Abstract
Negative feedback immune mechanisms are essential for maintenance of hepatic homeostasis and prevention of immune-mediated liver injury. We show here that scavenger receptor A (SRA/CD204), a pattern recognition molecule, is highly upregulated in the livers of patients with autoimmune or viral hepatitis, and of mice during concanavalin A (Con A)-induced hepatitis (CIH). Strikingly, genetic SRA ablation strongly sensitizes mice to Con A-induced liver injury. SRA loss-increased mortality and liver pathology correlate with excessive production of IFN-γ and heightened activation of T cells. Increased liver expression of SRA primarily occurs in mobilized hepatic myeloid cells during CIH, including CD11b+Gr-1+ cells. Mechanistic studies establish that SRA on these cells functions as a negative regulator limiting T cell activity and cytokine production. SRA-mediated protection from CIH is further validated by adoptive transfer of SRA+ hepatic mononuclear cells or administration of a lentivirus-expressing SRA, which effectively ameliorates Con A-induced hepatic injury. We also report for the first time that CIH and clinical hepatitis are associated with the increased levels of soluble SRA. This soluble SRA displays a direct T cell inhibitory effect and is capable of mitigating Con A-induced liver pathology. Our findings demonstrate an unexpected role of SRA in attenuation of Con A-induced, T cell-mediated hepatic injury. We propose that SRA serves as an important negative feedback mechanism in liver immune homeostasis, and may be exploited for therapeutic treatment of inflammatory liver diseases.
Keywords: Liver injury, CD204, T cell, Interferon-γ, myeloid cells
Introduction
Liver diseases are associated with significant mortality and represent one of the major global health problems. Immune-mediated hepatic injury is involved in liver diseases triggered by a variety of agents, including autoimmune hepatitis, viral hepatitis, and certain drugs (1). Although pathophysiological and immunological mechanisms underlying the disease progression have not been completely understood, activation of T cells producing hepatotoxic cytokines, such as interferon-γ (IFN-γ), is a critical event in inflammatory liver diseases (2–4). Given that excessive T cell activation is commonly associated with pathology in immune-mediated liver injury, it is of critical importance to elucidate molecular pathways that counter-regulate its activity. Knowledge of these immune modulators may lead to development of novel approaches to mitigating T cell activation-induced liver diseases.
Concanavalin A (Con A)-induced hepatitis (CIH) is a well-established experimental mouse model that has been used extensively to study the pathophysiology of immune-mediated hepatic injury and various aspects of T cell-mediated liver diseases, e.g., autoimmune or viral hepatitis (5). Intravenous injection of mice with Con A induces polyclonal activation of T cells, leading to clinical and histological symptoms of acute hepatitis, including leukocyte and lymphocyte infiltration in the liver, necrosis of hepatocytes, elevated levels of alanine transaminase (ALT) activity and inflammatory cytokines (e.g., IFN-γ) (5–7).
The scavenger receptor A (SRA, also termed CD204) is expressedprimarily on myeloid cells, such as macrophages and dendritic cells (DCs) (8). This receptor is also present on liver sinusoidal endothelial cells (LSECs) (9). SRA has been shown to function asan innate pattern recognition receptor sensing a broad spectrum of ‘self’ and ‘non-self’ ligands, including modified or altered molecules, pathogen-associated molecules and apoptotic cells (8). An important role of SRA in atherosclerosis and host defense has been documented (10, 11). Studies emerging from the field of tumor immunology recently showed that SRA acts as a suppressor of antigen presentation, T cell activation and antitumor immunity by modulating the intrinsic immunogenicity of antigen-presenting cells, e.g., DCs (12–16). These findings support diverse functions of this molecule under different physiological and pathological conditions.
Our initial observation of strong upregulation of SRA in human autoimmune hepatitis and a murine model of experimental hepatitis prompted us to study a potential role of this molecule in regulation of liver injury. We show that SRA−/− mice are highly susceptible to CIH, which may be attributed to the heightened T cell activation and excessive IFN-γ production in the absence of SRA. We provide compelling evidence that SRA on mobilized myeloid cells or in a soluble form is involved in direct inhibition of T cell activity and subsequent hepatic injury. Our results uncover an essential mechanism of SRA-mediated protection of liver damage, which provides insight into liver pathophysiology and offers new opportunities to develop future therapeutic strategies.
Experimental procedures
Mice
C57BL/6J mice, SRA knockout mice (SRA−/−, backcrossed at least 12 generations to the C57BL/6J background) and IFN-γ−/−mice were obtained from the Jackson Laboratory (Bar Harbor, ME). SRA−/−IFN-γ−/− mice were created by crossbreeding SRA−/− mice and IFN-γ−/− mice. All experiments and procedures involving mice were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Patient samples
Paraffin-embedded liver tissues were obtained from patients who were clinically, biochemically, and histologically diagnosed for autoimmune hepatitis (n=8), acute viral hepatitis (n=6) or chronic type B hepatitis (n=12) between January 2008 and December 2010 in the Nanfang Hospital affiliated with Southern Medical University. The normal controls (n=6) were adjacent liver tissues obtained from patients who underwent surgery for liver cancer. Serum was obtained from healthy blood donors (n=30) at least 3 months after the last donation or from patients with infection of hepatitis B virus (n=30) during routine medical examination. All patients gave informed consent. The study was reviewed and approved by the Ethical Committee of Southern Medical University.
