Abstract
Repeated exposure to psychostimulant drugs is associated with long-lasting changes in cognition, particularly in behavioral tasks that are sensitive to prefrontal cortex function. Adolescents may be especially vulnerable to these drug-induced cognitive changes because of the widespread adaptations in brain anatomy and function that are characteristic of normal development during this period. Here, we used a differential reinforcement of low rates of responding task in rats to determine if amphetamine exposure during adolescence would alter behavioral inhibition in adulthood. Between postnatal day 27 and 45, rats received every other day injections of saline or amphetamine (3 mg/kg). At postnatal day 125, rats were trained progressively through a series of four reinforcement schedules (DRL 5, 10, 15 and 30 sec) that required them to withhold responding for the appropriate amount of time before a lever press was reinforced. Relative to controls, amphetamine-treated rats displayed transient deficits in behavioral inhibition (i.e., decreases in efficiency ratio) that were only evident at DRL 5. In addition, they had increased responding during non-reinforced periods, which suggested increased perseveration and propensity to attribute incentive salience to reward-paired cues. Following challenge injections with amphetamine (0.25–1 mg/kg, i.p.), which were given 10 min before the start of DRL 30 test sessions, both groups exhibited dose-dependent decreases in efficiency. These results suggest that amphetamine-induced alterations in incentive-motivation and perseveration are more robust and longer-lasting than its effects on impulse control.
Keywords: Amphetamine, Adolescence, Impulsivity, Development, Psychostimulants, Prefrontal Cortex
Most drug use begins during adolescence (SAMHSA, 2011), which is generally accepted to begin at approximately 12 years of age and may extend through the early twenties (Dahl, 2004). Unfortunately, early initiation of drug use is associated with a greater likelihood of dependence (Grant & Dawson, 1997) and continued use into late adulthood (Merline, O'Malley, Schulenberg, Bachman, & Johnston, 2004). The substantial neural and behavioral development that occurs during adolescence (Sisk & Foster, 2004; Spear, 2000) likely modulates the effects of drugs of abuse taken during this time (Anderson, Bari, & Pierce, 2003). Corticolimbic regions, which include areas such as the prefrontal cortex (PFC) and nucleus accumbens (NAc), are among the last brain circuits to fully mature in both humans and rodents (Casey, Giedd, & Thomas, 2000). This could make them particularly vulnerable to the neuroadaptive effects of drugs and, in turn, contribute to the impairments in cognitive functioning that are frequently observed in drug abusers (Rogers & Robbins, 2001; Selby & Azrin, 1998).
Longitudinal studies in humans have demonstrated that development of gray matter in the PFC follows an inverted U-shape, peaking in early adolescence and declining to stable levels by adulthood (Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans, & Rapoport, 1999). In rats, this development has been characterized as a loss of neurons in the medial PFC (mPFC) during the adolescent period (Markham, Morris, & Juraska, 2007), which in rodents is defined differently by various investigators but is generally considered to encompass postnatal days (PNDs) 21 through 60 (Tirelli, Laviola, & Adriani, 2003). Additionally, studies demonstrate synaptic pruning that occurs during adolescence in the PFC (Huttenlocher & Dabholkar, 1997). In conjunction with these broad anatomical changes, neurotransmitters systems are also undergoing significant modifications during development. In the rodent PFC and NAc, for example, dopamine D1 and D2 receptors are overproduced and peak around PND 40, subsequently declining by PND 100–120 (Andersen, Thompson, Rutstein, Hostetter, & Teicher, 2000; Brenhouse, Sonntag, & Andersen, 2008). From the pre-pubertal through the adolescent period, dopaminergic projections to the PFC continue to increase and do not stabilize to adult-like levels until approximately PND 60 (Kalsbeek, Voorn, Buijs, Pool, & Uylings, 1988). Also during this time, the density of monoamine transporters in the PFC and NAc is in flux (Moll, Mehnert, Wicker, Bock, Rothenberger, Ruther, & Huether, 2000). These changes in dopamine systems may make adolescents especially vulnerable to long-lasting neuroadaptations in response to psychostimulants, such as amphetamine (AMPH), that have potent effects on extracellular levels of dopamine and other monoamines. One piece of indirect evidence for this comes from a recent study showing that adolescent rats given relatively low doses of AMPH exhibited enhanced locomotor responses (i.e., sensitization) when AMPH challenges were given in young adulthood (Mathews, Kelly, & McCormick, 2011).
One of the potential consequences of repeated psychostimulant exposure is a change in cognitive functioning. For example, human AMPH abusers exhibit deficits in PFC-sensitive tasks (Goldstein & Volkow, 2002) that assess attention (McKetin & Mattick, 1998; Ornstein, Iddon, Baldacchino, Sahakian, London, Everitt, & Robbins, 2000), behavioral inhibition (Monterosso, Aron, Cordova, Xu, & London, 2005), and decision-making (Rogers, Everitt, Baldacchino, Blackshaw, Swainson, Wynne, Baker, Hunter, Carthy, Booker, London, Deakin, Sahakian, & Robbins, 1999). Pre-clinical studies suggest similar deficits following repeated exposure to AMPH in primates (Castner, Vosler, & Goldman-Rakic, 2005) and rodents (Featherstone, Rizos, Kapur, & Fletcher, 2008; Fletcher, Tenn, Rizos, Lovic, & Kapur, 2005; Fletcher, Tenn, Sinyard, Rizos, & Kapur, 2007). Importantly, cognitive deficits in drug abusers have been shown to be related to poorer treatment outcomes (McKellar, Harris, & Moos, 2006) as well as poorer scores on skills necessary for day-to-day function (Henry, Minassian, & Perry, 2010).
