPs were administered to both cultures. A control group was also setup without the test compound. Studies were carried out in triplicate measurements in conical plastic containers (1-L and 2-L inner volume). Exposures were conducted in 500 mL and 1500 mL seawater for Artemia nauplii and adults, respectively. Aeration was provided by a line extending to the bottom of the conical flask to prevent settling of NPs from suspension during the course of the exposure. Details of the experimental conditions are summarized in Table 1. Light regime of 16:8 h light:dark and at a temperature of 24 ± 2 °C were maintained. The pH of the medium was measured at the beginning and at the end of the exposure. No food was provided during the course of the exposure.
Table 1.
Experimental conditions for acute exposure of Artemia to uncoated TiO2 NPs (D50 = 10-30 nm). Water temperature and salinity were maintained at 24±2 °C, 29-30‰ respectively. Each exposure is made in triplicate in seawater
| Duration | TiO2 NP concentration (mg/L) |
pH | Total number of Artemia |
|
|---|---|---|---|---|
| Nauplii (103) | Adult | |||
| 24 h | 0 | 8.1 - 8.4 | 30.5 | 230 |
| 10 | 8.1 - 8.5 | 31.5 | 231 | |
| 50 | 8.3 - 8.5 | 30.2 | 335 | |
| 100 | 8.1 - 8.4 | 30.2 | 332 | |
|
| ||||
| 96 h | 0 | 8.2 - 8.5 | 31.5 | 311 |
| 10 | 8.1 - 8.6 | 32.5 | 239 | |
| 50 | 8.3 - 8.7 | 32.2 | 305 | |
| 100 | 8.2 - 8.6 | 31.9 | 295 | |
Chemical analysis
At the end of the exposure, Artemia were sampled and thoroughly washed with deionized water through 40-μm plankton net. The cleaned samples were then filtered by 0.45-mm Whatman filter paper. For instrumental analysis, about 0.1 g of wet Artemia (nauplii and adult) was weighed and digested in teflon vessels in 2 mL concentrated HNO3 and 0.5 mL HF for 2 h using digestion block (DigiPrep MS, SCP Science) at 160 °C according to protocols described elsewhere (Arslan et al. 2000, 2011). Once completely digested, the contents were diluted to 10 mL with deionized water. The sample solutions were further diluted 10-fold for analysis. For quality control, pure TiO2 NP samples (ca. 10 mg, n=5) were digested in 3 mL HNO3 and 1.0 mL HF similarly and diluted to 10 mL with water. These samples were diluted 1000-fold before analysis. All samples were analyzed for titanium (Ti) concentration by inductively coupled plasma mass spectrometry (ICP-MS) using a Varian 820MS ICP-MS instrument (Varian, Australia). Titanium standard solutions in the range of 0.2 to 5.0 μg/mL were used for instrument calibration. These standard solutions were prepared in 5% HNO3 and contained trace HF (e.g., < 0.1%). Titanium concentration was converted to TiO2 content to determine the total accumulation across different doses of exposure.
Biochemistry
Thiobarbituric acid-reactive substances (TBARS) were measured to determine the lipid peroxidation products as a measure of oxidative stress. The values were expressed as total malondialdehyde (MDA) concentration per gram of Artemia. MDA concentration was measured as described by Van Ye et al. (1993). For MDA measurement, 0.1 g Artemia was washed with cold water and then assayed using the MDA kit (Northwest Life Science Specialties, LLC, Vancouver, WA). Samples were homogenized in 2 mL phosphate buffer (pH 7.2) by ultrasonic homogenizer and then centrifuged at 6,000 rpm for 10 min. The resulting sample supernatant was immediately processed for biochemical assay, where 10 μL butylated hydroxytoluene reagents (BHT), 0.25 mL of sample supernatant, 0.25 mL of phosphoric acid reagent, and 0.25 mL of thiobarbituric acid (TBA) reagent were added to a vial, respectively. A set of stock tetramethoxypropane standards in the range of 0 to 8 μM was prepared freshly in methanol. To prepare calibration standards, 0.25 mL of the appropriate standard solution was processed similar to the sample supernatants as described above. All samples and standards were incubated at 90 °C for one hour and centrifuged after cooling at 13,000 rpm for 10 min to precipitate suspending tissue. The reaction mixtures were then transferred to UV-visible spectrophotometer cuvettes and the absorbances were measured at 532 nm. Measurements were performed in triplicate for controls and experimental groups.