Cells
Liver mononuclear cells (MNCs) were prepared as described previously (17). Naïve T cells were isolated from lymph nodes using CD3 or CD8 T Cell Immunocolumns (Cedarlane Laboratories, Burlington, NC). CD4+ T cell were purified using anti-CD4-conjugated magnetic beads (#130-095-248, Miltenyi Biotec, Auburn, CA). Myeloid-derived suppressor cells (MDSCs) were purified as CD11b+ cells from the liver MNCs using anti-CD11b magnetic beads (#130-049-601, Miltenyi Biotec) as previously described (18, 19). The purity of the MDSC population was typically higher than 85%.
Reagents
SRA polyclonal Abs for immunoblotting and immunofluorescence were purchased from R&D Systems (Minneapolis, MN). SRA monoclonal Ab (2F8) for uorescence-activated cell sorter (FACS) analysis and biotin-labeled 2F8 were purchased from AbD Serotec (Raleigh, NC). Anti-human SRA polyclonal Abs were purchased from Sigma-Aldrich (St. Louis, MO). Anti-SE1 Abs were from Novus Biologicals (Littleton, CO). Mouse monoclonal Abs to CD3 (145-2C11), CD69 (H1.2F3), IFN-γ (XMG1.2), CD11b (M1/70), Gr-1 (RB6-8C5), and isotype control rat IgG1 (RTK2071) were from BioLegend (San Diego, CA). Mouse recombinant SRA and gp100 proteins were expressed in Sf21 insect cells using the BacPAK™ baculovirus expression system (Clontech, Palo Alto, CA) as previously described (20). Lentivirus-encoding SRA was packaged by co-transfecting pLVX-SRA vector with pRSV-Rev, pMD.2G and pMDLg/pRRE into Phoenix cells (21).
CIH
Age-matched mice received an intravenous (i.v.) injection of Con A (C2010, Sigma-Aldrich) at the doses of 15 mg/kg or 25 mg/kg body weight as indicated. The choice of these two different doses was based on our preliminary studies. The high dose (i.e., 25 mg/kg) was used to generate survival curves in WT and SRA−/− mice. Due to the high sensitivity of SRA−/−mice, administration of the low dose (i.e., 15 mg/kg) of Con A permits assessment of liver pathology and other parameters at different time points following Con A challenge. Liver samples were fixed in 4% paraformaldehyde, embedded in paraffin, and stained by Hematoxylin and Eosin (H&E). Alanine aminotransferase (ALT) activity in serum samples was determined using a kit from Cayman (Ann Arbor, MI). Intracellular staining for IFN-γ was performed in the presence of brefeldin A (Biolegend) as previously described (13), followed by staining with anti-CD3 and anti-IFN-γ Abs before analysis using a FACSCalibur.
MDSC suppression assay
The suppressive function of MDSCs was assessed based on their ability to inhibit CD3/CD28 engagement-induced T cell activation. Splenocytes were plated at 2×105 cells/well in 1 μg/ml of anti-CD3 Abs (2C11, eBioscience, San Diego, CA)-coated plates in the presence of 0.5 μg/ml of anti-CD28 Abs (Biolegend). Isolated MDSCs were added to the wells at different ratios. Cell proliferation was determined 72 hours later after incubating with 3H-thymidine for the last 16 hours.
In vitro T cell stimulation
For Con A-stimulated T cell proliferation, hepatic MNCs (4 × 105 per well) were cultured with 1 or 2.5 μg/ml Con A in flat-bottomed 96-well plates for 72 hours before harvesting for 3H-thymidine incorporation assays. Supernatants were collected at 48 hours for cytokine assays using an enzyme-linked immunosorbent assay (ELISA) kit.
ELISA analysis of soluble SRA
Microtiter wells (Nunc, Kamstrup, Denmark) pre-coated with 1 μg/ml of goat anti-mouse SRA polyclonal Abs (R&D system) were blocked with 1% (w/v) BSA in PBS for 1 hour, and incubated with serum diluted in PBS containing 1% BSA and 0.05% Tween 20 for 2 hours at room temperature. Plates were then incubated with 1 μg/ml of biotin-labeled rat anti-mouse SRA monoclonal Abs, followed by colorimetric assays. The soluble SRA levels in human serum samples were determined using an ELISA kit from Uscn Life Sciences Inc (Wuhan, China).
Treatment of CIH
Hepatic MNC transfer was performed as described previously (7). 5 ×106 cells in 50 μl saline were injected into the lateral left lobe of the liver at a rate of 10 μl/second prior to administration with Con A at a dose of 15 mg/kg. For treatment using SRA-expressing lentiviruses, 2×107 transducing units (TU) of viruses were delivered i.v. 5 days and 2 days before Con A injection. For treatment with SRA protein, 200 μg SRA protein was injected i.v. 1 hour before and 2 hours after Con A administration.
Statistical analysis
Samples were run in triplicate in all assays. Data are presented as mean ± S.D. The statistical analysis was conducted by the Student t-test for transaminase levels, T cell proliferation, cytokine production and soluble SRA levels. Comparison of the survival curves was performed using the Log-Rank (Mantel–Cox) test. p < 0.05 was considered significant.