Impulse control is one executive function that is mediated in large part by the PFC and its dysfunction has long been implicated in the cycle of drug dependence (Brady, Myrick, & McElroy, 1998; de Wit, 2009; Jentsch & Taylor, 1999). In psychostimulant users, impulsivity is positively correlated with amount of drug use, self-reported withdrawal, and reduced treatment compliance (Moeller, Dougherty, Barratt, Schmitz, Swann, & Grabowski, 2001). Some studies in animals have shown that repeated exposure to cocaine impairs behavioral inhibition (Winstanley, Bachtell, Theobald, Laali, Green, Kumar, Chakravarty, Self, & Nestler, 2009) and increases impulsive choice (Dandy & Gatch, 2009; Mendez, Simon, Hart, Mitchell, Nation, Wellman, & Setlow, 2010; Roesch, Takahashi, Gugsa, Bissonette, & Schoenbaum, 2007), while others have reported improved response inhibition (Krueger, Howell, Oo, Olausson, Taylor, & Nairn, 2009) or no significant changes in impulsive choice following AMPH treatment (Stanis, Marquez Avila, White, & Gulley, 2008). One study using nicotine found that pretreatment impaired impulsive action, but had no significant effects on impulsive choice (Counotte, Spijker, Van de Burgwal, Hogenboom, Schoffelmeer, De Vries, Smit, & Pattij, 2009). With one exception (Counotte, Spijker, Van de Burgwal, Hogenboom, Schoffelmeer, De Vries, Smit, & Pattij, 2009), these studies were all done in animals exposed to drugs during adulthood. Thus, it is not clear if adolescent-exposed animals would be more sensitive to drug-induced changes in impulsivity.
In the current study, we used a differential reinforcement of low rates of responding (DRL) task to determine the effects of AMPH exposure when rats were adolescents on impulse control when they matured to adulthood. In this task, rats are required to withhold an instrumental response for a specified period of time before that response is reinforced. Disruptions in DRL performance have been reported in rats exposed to AMPH in adulthood (Peterson, Wolf, & White, 2003) and in rats or mice with alterations of mPFC function induced by lesions or local depletions of dopamine (Cho & Jeantet, 2010; Nalwa & Rao, 1985; Sokolowski & Salamone, 1994). We hypothesized that, compared to controls, rats treated with AMPH during adolescence would exhibit deficits in the ability to inhibit lever press responding.
Methods
Subjects
Subjects were twenty-four male Sprague-Dawley rats born from breeders (Harlan; Indianapolis, IN, USA) maintained in our facility. On PND 23, rats were weaned and housed 2–3 per cage until PND 75, at which time they were individually housed for the duration of the experiment. Rats were kept on a 12-hour light/dark cycle (lights on at 8 AM) with water available ad libitum. Starting on PND 120, rats were food restricted and maintained at ≥ 85% of their free-feeding body weight for the duration of the experiment. Every 3–4 weeks, each rat’s target weight was increased 5% to account for normative changes in body weight that are typical in free-fed Sprague-Dawley rats. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana-Champaign, and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Apparatus
Standard operant chambers (Coulbourn Instruments; Whitehall, PA, USA) that were housed inside sound-attenuating cubicles were used in this experiment. The cubicles were equipped with fans that provided ventilation and masked extraneous noise. One wall of each operant chamber was equipped with a centrally located food trough outfitted on either side with two retractable levels that were equidistant (87 mm) from the trough. White cue lights were located above each lever and a 2.9 kHz Sonalert speaker (not used in this experiment) was located directly above the food trough. A white houselight was located near the chamber ceiling on the wall opposite the levers. Entries into the food trough were monitored using infrared detectors and a trough cue light was used to signal pellet delivery. Graphic State (v3.1; Coulbourn Instruments) was used for automated chamber control and data collection.
Saline or AMPH exposure
Rats were injected (i.p.) with either 0.9% saline or 3 mg/kg d-AMPH every other day from PND 27–45 for a total of 10 injections. Injections were given at a volume of 1 ml/kg and the dose of AMPH was calculated as the weight of the salt. For this treatment, animals were transported to a testing room, given their assigned injection, and placed for 60 min in an acrylic tub (46 × 25 × 22 cm) lined with hardwood bedding. Rats were subsequently returned to their home cage. We have previously used this treatment procedure in adult rats to demonstrate long-lasting sensitization (> 3 months) to the stereotypy-inducing effects of AMPH (Stanis, Marquez Avila, White, & Gulley, 2008).