Statistical analysis
All experiments were repeated three times independently, and data were recorded as the mean with standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons was used to detect significant differences in mortality and accumulation rates among the controls and treatments. In all data analyses, a p-value of 0.05 was considered statistically significant.
Results and discussion
Stability of TiO2 NPs in water
Aggregation for TiO2 NPs in aqueous suspensions has been reported previously (Adams et al. 2006; Zhu et al. 2008, 2010). Sonication has been used to achieve better dispersion and to prevent aggregation (Zhu et al. 2010; Xiong et al. 2011, Zhao et al. 2011). In this study, similar strategy was used and TiO2 NPs were exposed to ultrasounds in a sonicator bath for 10 min to improve dispersion in water. The TEM images collected from the dried NP suspensions are illustrated in Fig. 1. Aggregation was minimal in freshly prepared stock suspension (Fig. 1A). The size of NPs ranged from 8 to 40 nm (see Fig. 1A and Table 2) which were within the manufacturer’s estimate (e.g., 10-30 nm). However, the DLS measurements of the same NP suspensions indicated instantaneous aggregation in water. Hydrodynamic sizes ranged from 210 to 1833 nm with an estimated mean size of 371, 498, and 589 nm for 10, 50 and 100 mg/L TiO2 NPs, respectively (see Table 2). The size of aggregates tended to increase with TiO2 concentration. These results are consistent with previous findings (Zhu et al. 2010; Zhao et al. 2011) and are due to the hydration and reduction of electrostatic repulsion (surface charge) when TiO2 NPs are dispersed in water. Although aeration provided effective means of mixing to maintain homogeneity of the suspensions, aggregation occurred at all concentrations of TiO2 NPs.
Fig. 1.
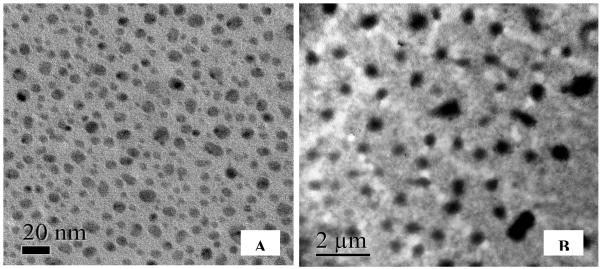
Size distribution of TiO2 NPs in exposure medium (pH 8.3). TEM Images are gathered from dried aliquots of 10 mg/L TiO2 NPs by multiple scans from different coordinates of the TEM sample plate. (A) Immediately after preparation of NP suspension, (B) 12 h after the start of exposure. Note that TiO2 NPs aggregated to micrometer size particles in 12 h.
Table 2.