Results
Hepatic SRA expression increases dramatically during human and murine liver injury
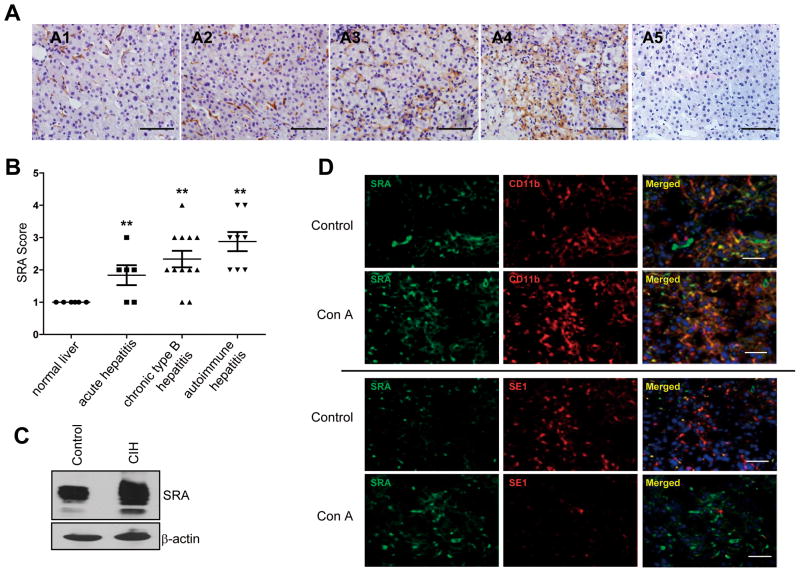
We initially assessed hepatic SRA expression in patients with autoimmune hepatitis or viral hepatitis. Immunohistochemical staining showed that the levels of SRA in the liver was highly elevated in patients with autoimmune hepatitis and chronic type B hepatitis (Fig. 1A and 1B). Similarly, hepatic SRA expression increased sharply in the model of Con A-induced experimental hepatitis, as indicated by immunoblotting (Fig. 1C) and immunofluorescence staining (Supplemental Fig. S1A).
Figure 1. Upregulation of SRA in human hepatitis and the mouse model of experimental hepatitis.
(A) Representative immunohistochemical staining of liver sections for SRA expression. (A1) normal human liver; (A2) acute viral hepatitis; (A3) chronic type B hepatitis; (A4) autoimmune hepatitis; (A5) Normal serum staining from A4. (B) Slides were examined and scored independently by the pathologists in a blinded fashion. SRA expression was quantitated using a grading system based on the intensity of staining and classified into four groups: 0 (undetected), 1 (very low), 2 (low), 3 (high), 4 (very high). p<0.01, compared to normal human liver. (C) Immunoblotting analysis of hepatic SRA expression in WT mice with or without Con A (15 mg/kg) treatment. (D). Expression pattern of SRA on myeloid cells and LSECs during CIH. Liver sections from control mice or those receiving Con A (n=5) were subjected to double staining with anti-SRA and anti-CD11b or anti-SE1 Abs. DAPI was used to counterstain nucleus (scale bar, 50 μm).
Considering that SRA is present on myeloid cells and LSECs, we next examined the expression pattern of SRA during CIH. SRA was mainly expressed on CD11b+ myeloid cells and also on LSECs to a less degree prior to Con A challenge (Fig. 1D). Similar result was obtained using FACS analysis (Supplemental Fig. S1B). The levels of CD11b+SRA+ myeloid cells in the livers elevated profoundly in response to Con A injection, whereas the SE1+ LSECs decreased substantially. The finding of diminished LSECs during CIH is consistent with the previously report on Con A-induced damage of LSECs (22). As a result, our subsequent studies focused on SRA-expressing myeloid cells.
Mice deficient in SRA are highly susceptible to CIH
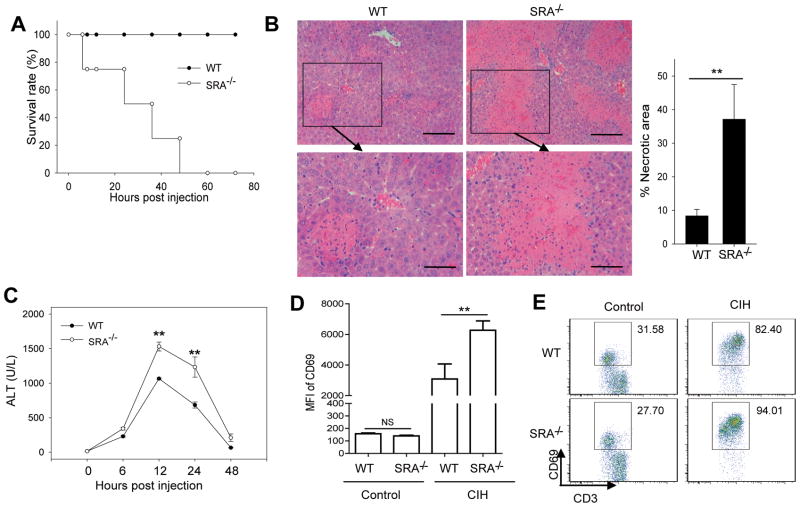
To investigate the impact of SRA on Con A-induced mortality, WT and SRA−/−mice were given a high dose of Con A (i.e., 25 mg/kg) via tail vein injection. Surprisingly, all of the SRA−/−mice died within 72 hours, whereas similarly treated WT mice survived (Fig. 2A). We also made the same observation using WT and SRA−/−littermates generated from SRA heterozygotes, i.e., SRA+/−(Supplemental Fig. S2). Intriguingly, all SRA+/−mice survived the challenge with such a high dose of Con A, suggesting that a single allele of SRA gene is able to afford protection. To evaluate the effect of SRA on liver injury, mice received 15 mg/kg of Con A, followed by histological and serum analysis. Con A-treated SRA−/−mice displayed significantly more hepatocyte necrosis and disseminated hemorrhage than those from WT counterparts. The necrotic area of livers from SRA−/−mice was much higher compared to that from WT mice (Fig. 2B). Sera collected from SRA−/−mice at different times points also showed significantly increased levels of ALT activity, a liver injury marker (Fig. 2C). Analysis of hepatic MNCs showed that hepatic T cells displayed a higher level of activation in SRA−/−mice than those in WT mice, as indicated by a strong elevation of CD69 expression (Fig. 2D) and a modest increase of the frequency of CD69+ T cells (Fig. 2E). Although there was an increase in hepatic leukocytes upon Con A injection, no significant difference was seen between WT and SRA−/−mice in the percentage of T, NKT, NK cells or regulatory T cells (Supplemental Fig. S3).