Instrumental training
On PND 125, rats had their first of two “magazine training” sessions where they were familiarized with the process of retrieving food (45 mg pellets; Bioserv, F0021; Frenchtown, NJ, USA) from the trough. During these sessions (30 min/day), pellets were dispensed on a random time (RT) 30-sec schedule. Following magazine training, rats were trained to lever press in 30-min autoshaping sessions where the active lever (counterbalanced across groups) was inserted into the chamber on an RT 30-sec schedule. Lever press responses made within 15 sec were immediately reinforced and the lever was retracted. If no response was made in this time period, the lever was retracted and a food pellet was dispensed. These sessions continued until rats made at least 45 responses for two consecutive sessions. In cases where rats failed to make ≥ 15 lever press responses during the first three autoshaping sessions (n = 16), they were shaped manually via successive approximations. In the final phase of lever-press training, rats were reinforced on a fixed ratio 1 (FR 1) schedule of reinforcement in 60-min sessions. Once they made ≥ 300 responses in two consecutive sessions they were moved to the first DRL schedule.
DRL: Training and stable baseline
Rats were trained progressively through four schedules, starting with DRL 5. For each trial on DRL 5, the houselight was illuminated and both the active lever and an inactive lever were inserted into the chamber. Responses on the active lever that occurred after a 5-sec delay were reinforced with food pellet delivery, which coincided with a 2-sec period during which the houselight was extinguished and the cue light inside the trough was illuminated. Responses occurring before 5 sec elapsed led to a resetting of the delay timer; there were no cues indicating that an inappropriate response had been made other than the lack of reinforcement. Subsequent trials began immediately after the conclusion of the pellet-delivery period. Responses on the inactive lever had no programmed consequences. Sessions ended after 60 min or 200 reinforcements were earned, whichever came first.
In order to progress to successive schedules of DRL 10, 15 and 30, wherein responses had to be separated by 10, 15, or 30 sec, respectively, rats were required to meet a performance criterion at each schedule. This criterion had two requirements: (1) completion of at least five training sessions, and (2) at least 35% efficiency for two consecutive sessions. Efficiency was calculated as reinforced lever presses/total lever presses × 100. After rats reached DRL 30, they remained on this schedule for a total of 30 sessions before they began a series of vehicle and drug test sessions.
DRL: AMPH challenge
Ten min prior to the start of a test session at DRL 30, rats were given either vehicle (saline) or AMPH (0.25, 0.5, or 1 mg/kg, i.p.). The order of injections was VVDDDVDDD (V=saline, D=AMPH), with an injection-free daily session following each AMPH dose. The order of AMPH doses was randomly assigned using a modified Latin Square Design and all doses were tested twice. Injections were given at a volume of 1 ml/kg and the dose of AMPH was calculated as the weight of the salt.
Data Analysis
For each DRL session, we recorded the frequency of lever pressing during each 1-sec time bin (inter-response time; IRT) during the delay period and a period of equal duration following the delay. Sessions to criterion, which was defined as the second of two consecutive sessions during which rats achieved ≥ 35% efficiency, was recorded for DRL 5, 10 and 15 schedules. Using an analysis adapted from Bizot (1998), the pattern of responding during DRL 30 was categorized into “burst”, “premature”, and “timing error responses” to include lever presses occurring during 1–3 sec IRTs, 4–27 sec IRTs, and 28–30 sec IRTs, respectively. Other performance characteristics that were analyzed include percent efficiency, total lever presses, reinforced lever presses, and lever presses occurring during the 2-sec pellet delivery period (i.e., non-reinforced, non-punished responses). The latter measure, which is an indicator of perseverative responding, was calculated as a proportion of the total number of reinforcements earned during the session. This normalization procedure was used to account for individual differences in the number of reinforcements earned. These measures were analyzed using mixed-factor repeated measures ANOVAs with DRL schedule or session as the repeated measure and group (saline-exposed or AMPH-exposed) as the between-subjects factor.
For the AMPH challenge portion of the study, data from the two drug challenges at each dose were averaged to give a single value for each dose of AMPH. For vehicle tests, the data obtained during the session just prior to the first AMPH test in each series of doses were not significantly different across tests (i.e., baseline responding did not change). Thus, a single value that was the average of these sessions was used to evaluate the effects of AMPH on responding. Measures from these sessions were analyzed with mixed-factor repeated measures ANOVA with dose as the repeated measure and group as the between-subjects factor.
All statistical tests were performed with SAS (version 9.2). Alpha level was set to p < 0.05 and Fisher LSD post-hoc tests were used to further investigate significant main effects and interactions. For datasets where residuals were not normally distributed, a power transformation was performed. During the course of the study, four rats were removed due to technical complications with data collection (n = 3 saline-exposed and n = 1 AMPH-exposed).
