Abstract
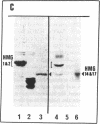
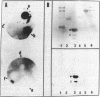
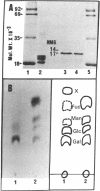
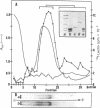
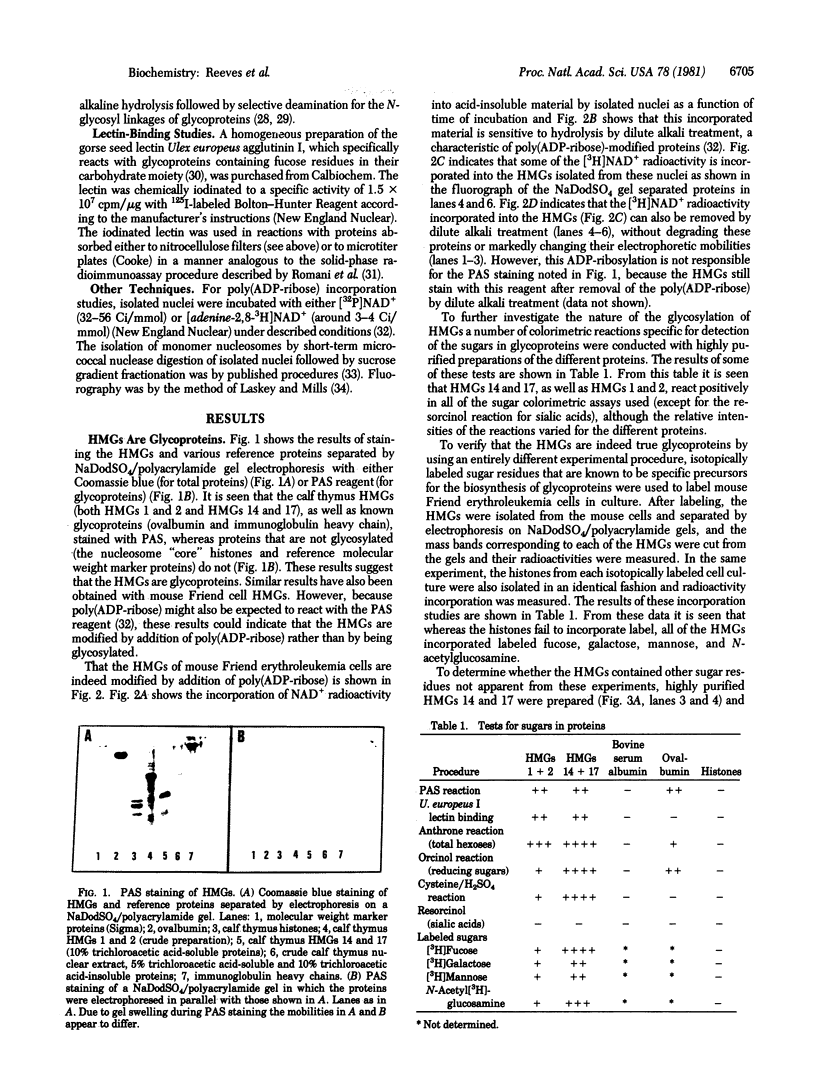
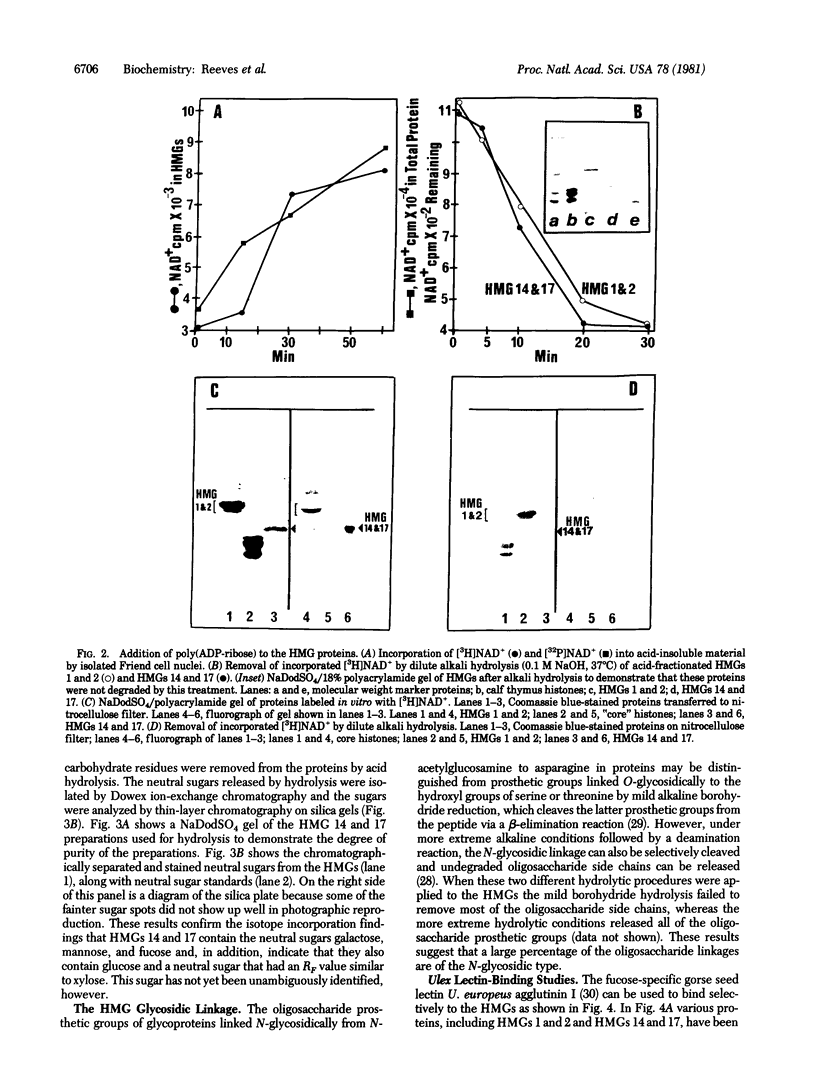
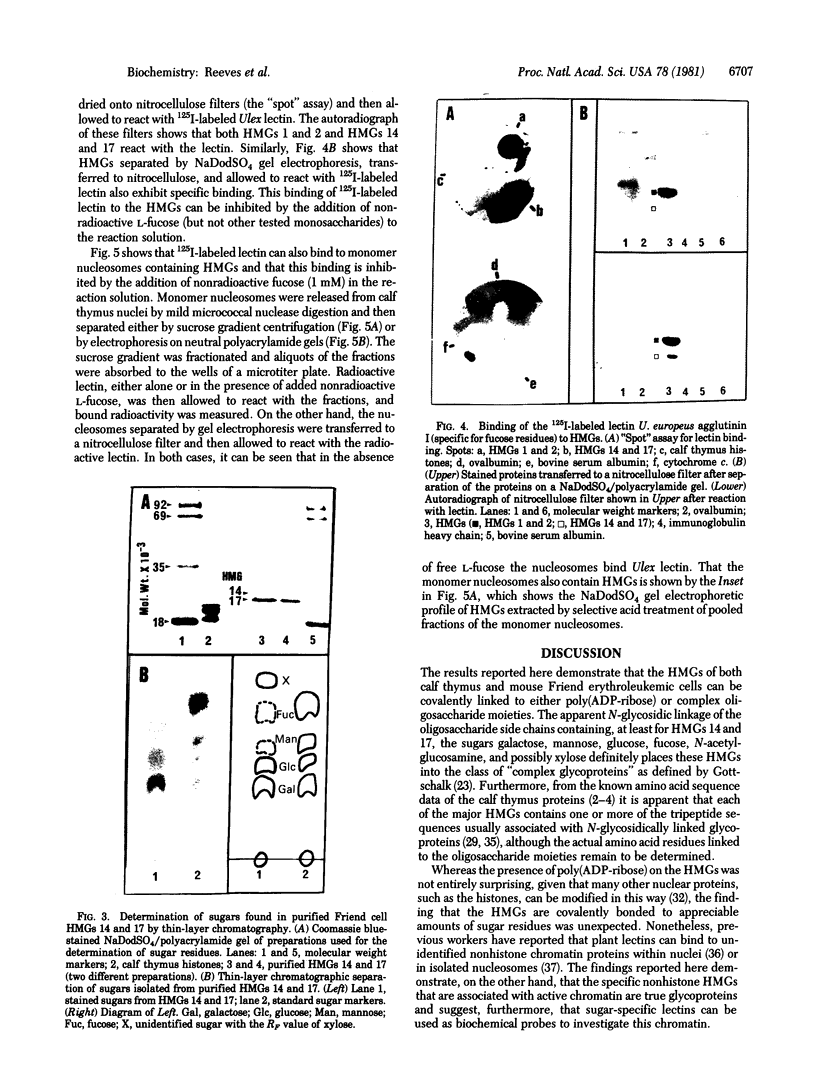
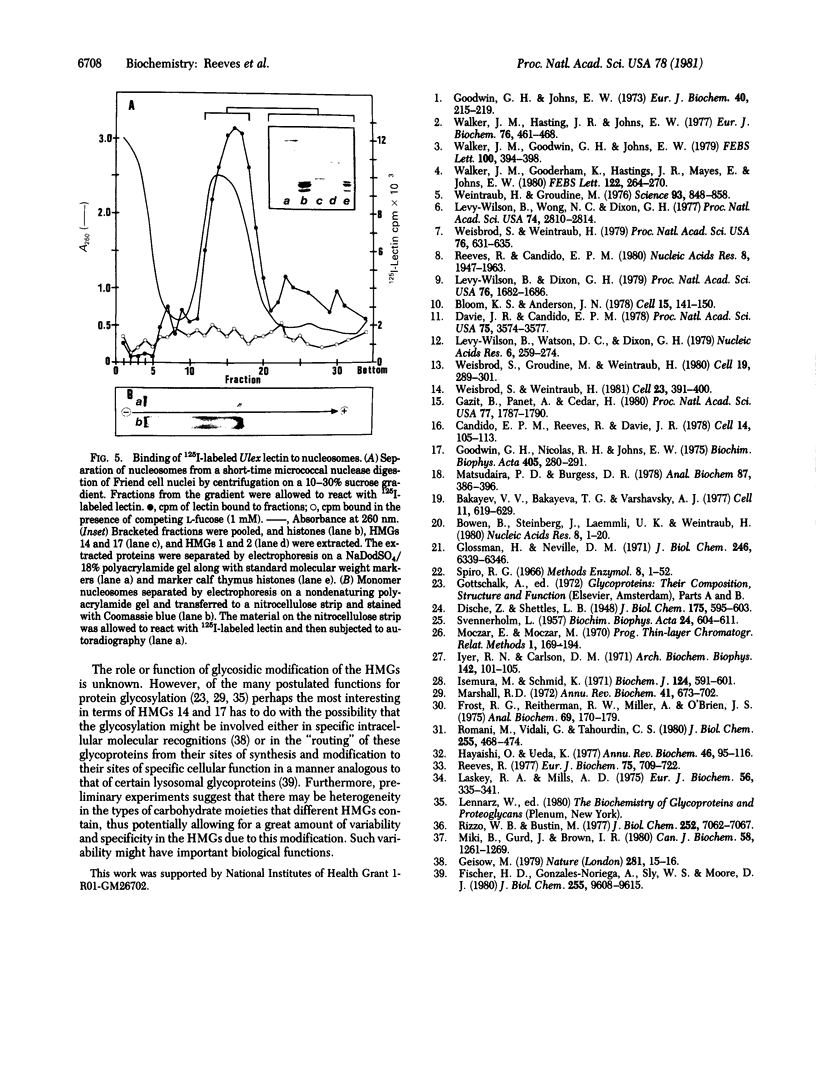
This paper reports the results of numerous biochemical analyses which indicate that the "high mobility group" proteins (HMGs) of mouse and bovine cells are bona fide glycoproteins and can, in addition, be modified by poly(ADP-ribose) addition in vitro. The sugars N-acetylglucosamine, mannose, galactose, glucose, fucose, and one unknown sugar (possibly xylose) have been identified in purified preparations of HMGs 14 and 17. Furthermore, the fucose-specific lectin Ulex europeus agglutinin I bound both to the isolated HMGs and to monomer nucleosomes containing HMGs released from "active chromatin" by micrococcal nuclease digestion. Selective alkaline borohydride reductive cleavages of the HMGs suggested that the oligosaccharide prosthetic groups are primarily bound to