Figure 2. SRA ablation renders mice more susceptible to CIH.
(A) WT and SRA−/−mice (n=8) were injected with Con A at the dose of 25 mg/kg, and overall survival of mice was determined. (B) 20 hours after Con A injection (15 mg/kg), histological analysis of mouse livers (n=5) was performed using H&E staining. Bottom panels show the higher magnification views of the necrotic area. Scale bar (top), 100 μm; Scare bar (bottom), 50 μm. The percentage of necrotic area was quantitated using ImageJ software. (C) Serum ALT levels were assessed at the different time points as indicated following Con A (15 mg/kg) injection. (D, E) SRA ablation results in increased T cell activation. Mean fluorescence intensity (MFI) of CD69 on T cells and the percentage of CD69+ T cells before and after Con A injection is assessed by FACS analysis of liver MNCs. ** p<0.01; NS, not significant. Data are representative of three independent experiments with similar results.
Hypersensitivity of SRA−/−mice to CIH is associated with excessive IFN-γ production
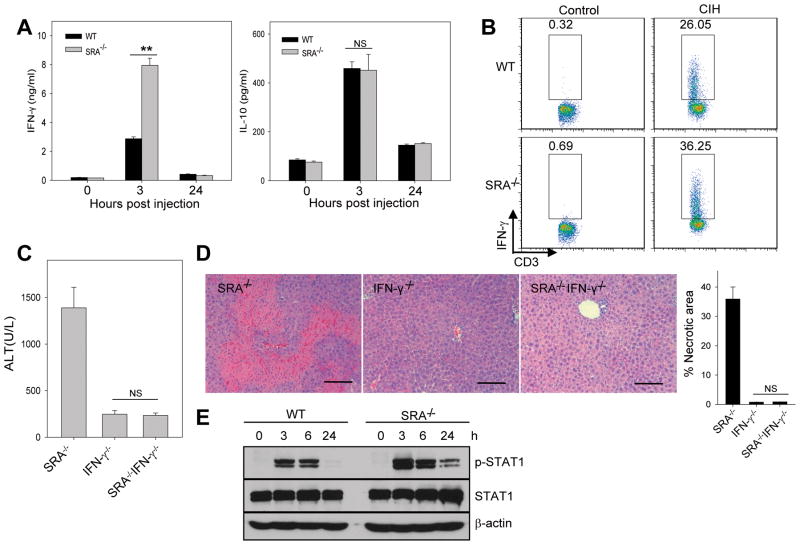
Compared with WT mice, Con A-treated SRA−/−mice showed significantly higher levels of IFN-γ, not IL-10 (Fig. 3A). Con A injection also resulted in a marked increase in the frequency of IFN-γ-producing T cells infiltrating the livers of SRA−/−mice (Fig. 3B). We further showed that IFN-γ−/−and SRA−/−IFN-γ−/−mice were protected from CIH, as indicated by the serum levels of ALT (Fig. 3C) and liver pathology (Fig. 3D). In addition, enhanced STAT1 phosphorylation at Tyr701 was observed in the liver of Con A-treated SRA−/−mice (Fig. 3E). These data suggest that excessive production of IFN-γ (6, 23) and subsequent STAT1 activation (24) associated with the SRA loss may contribute to increased hepatic injury.
Figure 3. SRA ablation leads to increased IFN-γ production during CIH.
(A) Serum levels of IFN-γ and IL-10 were determined at 3 and 24 hours after Con A (15 mg/kg) injection to mice (n=5). (B) Increased frequency of IFN-γ-producing T cells, analyzed 14 hours after Con A injection using intracellular cytokine staining. (C, D) IFN-γ deficiency abolishes SRA loss-promoted CIH. SRA−/−, IFN-γ−/−and SRA−/−IFN-γ−/−mice (n=3) were injected with Con A. ALT levels (C) and liver histology (D) were examined 20 hours after Con A injection (scale bar, 100 μm). Necrotic area was quantitated and calculated based on at least 30 random fields from the liver samples of three mice. (E) Increased STAT-1 activation in the livers from Con A-treated SRA−/−mice. Levels of phospho-STAT1 and total STAT1 protein were analyzed using immunoblotting. ** p<0.01; NS, not significant. The data shown represent two independent experiments.
SRA loss impairs the immunosuppressive activity of hepatic CD11b+Gr-1+ MDSCs
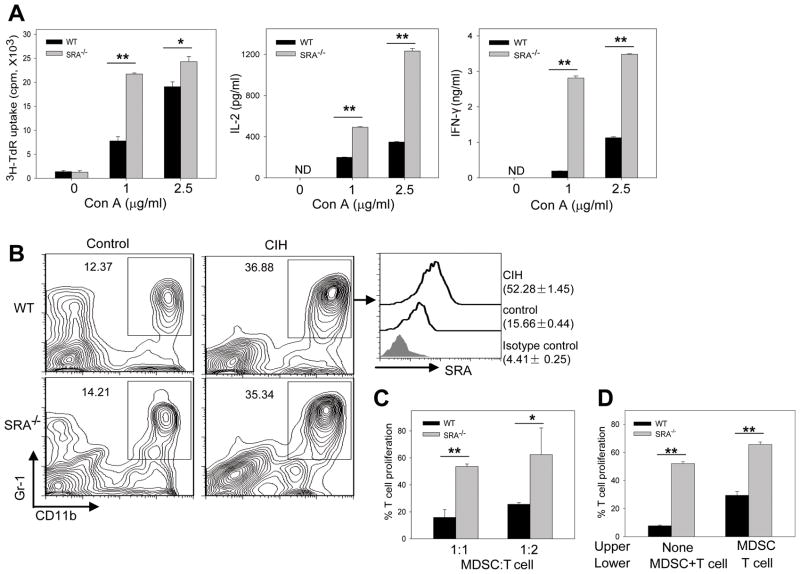
Hepatic MNCs contain a significant number of T cells and SRA-expressing myeloid cells. Upon stimulation with Con A, the MNCs from SRA−/−mice showed a more robust proliferation, and produced significantly more IL-2 and IFN-γ than those from WT mice (Fig. 4A).