Results
DRL: Training and stable baseline
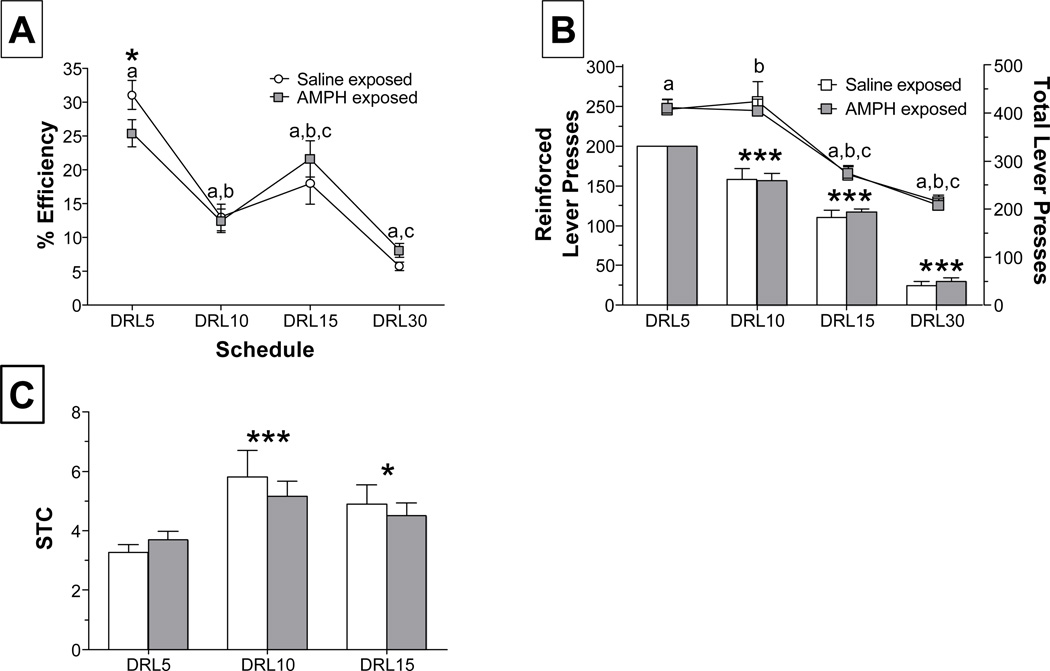
In order to assess baseline performance, measures of instrumental responding, efficiency, and sessions to criterion were analyzed. Analysis of response efficiency during the first session of each DRL schedule revealed that AMPH-treated rats performed worse relative to controls, but this was only significant at DRL 5 (Fig. 1-A). A two-way repeated measures ANOVA revealed a significant main effect of session (F3,60 = 43.4, p < 0.001) and a nearly significant interaction (F3,60=2.20, p = 0.0984). Investigation of this interaction revealed that saline-exposed rats performed better than AMPH-exposed rats at DRL 5 only (p = 0.052). Figure 1-B shows the total number of lever presses (line graph) and reinforced responses (bar graph) averaged across the fourth and fifth training session at each DRL schedule. These sessions were chosen for analysis because data are available for each rat in every group; after the fifth session, rats that met criterion were moved to the next schedule. As expected, we observed a significant decrease in response output as training progressed for both total (F3,60 = 68.8, p < 0.001) and reinforced (F3,60 = 313.8, p < 0.001) responses. There was no effect of AMPH exposure on either of these measures. In addition, the number of training sessions needed to reach criterion did not differ between groups (Fig. 1-C), but there was a significant main effect of DRL schedule (F2,41 = 9.4, p < 0.001). Sessions to criterion data are not shown for DRL 30 because all rats were trained for 30 sessions, regardless of performance.
Figure 1.
Performance across DRL schedules in rats exposed to saline or 3 mg/kg AMPH during adolescence (n = 9–13 rats/group). (A) Percent efficiency (reinforced lever presses/total lever presses×100) during the first session across all DRL schedules. Matching letters indicate significant differences between schedules, p < 0.01 ; * p = 0.052, between group (B) Lever press responses occurring after the delay period (reinforced lever presses; bar graph) and throughout the session (line graph) are presented as the mean and SEM for the fourth and fifth session at each DRL schedule. Matching letters indicate significant differences, p < 0.001; ***p < 0.001, compared to DRL 5 (C) Sessions required to reach a criterion of ≥ 35% efficiency for 2 consecutive sessions. * p < 0.05 and *** p < 0.001, vs. DRL 5.
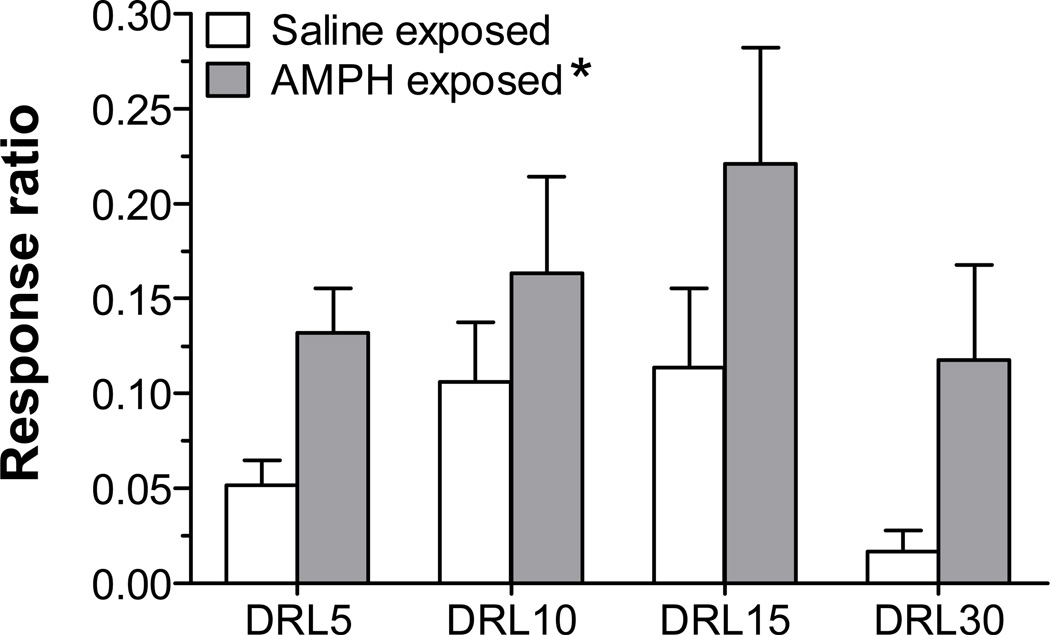
As an additional assessment of task behavior, we examined cue-associated perseverative responding by analyzing the number of responses elicited during the 2-sec pellet delivery period. During this time, both the cues paired with food delivery (houselight off, trough light on) and the food reward itself were present. As shown in Fig. 2, the rate of responding during this period differed as a function of pre-treatment group. A repeated measures 2-way ANOVA indicated a main effect of group (F1,60 = 7.3, p < 0.01), demonstrating that AMPH-treated rats had higher rates of responding at all DRL schedules.