these proteins by N-glycosidic linkages. The unexpected finding that the HMGs contain covalently bound complex carbohydrate moieties allows for a potentially great amount of variability and specificity in these proteins that may have important biological implications.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Frost R. G., Reitherman W., Miller A. L., O'Brien J. S. Purification of Ulex europeus hemagglutinin I by affinity chromatography. Anal Biochem. 1975 Nov;69(1):170–179. doi: 10.1016/0003-2697(75)90578-3. [DOI] [PubMed] [Google Scholar]
- Gazit B., Panet A., Cedar H. Reconstitution of a deoxyribonuclease I-sensitive structure on active genes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1787–1790. doi: 10.1073/pnas.77.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. Sugars and intracellular recognition. Nature. 1979 Sep 6;281(5726):15–16. doi: 10.1038/281015a0. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973 Dec 3;40(1):215–219. doi: 10.1111/j.1432-1033.1973.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Isemura M., Schmid K. Studies on the carbohydrate moiety of 1 -acid glycoprotein (orosomucoid) by using alkaline hydrolysis and deamination by nitrous acid. Biochem J. 1971 Sep;124(3):591–604. doi: 10.1042/bj1240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R. N., Carlson D. M. Alkaline borohydride degradation of blood group H substance. Arch Biochem Biophys. 1971 Jan;142(1):101–105. doi: 10.1016/0003-9861(71)90263-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Watson D. C., Dixon G. H. Multiacetylated forms of H4 are found in a putative transcriptionally competent chromatin fraction from trout testis. Nucleic Acids Res. 1979 Jan;6(1):259–274. doi: 10.1093/nar/6.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Gurd J. W., Brown I. R. Characterization of mononucleosomes and associated glycoproteins from Ehrlich ascites tumour cells. Can J Biochem. 1980 Nov;58(11):1261–1269. doi: 10.1139/o80-169. [DOI] [PubMed] [Google Scholar]
- Reeves R., Candido E. P. Partial inhibition of histone deacetylase in active chromatin by HMG 14 and HMG 17. Nucleic Acids Res. 1980 May 10;8(9):1947–1963. doi: 10.1093/nar/8.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo W. B., Bustin M. Lectins as probes of chromatin structure. Binding of concanavalin A to purified rat liver chromatin. J Biol Chem. 1977 Oct 25;252(20):7062–7067. [PubMed] [Google Scholar]
- Romani M., Vidali G., Tahourdin C. S., Bustin M. Solid phase radioimmunoassay for chromosomal components. J Biol Chem. 1980 Jan 25;255(2):468–474. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Gooderham K., Hastings J. R., Mayes E., Johns E. W. The primary structures of non-histone chromosomal proteins HMG 1 and 2. FEBS Lett. 1980 Dec 29;122(2):264–270. doi: 10.1016/0014-5793(80)80453-4. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Goodwin G. H., Johns E. W. The primary structure of the nucleosome-associated chromosomal protein HMG 14. FEBS Lett. 1979 Apr 15;100(2):394–398. doi: 10.1016/0014-5793(79)80378-6. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Hastings J. R., Johns E. W. The primary structure of a non-histone chromosomal protein. Eur J Biochem. 1977 Jun 15;76(2):461–468. doi: 10.1111/j.1432-1033.1977.tb11616.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]