Figure 4. Upregulation of SRA on hepatic CD11b+Gr-1+ MDSCs correlates with their immunosuppressive activity during CIH.
(A) SRA−/−hepatic MNCs are more responsive to Con A stimulation. Hepatic MNCs (4 × 105 per well) were cultured with Con A at different concentrations. T cell proliferation and cytokine levels were determined by 3H-thymidine incorporation and ELISA assays, respectively. Data are representative of three independent experiments. ** p<0.01; * p<0.05; ND, not detectable. (B) Elevation of SRA expression on hepatic CD11b+Gr-1+ myeloid cells. Hepatic MNCs from WT and SRA−/−mice (n=5) were stained for CD11b and Gr-1, followed by FACS analysis (left). SRA expression on CD11b+Gr-1+ cells from WT mice, as indicated by MFI, was also examined (right). (C) SRA absence on hepatic MDSCs reduces their immunosuppressive activity. Splenocytes were stimulated with anti-CD3/CD28 Abs in the presence of hepatic MDSCs isolated from Con A-injected WT and SRA−/−mice. (D) SRA-mediated immune suppression by MDSCs requires interaction with T cells. T cells were co-cultured with MDSCs in the lower chambers of the transwell plates, or separated from MDSCs by seeding MDSCs to the upper chambers. T cell proliferation in the absence of MDSC in (C and D) was set as 100 %. * p<0.05; ** p<0.01. All samples were run in triplicate. Data shown represent two independent experiments.
The CD11b+Gr-1+ MDSCs were recently reported to play a regulatory role in the murine models of experimental autoimmune hepatitis (19, 25) and chronic hepatitis B virus infection (26). We found that Con A administration resulted in a similar accumulation of hepatic MDSCs in WT and SRA−/−mice (Fig. 4B). However, surface expression of SRA was highly elevated on hepatic MDSCs in WT mice (Fig. 4B).
Interestingly, hepatic MDSCs from WT mice suppressed T cell activation more efficiently than those from SRA−/−mice (Fig. 4C). In the transwell assays, separation of hepatic MDSCs from responder T cells reduced the inhibitory effect of WT and SRA−/−MDSCs. However, interference with MDSC-T cell contact abrogated the suppressive activity of WT MDSCs more dramatically than SRA−/−MDSCs, as indicated by the extent of reversal of MDSC-induced T cell inhibition (3.86±0.59 fold difference versus 1.26±0.06 fold difference) (Fig. 4D).
Elevation of hepatic SRA expression reduces the severity of CIH
To determine whether SRA-expressing hepatic myeloid cells were able to reduce Con A-induced liver injury in SRA−/−mice, hepatic MNCs isolated from WT mice were injected into SRA−/−mice. Transfer of SRA+ hepatic MNCs ameliorated Con A-induced liver injury (Fig. 5A and 5B). Quantitative analysis indicates that approximately 42.11% of the liver was necrotic in Con A-treated SRA−/−mice compared to 7.92% in those receiving SRA+ hepatic MNCs (Fig. 5A).
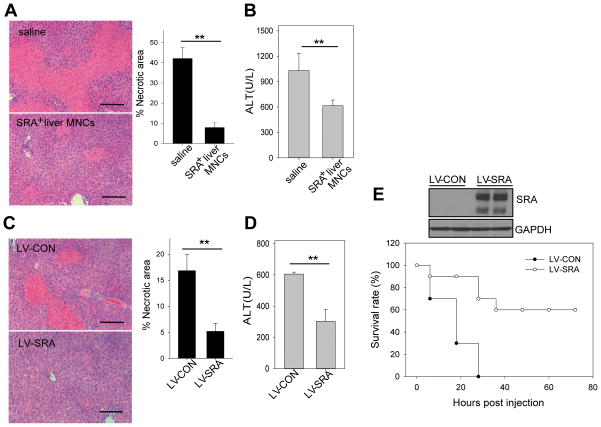
Figure 5. Increased levels of hepatic SRA expression protect mice from CIH.
(A, B) Adoptive transfer of SRA+ hepatic MNCs ameliorates CIH in SRA−/−mice. SRA−/−mice (n=5) were injected with WT hepatic MNCs (5×106 cells) 2 hours before Con A (15 mg/kg) injection. Mice were examined 20 hours later for liver histology (A) and serum ALT levels (B). (C, D) Lentiviral-mediated overexpression of SRA reduces CIH in WT mice. WT mice (n=5) were i.v. injected with 2×107 TU LV-CON or LV-SRA 6 days and 3 days before Con A (15 mg/kg) administration. Liver histology (C) and ALT levels (D) were examined. Scale bar, 100 μm. (E) Enhanced survival of Con A-challenged SRA−/−mice by upregulation of hepatic SRA. SRA−/−mice (n=10) were injected with LV-SRA or LV-CON before Con A (25 mg/kg) administration. Lentivirus-mediated hepatic SRA expression was examined prior to Con A injection by immunobloting. ** p<0.01. Data shown are representative of three independent experiments.