Figure 2.
Responses made during the 2-sec pellet delivery period, which are not reinforced and provide a measure of cue-associated perseverative responding. Data are represented as response ratio (lever presses during pellet delivery/ reinforcements earned) averaged over the fourth and fifth session at each DRL schedule (n=9–13 rats/group). * p < 0.05, vs. saline-exposed rats (collapsed across DRL schedule).
DRL: AMPH challenge
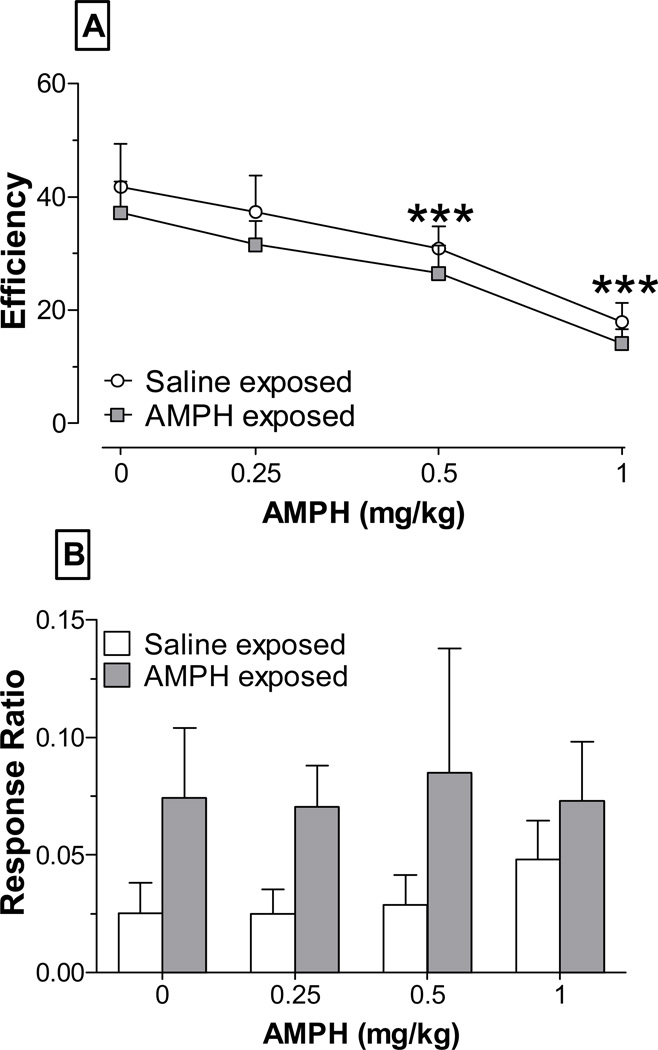
Following 30 sessions at DRL 30, rats were challenged with saline or AMPH (0.25–1 mg/kg). We found that the total number of lever presses increased, whereas reinforced lever presses decreased, as a function of increasing AMPH dose (data not shown). Consequently, AMPH led to dose-dependent decreases in percent efficiency (Fig 3-A). AMPH pre-exposure did not influence this effect, however, as ANOVA revealed only a significant main effect of dose (F3,54 = 23.2, p < 0.001).
Figure 3.
Efficiency (A) and perseverative responding (B) at DRL 30 following saline (0 mg/kg) or AMPH challenge (0.25, 0.5, and 1 mg/kg). Data are from 8–12 rats/group. *** p < 0.001, vs. saline (collapsed across group)
Consistent with results obtained during the training sessions, there were differences between the pre-treatment groups in cue-associated perseverative responding following AMPH challenge. Specifically, AMPH-exposed rats tended to have increased responding during this period at each dose of AMPH tested (Fig. 3-B). However, these group differences were not statistically significant as the overall ANOVA revealed no main effects and no interaction.