Size distributions of aqueous suspensions of TiO2 NPs. TEM and DLS measurements from stock suspensions were taken immediately after preparation. The measurements from exposure medium were taken 12 h later following the addition of appropriate volumes of stock suspensions to exposure tanks. Values in parenthesis are the mean value
| Fresh stock suspension | Exposure medium | |||
|---|---|---|---|---|
|
| ||||
| TiO2 NP concentration (mg/L) |
Dry size (TEM, nm) |
Hydrodynamic size (DLS, nm) |
Dry size (TEM, nm) |
Hydrodynamic size (DLS, nm) |
| 10 | 8–35 (22) |
270-1239 (371) |
> 200 | 280-1455 (461) |
| 50 | 10–40 (28) |
210-1330 (498) |
> 200 | 345-1530 (610) |
| 100 | 12–40 (32) |
212-1833 (589) |
> 200 | 396-2334 (740) |
The TEM image recorded 12 h later from exposure medium containing 10 mg/L TiO2 NPs is illustrated in Fig. 1B. Considerably larger aggregates and strips of TiO2 were observed that were more stable (e.g., remained as large particles) compared with the fine NPs shown in Fig. 1A. Size of dry particles ranged from several hundred nanometers to microns in diameter. Hydrodynamic diameter of the particles also increased ranging from 280 to 2334 nm (see Table 2). This kind of temporal increase in the size of the aggregates of TiO2 NPs was also reported by Zhu et al. (2010) for 10 mg/mL suspension of uncoated TiO2 NPs (21 nm). Moreover, they renewed the suspensions daily to maintain NP stability and concentration, but the effects were not very different from that of continuous mixing used in this study. The median size of the aggregates increased from 580.5 to 2349 and then to 3526 nm within 1, 12 and 24 h, respectively.
Accumulation of TiO2 NPs
Artemia are filter-feeders as daphnids that can readily ingest fine particles smaller than 50 μm (Hund-Rinke and Simon 2006; Zhu et al. 2010). Accumulation of TiO2 NPs was performed qualitatively on each group at the conclusion of the exposure under a phase contrast microscope (Micromaster (Model 12-575-252, Fisher Scientific) equipped with a digital camera. Images were captured by Micron Imaging software package from live Artemia placed in petri-dishes. Compared with the controls, the guts of exposed Artemia were filled particles (Fig. 2). The ingested TiO2 particles appeared as a long strip of particles suggesting that even larger aggregates of TiO2 formed inside the guts.
Fig. 2.
Phase contrast microscopy images of the TiO2 NPs inside Artemia nauplii and adults. The images were taken 24 h after the start of exposure. The guts are completely empty in controls. Aggregates of TiO2 are visible as a dark line inside the guts of the treatments.
Total TiO2 content (wet weight) determined by ICP-MS analysis of Artemia samples is illustrated in Fig. 3 along with the elimination rates. For nauplii, the mean values were 0.47, 2.65 and 3.19 mg/g for 24 h exposure, and 1.29, 3.87 and 4.43 mg/g for 96 h exposure to 10, 50 and 100 mg/L suspensions of TiO2 NPs, respectively. Adults showed significantly higher levels that were 2.30, 3.88 and 4.19 mg/g in 24 h, and 4.38, 5.63 and 6.20 mg/g in 96 h when exposed 10, 50 and 100 mg/L suspensions of TiO2 NPs, respectively. No significant TiO2 was detected in the controls. Total TiO2 content increased with increasing concentration and exposure time indicating a dose and time dependent accumulation that exhibited a plateau beyond 24 h in suspensions of higher concentration (> 50 and 100 mg/L TiO2). TiO2 levels accumulated within 24 h and 96 h were not statistically different for nauplii nor for adults (p>0.05) (Fig. 3). This effect was indicative of reduced accumulation as the guts were full of TiO2 aggregates as shown by the images in Fig. 2.
Fig. 3.
Accumulation and elimination profiles of TiO2 NP suspensions by Artemia nauplii and adults. Values are given as average ± standard deviation of triplicate exposures. Bars with the same letter are not significantly different (p>0.05). The bars for elimination indicate the average loss in ingested TiO2 content within 24 h in clean seawater at the conclusion of exposure for 24 h and 96 h.