To validate the protective effect of SRA, we constructed SRA-expressing lentiviruses (Supplemental Fig. S4A). While hepatocyte necrosis was prominent in WT mice injected with Con A (15 mg/kg), such damage was markedly reduced in mice receiving SRA-expressing lentiviruses (Fig. 5C), which was associated with decreased serum ALT activity (Fig. 5D) and increased hepatic SRA expression (Supplemental Fig. S4B).
Considering that 25 mg/kg of Con A has been defined as the lethal dose for SRA−/−mice during our studies, we further examined whether SRA-expressing viruses could reduce the mortality of SRA−/−mice. As expected, all LV-CON-treated mice succumbed to challenge. In contrast, 60% of LV-SRA-treated mice survived (Fig. 5E), which positively correlated with hepatic SRA expression. Immmunofluorescence staining showed that lentiviruses-delivered SRA was expressed on both CD11b+ myeloid cells and SE1+ LSECs (Supplemental Fig. 4C).
CIH and clinical hepatitis is associated with increased levels of soluble SRA
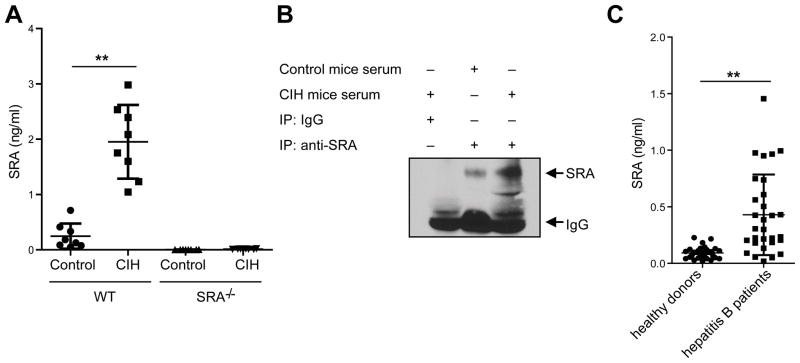
We made a surprising finding that Con A-treated WT mice showed a substantial increase in the serum levels of SRA compared with untreated controls (Fig. 6A). The specificity of anti-SRA Abs in detecting soluble SRA was confirmed using sera from SRA−/−mice with or without Con A treatment (Fig. 6A). The increase of soluble SRA in response to Con A administration was further validated using immunoprecipitation assays (Fig. 6B).
Figure 6. Liver injury is associated with soluble SRA.
(A) WT mice (n=8) were injected with Con A (15 mg/kg). Serum levels of SRA were measured 24 hours later by ELISA. Sera collected from SRA−/−mice before and after Con A injection were used as negative controls. (B) The presence of SRA in sera was confirmed by immunoprecipitation assays using anti-SRA Abs. ** p<0.01. Data represent three independent experiments. (C) Presence of soluble SRA in patients with hepatitis B virus (n=30) compared to healthy donors (n=30).
We subsequently addressed the question as to whether such a soluble form of SRA existed in patients with hepatitis. ELISA analysis of the sera from patients with hepatitis B virus (n=30) showed that the levels of soluble SRA in these patients were significantly higher than that in healthy donors (Fig. 6C).
Extracellular SRA protein exhibits T cell inhibitory activity in vitro
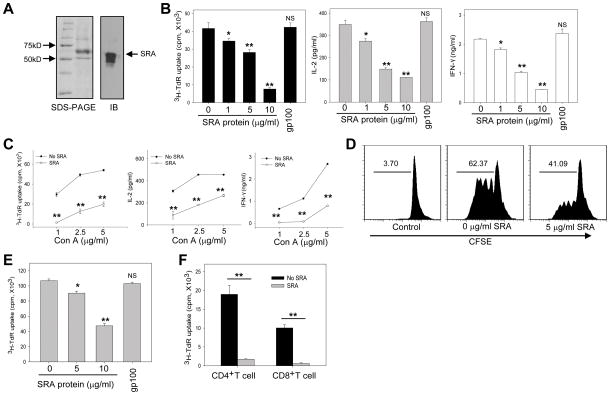
To further examine the ability of SRA to suppress T cell activation during CIH, we prepared recombinant SRA protein using the baculovirus-insect cell system (Fig. 7A). SRA protein inhibited hepatic MNCs proliferation and cytokine (IL-2 and IFN-γ) production stimulated by Con A in a dose-dependent manner (Fig. 7B). In contrast, gp100 protein that was prepared in the same fashion had little effect on T cells, indicating the specificity of the inhibitory activity of SRA (Fig. 7B). SRA protein also reduced hepatic T cell activation when stimulated with different concentrations of Con A (Fig. 7C). The result was also confirmed by an independent proliferation assay of CFSE-labeled CD3+ T cells (Fig. 7D).
Figure 7. Extracellular SRA protein inhibits T cell activation.
(A) Preparation of recombinant SRA protein using baculovirus-insect cell system, analyzed by SDS-PAGE and immunoblotting. (B) Reduced hepatic MNC proliferation by SRA protein. 4×105 purified MNCs were stimulated with Con A (2.5 μg/ml) in the presence of indicated concentrations of SRA protein. Cell proliferation and cytokine production were determined. gp100 protein was used as an irrelevant protein control. (C) Effect of extracellular SRA (10 μg/ml) on hepatic MNCs proliferation-simulated by Con A at different concentrations. (D) Inhibition of Con A-induced T cell proliferation by SRA protein. CFSE (5 μM)-labeled hepatic MNCs were stimulated with Con A in the presence of SRA protein for 72 hours. T cell proliferation was assessed by FACS analysis of CD3+ T cells based on the dilution of CFSE intensity. Representative histograms from two independent experiments are shown. (E) Purified T cells were stimulated with anti-CD3/CD28 Abs in the presence of SRA protein, followed by 3H-thymidine incorporation assays. (F) Proliferation of purified CD4+ or CD8+ T cells in the presence of SRA protein. * p<0.05; ** p<0.01; NS, not significant, compared to the group without SRA protein. All samples were run in triplicate. Data shown represent three independent experiments.