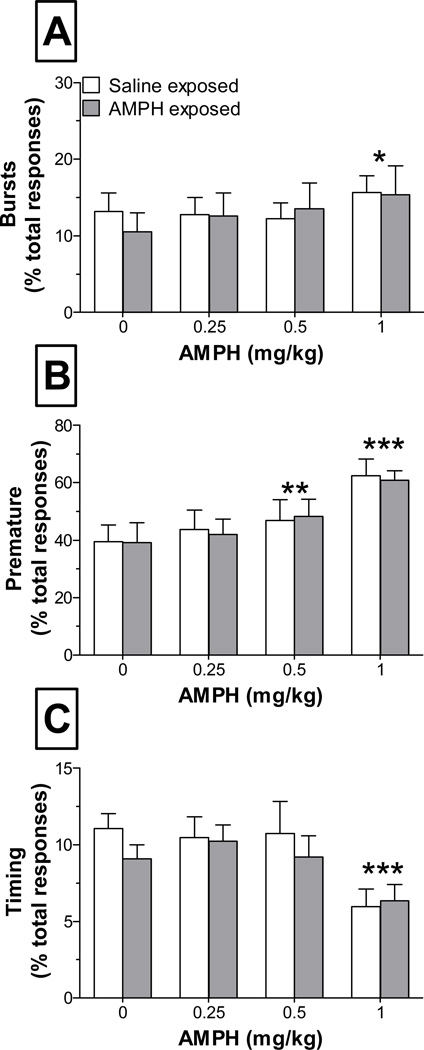
In order to assess how the temporal characteristics of lever press responding changed in response to AMPH challenge, we analyzed IRTs in three different intervals. Burst responses (1–3 sec IRTs) and premature responses (4–27 sec IRTs) significantly increased in a dose-dependent manner following AMPH challenge. Two-way repeated measures ANOVAs revealed a significant main effect of dose for both bursting (F3,54=3.1, p < 0.05; Fig. 4-A) and premature responses (F3,54 = 20.8, p < 0.001; Fig. 4-B). In contrast, timing-error responses (28–30 sec IRTs) were significantly reduced as a function of dose, as indicated by a main effect of dose (F3,54 = 6.9, p < 0.001; Fig. 4- C).There was no effect of AMPH pre-exposure on these measures, as verified by no significant main effects of group or interactions between dose and group.
Figure 4.
Temporal characteristics of responding at DRL 30 following administration of saline (0 mg/kg) or AMPH (0.25, 0.5, and 1 mg/kg). Burst responses (A) are those that occurred ≤ 3 sec following a previous response, premature responses (B) are those that occurred 4–27 sec following a previous response, and timing-error responses (C) are those that occurred 28–30 sec following a previous response (i.e., just prior to the conclusion of the delay period). *** p < 0.001, * p < 0.05 and ** p < 0.01, vs. saline (collapsed across group)
Discussion
In this study, we investigated the long-term effects of AMPH exposure during adolescence on behavioral inhibition in adulthood. We found that compared to saline-treated controls, AMPH-treated rats displayed decreases in response efficiency during the initial training at DRL 5. This deficit was ameliorated by further training, however, as there were no group differences in performance when rats were moved to DRL 10, 15 and 30. We also observed an effect of AMPH exposure on responding during the reinforcement period, which was the portion of each trial immediately following a reinforced lever press that included the three coincident events of food pellet delivery to the trough, illumination of a trough cue, and extinguishing of the house light. Lever press responses during this time, which had no programmed consequences, were significantly increased in AMPH-exposed rats, suggesting drug-induced increases in perseveration and responsiveness to stimuli with incentive motivational properties. In the final AMPH challenge test, which was done at DRL 30, rats in both groups displayed a dose-dependent impairment in performance (i.e., decreased percent efficiency). This impairment, which was characterized by a shift from timing-error responses towards bursting and premature responses, was similar in the saline- and AMPH-exposed groups. Together, these results suggest that the effects of adolescent AMPH exposure on impulsive action can be overcome with training, but that changes in perseveration and responsiveness to rewarding stimuli are longer-lasting.
Our finding of a transient effect of AMPH-exposure on impulse control is consistent with some, but not all, of the previously published studies of chronic drug exposure on impulse control. For example, Peterson et al. (2003) demonstrated that pre-treatment with 5 mg/kg AMPH for five days led to significant decreases in efficiency when rats were tested at DRL 30. Here, we only observed similar deficits in AMPH pre-exposed rats during the initial training at DRL 5. These different results may be due to the timing of AMPH exposure relative to DRL training, as rats in the Peterson et al. study were treated after they had significant experience at DRL 5, 10, 20 and 30. In addition, there are significant differences in the period between DRL testing and withdrawal (2 days compared to ~ 3 months in the current study). Our findings are consistent with previous studies demonstrating that rats exposed to cocaine during adolescence and tested after a prolonged withdrawal display drug-induced deficits that dissipate after long periods of withdrawal (Black, Maclaren, Naydenov, Carlezon, Baxter, & Konradi, 2006; Santucci, Capodilupo, Bernstein, Gomez-Ramirez, Milefsky, & Mitchell, 2004). Furthermore, the deficit we observe is consistent with alterations in mPFC functioning (Sokolowski & Salamone, 1994), which may not be surprising given the significant ongoing changes in the mPFC (Andersen et al., 2000; Kalsbeek et al., 1988; Markham et al., 2007) during the period when the rats were treated with AMPH.