Elimination of ingested TiO2 NPs
At the conclusion of the exposure, Artemia were placed into freshly prepared seawater and allowed to clean up the guts from particles for 24 h. Then they were washed and digested similarly in acid to determine the change or loss in the TiO2 content. The results are illustrated in Fig. 3. For nauplii, the concentration of TiO2 decreased by 0.015 - 0.42 mg/g and 0.030 - 0.53 mg/g following 24-h and 96-h exposures, respectively. The adults showed similar elimination pattern; 0.11 - 0.52 mg/g for 24-h exposure and 0.22 - 0.74 mg/g for 96-h exposure. These concentrations correspond to about 3 to 12% reduction in TiO2 content. It is evident that Artemia were unable to eliminate the ingested particles. Likewise, D. magna had difficulty in getting rid of the particles from the guts after acute exposure to 1.0 mg/L suspensions of TiO2 NPs in static water (Zhu et al. 2010). Only a fraction of ingested TiO2 were excreted from the body in 24 h, though the efficiency improved up to 50% within 72 h. Presence of food in the medium improved the elimination efficiency, but a significant portion (ca. 20%) still remained in the guts (Zhu et al. 2010).
The TEM and DLS data (Fig. 1B and Table 2) clearly show that TiO2 NPs were no longer nanometer size particles but aggregates in the exposure medium. Nevertheless, Artemia, even nauplii, accumulated the aggregates from water readily within 24 h (Fig. 3). The large discrepancy between the accumulation and elimination rates could be due to the continuous aggregation of ingested particles inside the guts to yield massive TiO2 particles (see Fig. 2) that could not be excreted from the guts.
Effect of exposure time on mortality
The mortality values are summarized in Table 3. The controls for nauplii and adults exhibited about 3 to 5% mortality within 24 h and 96 h that were not statistically different (p>0.05). In 24 h, treatments exhibited similar rate of mortality to that of controls, but the values increased significantly in 96 h (p<0.05). For instance, mortality increased from 6 to 18% for nauplii and 5 to 14% for adults at 100 mg/L TiO2 NP suspension (LC50 > 100 mg/L). Though marginal, nauplii were more susceptible than adults during prolonged exposure suggesting that the effects depend on the state of maturity of the organism (p = 0.046). Still, the results suggest that aqueous suspensions of TiO2 NPs were not acutely toxic to Artemia at elevated levels (100 mg/L), even to the most vulnerable nauplii (LC50 > 100 mg/L). The effects observed on D. magna also refer to low acute toxicity with LC50 values higher than 100 mg/L (Warheit et al. 2007; Heinlaan et al. 2008; Wiench et al. 2009; Zhu et al. 2010). Similarly, D. magna were more vulnerable under prolonged exposure as were Artemia in this study. Though, TiO2 NPs were not acutely toxic on D. magna (LC50 > 100 mg/L) in 24 h, 72-h exposure to the same size NPs resulted in about 13% mortality at 0.1 mg/L level (LC50 = 2.02 mg/L).
Table 3.
Percent mortality rates for Artemia measured for 24 h and 96 h expo sure to different suspensions of uncoated TiO2 NPs
| TiO2 NP concentration (mg/L) |
Nauplii | Adult | ||
|---|---|---|---|---|
|
| ||||
| 24 h | 96 h | 24 h | 96 h | |
| 0 | 3 | 5 | 3 | 4 |
| 10 | 4 | 13 | 3 | 10 |
| 50 | 5 | 16 | 4 | 13 |
| 100 | 6 | 18 | 5 | 14 |
Effect of concentration of TiO2 NP suspension on mortality
Average mortality measured across 10-fold concentration gradient was largely dependent on the duration of the exposure rather than the TiO2 NP concentration of the suspension (Table 3). Compared with the controls, the suspensions had no toxic effects at any concentration within 24 h, but caused mortality during 96 h. The differences among 24-h mortalities were not significant for nauplii nor for adults (p>0.05), ranging between 3 and 6% when the concentration of the suspension increased from 10 to 100 mg/L (Table 3). This effect was thought to be due to the reduced surface area and catalytic activity as the NPs aggregated or >agglomerated to micro-scale particles in solution and inside the guts. The effects on D. magna were also consistent with these results (Warheit et al. 2007; Heinlaan et al. 2008). TiO2 NP suspensions as high as 20 g/L (two orders of magnitude more concentrated than those tested here) were reported to be nontoxic to D. magna (Heinlaan et al. 2008).