In addition, the presence of SRA protein in the extracellular space resulted in a reduction in T cell proliferation, assayed by 3H-thymidine incorporation (Fig. 7E) and IL-2 production (data not shown) triggered by anti-CD3/CD28 Abs in a dose-dependent manner. The inhibitory effect of SRA on T cell activation engaged by T cell receptor was similarly observed when purified CD4+ or CD8+ T cell subsets were used (Fig. 7F).
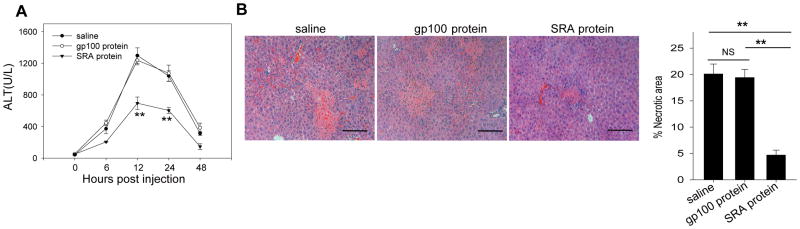
SRA protein ameliorates Con A-induced hepatic injury
To directly examine the potential effect of soluble SRA in vivo, WT mice were injected with recombinant SRA protein or gp100 protein as an irrelevant protein control prior to Con A administration. In contrast to saline or gp100-treated mice, mice receiving SRA protein showed markedly reduced serum ALT levels (Fig. 8A) and hepatic pathology (Fig. 8B). Interestingly, SRA protein also decreased the severity of tissue damage in the spleen after Con A injection (Supplemental Fig. S5), indicating a systemic effect of SRA treatment.
Figure 8. Soluble SRA protein partially protects mice from CIH.
WT mice (n=5) received two doses of SRA protein (200 μg) 1 hour before and 2 hours after Con A (15 mg/kg) injection. gp100 protein was used as an irrelevant protein control. Serum ALT (A) and histology of liver (B) were examined 20 hours following Con A administration. Quantitative data of the percentage of necrotic area are also presented. Scale bar, 100 μm. ** p<0.01. Data are representative of at least three independent experiments.
Discussion
Although emerging evidence indicates that SRA regulates inflammation and immune responses, the potential role of SRA in immune-mediated liver injury has not been determined. We are the first to report that SRA on hepatic myeloid cells is essential for maintenance of liver resistance against CIH. We have provided mechanistic insights into the SRA actions by demonstrating direct T cell inhibitory effects of SRA, which extends the previously documented role of SRA in limiting the immunostimulating activity of antigen-presenting cells (e.g., DCs). Our studies reveal a previously unknown feature of SRA functions in liver homeostasis, i.e., increased expression and release of SRA represents a novel mechanism involved in protection from Con A-induced hepatic injury.
Several lines of clinical and preclinical evidence support the connection between SRA and liver diseases. Hepatic SRA expression dramatically increases in patients with autoimmune hepatitis and viral hepatitis, which is mirrored by substantial elevation of liver SRA levels in the mouse model of hepatitis. We demonstrate for the first time that SRA−/−mice are much more susceptible to CIH than their WT counterparts, as evidenced by sharply reduced survival rate and increased liver pathology. The protective effect of SRA in CIH is validated using independent experimental systems, including adoptive transfer of SRA-expressing MNCs and lentivirus-mediated SRA overexpression in the liver. Increased hypersensitivity to CIH by the SRA ablation correlates with excessive IFN-γ production and enhanced STAT1 phosphorylation in the liver, which are known to be major contributors to CIH (6, 23, 27). Therefore, the discrepancy in the severity of CIH in WT and SRA−/−mice may be partially attributed to the different levels of IFN-γ.
Con A administration results in a profound liver infiltration by immunosuppressive CD11b+Gr-1+ MDSCs (25). This may be a natural mechanism to limit inflammation following Con A treatment. We show that SRA expression is strongly induced on these mobilized hepatic MDSCs during CIH, even though SRA ablation does not appear to alter the infiltration of these cells. Strikingly, lack of SRA on hepatic MDSCs reduced their immunosuppressive activity, suggesting that SRA may directly participate in the immunosuppressive action of hepatic MDSCs. Supporting evidence also came from the transwell study, in which disrupting SRA interactions with T cells led to a partial reversal of hepatic MDSC-mediated immune suppression. Another important aspect of our work is the comprehensive studies demonstrating that extracellular SRA exhibits T cell-suppressive activity, which provide direct evidence supporting the suppressive functions of SRA itself during contact with T cells. However, it remains to be defined as to whether SRA regulates MDSC functions via other mechanisms (e.g., altering cytokine expression).
Our study confirms that SRA is also present on LSECs, even though to a less extent, when compared with myeloid cells. Based on our findings that Con A-injection results in a marked reduction in the levels of LSECs, and concurrent elevation of hepatic SRA-expressing MDSCs with immunosuppressive activity, we propose that myeloid cell-associated SRA plays a significant role in protecting mice from CIH. Nonetheless, involvement of SRA-expressing LSECs in this process, especially in the early phase of response cannot be excluded.