There are at least two implications of our finding that rats pre-exposed to AMPH exhibit increased lever pressing during the period immediately following a reinforced response. First, the presence of reward-paired cues, which includes the stimuli associated with reward delivery (e.g., the pellet feeder motor and the sound of the pellet falling into the trough), the illumination of the trough light cue, the extinguishing of the house light, and the reward itself, may have stimulated the behavioral response associated with reward. This enhanced attribution of incentive salience to reward-related cues, and its associated increase in instrumental responding, has been previously demonstrated in rats exposed to AMPH in adulthood (Wyvell & Berridge, 2001). Other studies have demonstrated that Pavlovian conditioning is enhanced following repeated AMPH exposure in adulthood (Harmer & Phillips, 1998; Taylor & Jentsch, 2001). The present study is the first, to our knowledge, that demonstrates these effects can be seen in adult rats following drug exposure during adolescence. The attribution of incentive-salience appears to be dependent on dopamine transmission in the NAc (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011) and is sensitive to functional disruption of the NAc shell (Corbit, Muir, & Balleine, 2001). Additionally, Wan & Peoples (2008) demonstrated that repeated exposure to AMPH in adult rats led to increases in the firing rate of NAc neurons when a sucrose-associated cue was presented. The effect of AMPH on these behaviors is consistent with the known development of the NAc catecholamine system during the adolescent period in rodents (Andersen et al., 2000; Brenhouse et al., 2008; Lesting, Neddens, & Teuchert-Noodt, 2005; Tarazi, Tomasini, & Baldessarini, 1998). This explanation is also consistent with data demonstrating that rats given sensitizing doses of AMPH have higher levels of dopamine in NAc shell (Scholl, Feng, Watt, Renner, & Forster, 2009), as well as increased spine density and branching in the NAc (Robinson & Kolb, 1997). Taken together, the data suggest that repeated exposure to AMPH in adolescence enhances responsiveness to rewarding stimuli in adulthood and that this might be mediated by alterations in NAc functioning and/or dopamine transmission.
A second implication of the finding that AMPH-exposed rats continued lever pressing during the reinforcement period is that once they had initiated a successful response, these rats may have had difficulty inhibiting subsequent responses. This response perseveration may be due to long lasting, drug-induced alterations in the orbitofrontal region of the prefrontal cortex (OFC). Previous studies suggest that injury or lesions to the OFC result in perseverative responding in humans (Freedman, Black, Ebert, & Binns, 1998), primates (Clarke, Robbins, & Roberts, 2008; Dias, Robbins, & Roberts, 1996) and rodents (Bissonette, Martins, Franz, Harper, Schoenbaum, & Powell, 2008; McAlonan & Brown, 2003). Jentsch et al. (2002) demonstrated that repeated exposure to cocaine in monkeys results in perseverative responding on a reversal task, in which a previously rewarded response needs to be inhibited. Similarly, rats that initiated cocaine self-administration during adolescence displayed more deficits in an OFC-sensitive, win-shift task compared to their adult-onset counterparts (Harvey, Dembro, Rajagopalan, Mutebi, & Kantak, 2009). The existence of abnormalities in OFC-sensitive behaviors in AMPH-treated rats is also consistent with clinical studies in psychostimulant abusers that demonstrate deficits on tasks sensitive to OFC function (Rogers et al., 1999) and altered OFC glucose metabolism (Volkow, Fowler, Wolf, Hitzemann, Dewey, Bendriem, Alpert, & Hoff, 1991). Finally, it is consistent with the recent finding that there are robust changes in protein levels and markers of synaptic plasticity in the OFC following chronic atomoxetine treatment during adolescence (Sun, Cocker, Zeeb, & Winstanley, 2012).
Increases in the attribution of incentive salience and response perseveration may be two outcomes of repeated drug exposure that interact to enhance drug-seeking behavior (Jentsch & Taylor, 1999). Indeed, studies have demonstrated a role for the PFC (Lasseter, Ramirez, Xie, & Fuchs, 2009), as well as the NAc (McFarland & Kalivas, 2001), in the reinstatement of drug-seeking behavior as well as the initiation and maintenance of self-administration (Grakalic, Panlilio, Quiroz, & Schindler, 2010; Lyness, Friedle, & Moore, 1979; Roberts, Koob, Klonoff, & Fibiger, 1980). It is likely that other limbic-related, subcortical regions are also involved, given findings that the interconnected basolateral amygdala and OFC circuitry is critical for reinstatement of drug seeking (Lasseter, Wells, Xie, & Fuchs, 2011). Our findings are consistent with the model proposed by Jentsch & Taylor (1999), which suggests that deficits in impulse control (perseveration), combined with altered reward processing, may account for the transition to disordered drug intake. Although we did not assess drug seeking or other drug reward behaviors in the current study, our results lend support to this model by demonstrating the existence of long-lasting AMPH-induced alterations in impulse control and/or reward processing.