Exposure for 96 h resulted in elevated mortality in all suspensions relative to the controls (p<0.05). However, the differences among the treatments were marginal that ranged from 13 to 18% for nauplii (p = 0.043) and 10 to 14% for adults (p = 0.045) when NP concentration increased from 10 to 100 mg/L. Tukey’s multiple comparisons revealed that 96-h mortality rates between the adjacent treatments (e.g., 10 and 50 mg/L, and 50 and 100 mg/L) were not statistically different (p>0.05) indicating that the concentration of the NP suspensions had marginal toxic effects on Artemia. Still though, these results imply that prolonged exposure (e.g., 96 h) increases the risk of mortality on Artemia, regardless of its state of maturity and concentration of the NP suspension. The lethal effects observed could be attributed to the failure in eliminating the aggregates of TiO2 NPs from the guts and consequently depletion of food uptake from water.
Oxidative stress induced by suspensions of TiO2 NPs
Malondialdehyde (MDA) is a natural bi-product of lipid peroxidation and a robust biomarker of oxidative stress (Pascual et al. 2003; Sayeed et al. 2003). The MDA concentrations measured from the Artemia samples are summarized in Table 4. The data clearly demonstrate that the suspensions of TiO2 NPs were totally benign to Artemia in 24 h. No significant toxicity was observed from any of the suspensions to nauplii or adults (p>0.05). In 96 h, the MDA concentrations in treatments increased compared with the controls (p<0.05) that substantiated that prolonged exposure induced oxidative stress on Artemia. In addition, the MDA levels closely correlated with the mortality rates (r2 > 0.9 for nauplii and adults) indicating that the mortalities were due to the oxidative stress. These results are consistent with those reported for D. magna (Kim et al. 2010) and marine abalone (Zhu et al. 2011). Oxidative stress induced by TiO2 NPs in a chronic exposure resulted in mortalities on D. magna (Kim et al. 2010), while marine abalone showed no significant mortality. Nevertheless, lipid peroxidation levels were found to increase in the presence of TiO2 NPs at and above 1 mg/L levels (Zhu et al. 2011).
Table 4.
Oxidative stress levels associated with exposure to the suspensions of TiO2 NPs. Values are mean ± standard deviation for malondialdehyde concentration (nmol/g) in Artemia samples following 24 h and 96 h exposures
| TiO2 NP concentration (mg/L) |
Nauplii | Adult | ||
|---|---|---|---|---|
|
| ||||
| 24 h | 96 h | 24 h | 96 h | |
| 0 | 22.0 ± 0.61 | 25.4 ± 1.7 | 27.9 ± 1.2 | 30.6 ± 2.1 |
| 10 | 22.9 ± 0.33 | 41.6 ± 1.7 | 29.0 ± 1.4 | 56.2 ± 1.9 |
| 50 | 23.9 ± 0.38 | 44.1 ± 1.6 | 32.4 ± 2.8 | 60.7 ± 2.2 |
| 100 | 24.4 ± 1.05 | 44.1 ± 1.4 | 31.2 ± 2.5 | 61.2 ± 3.2 |
Lipid peroxidation levels increase with food deprivation (Pascual et al. 2003). The relationship is attributed to the increasing generation of oxygen free radicals as the antioxidant levels deplete as a result of starvation. Eventually, the symptoms exhibited by Artemia along with experimental data indicate that the suspensions of TiO2 NPs are nontoxic despite substantial accumulation. This result points to the fact that that the oxidative stress induced during prolonged exposure is associated with the impaired food uptake as a result of accumulation and deposition of TiO2 aggregates inside the guts. The lethal effects occurred during 96-h exposure could therefore be attributed to the oxidative stress caused by the deprivation from food or starvation rather than the chemical toxicity of the suspensions of TiO2 NPs.