An unexpected discovery in this study is that Con A administration results in high levels of soluble SRA. To our knowledge, it is also the first report of the presence of SRA in a soluble form in patients with infection of hepatitis B viruses. It is possible that soluble SRA molecules are shed by myeloid cells or other SRA-expressing cells (e.g., LSECs) to the circulation system in response to Con A-induced inflammation and tissue damage. An increase in the levels of soluble SRA in vivo may represent a novel marker of inflammation and injury. However, the molecular mechanism underlying the release of soluble SRA remains to be determined.
SRA upregulation on myeloid cells (e.g., MDSCs) and its release into the circulation during CIH represents a negative feedback mechanism for preventing excessive immune activation and cytokine (e.g., IFN-γ) production. It is possible that SRA executes its direct T cell suppressive functions via both membrane-associated form and soluble form. Engaging these dual mechanisms is expected to dampen T cell activity and reduce tissue damage more efficiently. We speculate that membrane-bound SRA preferentially limits activation of local T cells that have been recruited to the inflamed organs (e.g., liver), while soluble SRA in the circulation facilitates systemic tolerance in the peripheral or distant sites.
Our discovery of SRA as a suppressor of liver injury bodes well with recently reported SRA actions in attenuating the intrinsic immunogenicity of antigen-presenting cells (e.g., DCs) exposed to inflammatory signals and subsequent T cell response (13, 16, 21). In contrast, our findings in the current study emphasize a direct T cell regulatory effect of SRA on myeloid cells or in the extracellular space during immune activation (e. g., CIH), which provides new insights into the SRA functions as a novel immune modulator.
In summary, interference of SRA-mediated regulatory pathways results in hypersensitivity of mice to Con A-induced, T cell-mediated hepatic injury. Increased SRA expression on hepatic myeloid cells and secretion of soluble SRA are important for restraining T cell activation and inflammatory liver pathology. Since similar immune-mediated mechanisms are critical in autoimmune and viral hepatitis, these results are relevant for the pathogenesis of liver injury in humans. These findings substantiate the concept that SRA is a key regulator of liver homeostasis, thus, making it a potential target for modulation in the clinical settings of immune-mediated liver injury.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health (NIH) Grants CA154708, CA129111, American Cancer Society Scholarship (X.Y.W), NCI Cancer Center Support Grants to VCU Massey Cancer Center P30 CA16059, and Natural Science Foundation of China 30972679 (Z.C.). X.Y.W is a Harrison Endowed Scholar at the VCU Massey Cancer Center.
Abbreviations
- ALT
alanine transaminase
- Con A
Concanavalin A
- CIH
Con A-induced hepatitis
- CD
clusters of differentiation
- DC
dendritic cells
- LSECs
liver sinusoidal endothelial cells
- MDSC
myeloid-derived suppressor cell
- MNC
mononuclear cell
- CFSE
5-(and-6)- carboxyfluorescein diacetate, succinimidyl ester
- IL
interleukin
- IFN-γ
interferon gamma
- STAT1
signal transducer and activator of transcription
- ELISA
enzyme-linked immunosorbent assay
References
- 1.Dienes HP, Drebber U. Pathology of immune-mediated liver injury. Dig Dis. 2010;28:57–62. doi: 10.1159/000282065. [DOI] [PubMed] [Google Scholar]
- 2.Lohr HF, Schlaak JF, Gerken G, Fleischer B, Dienes HP, Meyer zum Buschenfelde KH. Phenotypical analysis and cytokine release of liver-infiltrating and peripheral blood T lymphocytes from patients with chronic hepatitis of different etiology. Liver. 1994;14:161–166. doi: 10.1111/j.1600-0676.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane IG. Pathogenesis of autoimmune hepatitis. Biomed Pharmacother. 1999;53:255–263. doi: 10.1016/S0753-3322(99)80096-1. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B. Intrahepatic T cells in hepatitis B: viral control versus liver cell injury. J Exp Med. 2000;191:1263–1268. doi: 10.1084/jem.191.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 9.Hansen B, Arteta B, Smedsrod B. The physiological scavenger receptor function of hepatic sinusoidal endothelial and Kupffer cells is independent of scavenger receptor class A type I and II. Mol Cell Biochem. 2002;240:1–8. doi: 10.1023/a:1020660303855. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 11.Areschoug Thomas, Gordon Siamon. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cellular Microbiology. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007;67:4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- 13.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, Kim HL, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–5828. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, Manjili MH, et al. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T cell response and antitumor immunity. Cancer Res. 2011;71:6611–6620. doi: 10.1158/0008-5472.CAN-11-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, Kane JM, 3rd, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J Immunol. 2011;187:2905–2914. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R, Tian Z, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. J Immunol. 2004;172:5648–5655. doi: 10.4049/jimmunol.172.9.5648. [DOI] [PubMed] [Google Scholar]
- 18.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 19.Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52:1350–1359. doi: 10.1002/hep.23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XY, Yi H, Yu X, Zuo D, Subjeck JR. Enhancing antigen cross-presentation and T-cell priming by complexing protein antigen to recombinant large heat-shock protein. Methods Mol Biol. 2011;787:277–287. doi: 10.1007/978-1-61779-295-3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, Subjeck JR, et al. Pattern pecognition scavenger receptor CD204 attenuates toll-like receptor 4-induced NF-{kappa}B activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem. 2011;286:18795–18806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, et al. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824–829. doi: 10.1002/hep.510240413. [DOI] [PubMed] [Google Scholar]
- 23.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 24.Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. doi: 10.1371/journal.pone.0018281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol. 2011;166:134–142. doi: 10.1111/j.1365-2249.2011.04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.