Conclusion
In this study, we used Artemia, crustacean filter feeder, as a test model to investigate the effects of exposure to aqueous suspensions of TiO2 NPs in marine ecosystems. The results demonstrate that TiO2 NPs rapidly aggregate in saltwater to form micro-scale particles. However, the formation of large particles had no effect on the accumulation; both nauplii and adults readily accumulated the micro-scale aggregates to elevated levels such that the guts were filled with particles within 24 h. Yet, neither nauplii nor adults showed any significant mortality or oxidative stress within 24 h exposure. Thus, it was concluded that the suspensions of TiO2 NPs were nontoxic to Artemia. Extended exposure to 96 h did induce oxidative stress manifested with marginal mortality. However, these effects were most likely due to the lack of food uptake since the guts were completely filled with the aggregates of TiO2 NPs.
Acknowledgement
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS); (Contract #W912HZ-10-2-0045). The views expressed herein are those of the authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies.
Contributor Information
Mehmet Ates, Department of Chemistry and Biochemistry, Jackson State University, PO Box 17910, Jackson, MS 39217 USA.
James Daniels, Department of Chemistry and Biochemistry, Jackson State University, PO Box 17910, Jackson, MS 39217 USA.
Zikri Arslan, Department of Chemistry and Biochemistry, Jackson State University, PO Box 17910, Jackson, MS 39217 USA.
Ibrahim O. Farah, Department of Biology, Jackson State University, PO Box 18540, Jackson, MS 39217 USA
References
- Adams LK, Lyon DY, Alvarez PJJ. Comparative ecotoxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Research. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Arslan Z, Ertas N, Tyson JF, Uden PC, Denoyer ER. Determination of trace elements in marine plankton by inductively coupled plasma mass spectrometry (ICP-MS) Fresenius’ Journal of Analytical Chemistry. 2000;366:273–282. doi: 10.1007/s002160050053. [DOI] [PubMed] [Google Scholar]
- Arslan Z, Ates M, McDuffy W, Agachan MS, Farah IO, Yu WW, Bednar AJ. Probing metabolic stability of CdSe nanoparticles: alkaline extraction of free cadmium from liver and kidney samples of rats exposed to CdSe nanoparticles. Journal of Hazardous Materials. 2011;192:192–199. doi: 10.1016/j.jhazmat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnemann DW, Kholuiskaya SN, Dillert R, Kulak AI, Kokorin AI. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Applied Catalysis B: Environmental. 2002;36:161–169. [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environmental Science & Technology. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Chatterjee R. The challenge of regulating nanomaterials. Environmental Science & Technology. 2008;42:339–343. [PubMed] [Google Scholar]
- Choi H, Stathatos E, Dionysiou DD. Sol-gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Applied Catalysis B: Environmental. 2006;63:60–67. [Google Scholar]
- Choi JY, Ramachandran G, Kandlikar M. The impact of toxicity testing costs on nanomaterial regulation. Environmental Science & Technology. 2009;43:3030–3034. doi: 10.1021/es802388s. [DOI] [PubMed] [Google Scholar]
- Farkas J, Christian P, Urrea JA, Roos N, Hassellöv M, Tollefsen KE, Thomas KV. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic Toxicology. 2010;96:44–52. doi: 10.1016/j.aquatox.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Farre M, Gajda-Schrantz K, Kantiani L, Barcelo D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Analytical and Bioanalytical Chemistry. 2009;393:81–95. doi: 10.1007/s00216-008-2458-1. [DOI] [PubMed] [Google Scholar]
- Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during ecotoxicty and analysis of washing. Environmental Science & Technology. 2009;43:8113–8118. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- Handy RD, Henry TB, Scown TM, Johnson BD, Tyler CR. Manufactured nanoparticles: their uptake and effects on fish-a mechanistic analysis. Ecotoxicology. 2008;17:396–409. doi: 10.1007/s10646-008-0205-1. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environmental Science & Pollution Research. 2006;13:225–232. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- Kanwar A. Brine shrimp (Artemia salina) a marine animal for simple and rapid biological assays. Journal of Chinese Clinical Medicine. 2007;2:236–240. [Google Scholar]
- Kim KT, Klaine SJ, Cho J, Kim SH, Kim SD. Oxidative stress responses of Daphnia magna exposed to TiO2 nanoparticles according to size fraction. Science of the Total Environment. 2010;408:2268–2272. doi: 10.1016/j.scitotenv.2010.01.041. [DOI] [PubMed] [Google Scholar]
- Lovern SB, Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environmental Toxicology & Chemistry. 2006;25:1132–1137. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- Mills A, Lepre A, Elliott N, Bhopal S, Parkin IP, O’Neill S. Characterization of the photocatalyst Pilkington Activ™: a reference film photocatalyst? Journal of Photochemistry and Photobiology A: Chemistry. 2004;160:213–224. [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nunes BS, Carvalho FD, Guilhermino LM, Van Stappen G. Use of the genus Artemia in ecotoxicity testing. Environmental Pollution. 2006;144:453–462. doi: 10.1016/j.envpol.2005.12.037. [DOI] [PubMed] [Google Scholar]
- OECD Organisation for Economic Co-operation and Development (OECD) Guideline for the Testing of Chemicals (Part 202) 2004.
- Pascual P, Pedradas JR, Toribio F, Lopez-Barea J, Peinado J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata) Chemico-Biological Interactions. 2003;145:191–199. doi: 10.1016/s0009-2797(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Persoone G, Van de Vell A, Van Steertegem M, Nayer B. Predictive value for laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquatic Toxicology. 1989;14:149–166. [Google Scholar]
- Sanchez F, Sanz F, Santa-Maria A, Ros J, De Vicente M, Encinas M, Vinagre E. Barahona M. Acute sensitivity of three age classes of Artemia salina larvae to seven chlorinated solvents. Bulletin of Environmental Contamination and Toxicology. 1997;59:445–451. doi: 10.1007/s001289900498. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Raisuddin S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicology and Environmental Safety. 2003;56:295–301. doi: 10.1016/s0147-6513(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Schmidt CW. Nanotechnology-related environmental, health, and safety research examining the national strategy. Environmental Health Perspectives. 2009;117:A158–A161. doi: 10.1289/ehp.117-a158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Hohenberg H, Pflücker F, Gärtner E, Will T, Pfeiffer S. Distribution of sunscreens on skin. Advanced Drug Delivery Reviews. 2002;54:157–163. doi: 10.1016/s0169-409x(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Sorgeloos P. Availability of reference Artemia cysts. Marine Ecology Progress Series. 1980;3:363–364. [Google Scholar]
- Van Ye TM, Roza AM, Pieper GM. Inhibition of intestine lipid peroxidation does not minimize morphologic damage. Journal of Surgical Research. 1993;55:553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- Vanhaecke P, Persoone G, Claus C, Sorgeloos P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicology and Environmental Safety. 1981;5:392–387. doi: 10.1016/0147-6513(81)90012-9. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicology Letters. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, Landsiedel R. Acute and chronic effects of nano- and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere. 2009;76:1356–1365. doi: 10.1016/j.chemosphere.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Science of Total Environment. 2011;409:1444–152. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Zeynalov EB, Allen NS. Effect of micron and nano-grade titanium dioxides on the efficiency of hindered piperidine stabilizers in a model oxidative reaction. Polymer Degradation and Stability. 2006;91:931–939. [Google Scholar]
- Zhao J, Wang Z, Liu X, Xie X, Zhang K, Xing B. Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. Journal of Hazardous Materials. 2011;197:304–310. doi: 10.1016/j.jhazmat.2011.09.094. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhu L, Li Y, Qi R, Duan Z, Lang YP. Comparative toxicity of several metal oxide nano-particle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. Journal of Environmental Science and Health, Part A. 2008;43:278–284. doi: 10.1080/10934520701792779. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chang Y, Chen Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere. 2010;78:209–215. doi: 10.1016/j.chemosphere.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta) Marine Pollution Bulletin. 2011;63:334–338. doi: 10.1016/j.marpolbul.2011.03.006. [DOI] [PubMed] [Google Scholar]