Following challenge injections with 0.25–1 mg/kg AMPH, we observed a dose-dependent decrease in percent efficiency. This result is generally consistent with previous studies (Britton & Koob, 1989; Cheng & Liao, 2007; Sable, Eubig, Powers, Wang, & Schantz, 2009; Sabol, Richards, Layton, & Seiden, 1995; Sanger, Key, & Blackman, 1974; but see Bardo, Cain, & Bylica, 2006). We did not, however, observe a differential effect of AMPH challenge in our AMPH-treated and control rats. Numerous studies have shown that acute AMPH disrupts DRL performance in control animals (Britton & Koob, 1989; Cheng & Liao, 2007; Sable et al., 2009; Sabol et al., 1995), and therefore, this finding is not surprising in light of the similar pattern of baseline performance at DRL 30 in our control and experimental groups. Given that DRL is sensitive to dopamine transmission in the mPFC (Sokolowski & Salamone, 1994), these results might suggest that AMPH challenge failed to alter dopamine levels differentially in control compared to AMPH-treated rats. While this would be consistent with microdialysis data demonstrating no differences in mPFC dopamine efflux following a challenge of 1 mg/kg AMPH in drug naïve and AMPH-sensitized rats (Peleg-Raibstein & Feldon, 2008), it is a hypothesis that would require further experiments to evaluate directly. Notably, previous studies have shown that rats pre-exposed to a high dose of methamphetamine (10 mg/kg) during PND 11–20 exhibit long-lasting changes in several markers of dopaminergic functioning that were measurable as late as PND 90 (Crawford, Williams, Newman, McDougall, & Vorhees, 2003). In addition, male rats given methamphetamine during adolescence exhibited reduced dopamine transporter immunoreactivity in the striatum and increases in cocaine-induced locomotor activity when they were tested in adulthood (McFadden, Yamamoto, & Matuszewich, 2011).
The pattern of responding following AMPH challenge shifted from late-occurring IRTs (timing-error responses) to earlier IRTs (bursting and premature responses) with increasing doses of AMPH. These changes are consistent with previous studies demonstrating that AMPH challenge shifts responding to shorter IRTs (Balster & Baird, 1979; Bizot, 1998; Sanger et al. 1974; Wiley, Compton, & Golden, 2000). Given that the greatest shift in responding was to those that were premature (4–27 sec IRTs) suggests that AMPH challenge produced alterations in timing, such that rats began to overestimate the amount of time elapsed since the previous response. This is not surprising, since alterations in timing at DRL schedules have been attributed to dopamine neurotransmission (Meck, 1996), such that dopamine agonists increase internal time estimations, while dopamine antagonists decrease internal time estimations (Maricq & Church, 1983). This is also consistent with findings that AMPH has the most robust effects on DRL performance when subjects are required to estimate the interval between responses rather than having a signaled interval (Wiley et al., 2000). The finding that the highest dose of AMPH increased burst responses (≤ 3 sec IRTs) suggests that this dose resulted in not only overestimation of time, but also reduced impulse control (Bizot, 1998).
In summary, the results of the current study suggest that AMPH-induced alterations in incentive-motivation and perseveration are more robust and longer-lasting than AMPH-induced deficits in impulse control. The interpretation that drug-induced alterations in corticolimbic circuitry were responsible for the changes in behavior observed here is consistent with findings demonstrating that these regions are still developing and making connections during the adolescent period (Andersen et al. 2000; Giedd et al., 1999; Huttenlocher & Dabholkar, 1997; Kalsbeek et al., 1988; Lesting et al., 2005; Tarazi et al., 1998). Moreover, even low doses of AMPH have the potential to induce long-lasting changes in the anatomy of brain regions such as the PFC (Diaz Heijtz, Kolb, & Forssberg, 2003). Given that these behavioral alterations are long-lasting, they could reflect a mechanism by which early initiation of drug use results in greater likelihood of transitioning to dependence. They could also provide the basis for understanding some of the cognitive deficits observed in psychostimulant abusers, such as deficits in decision-making (Rogers et al., 1999) and attention (McKetin & Mattick, 1998; Ornstein et al., 2000). For instance, it is possible that enhanced perseveration is responsible for deficits observed in decision-making tasks and that altered reward processing produces deficits in attention. It will be important in future studies to determine if the effects of AMPH we observed here are specific to adolescent exposure, as the current study did not utilize a comparison group of adult-exposed rats. Moreover, studies like this would help to elucidate the mechanisms specific to adolescent drug exposure that may contribute to the higher rates of dependence in early-onset drug abusers.
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (R01 DA029815). We thank Ben Marcus, Rakesh Marreddy, Sapan Shah, and Caitlyn Vidas for technical assistance.
References
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse (New York, N.Y.) 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Balster RL, Baird JB. Effects of phencyclidine, d-amphetamine and pentobarbital on spaced responding in mice. Pharmacology, Biochemistry, and Behavior. 1979;11:617–623. doi: 10.1016/0091-3057(79)90252-1. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Cain ME, Bylica KE. Effect of amphetamine on response inhibition in rats showing high or low response to novelty. Pharmacology, Biochemistry, and Behavior. 2006;85:98–104. doi: 10.1016/j.pbb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizot JC. Effects of various drugs including organophosphorus compounds (OPC) and therapeutic compounds against OPC on DRL responding. Pharmacology, Biochemistry, and Behavior. 1998;59:1069–1080. doi: 10.1016/s0091-3057(97)00519-4. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Myrick H, McElroy S. The relationship between substance use disorders, impulse control disorders, and pathological aggression. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 1998;7:221–230. [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Koob GF. Effects of corticotropin releasing factor, desipramine and haloperidol on a DRL schedule of reinforcement. Pharmacology, Biochemistry, and Behavior. 1989;32:967–970. doi: 10.1016/0091-3057(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biological Psychiatry. 2005;57:743–751. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Liao RM. Dopamine receptor antagonists reverse amphetamineinduced behavioral alteration on a differential reinforcement for low-rate (DRL) operant task in the rat. The Chinese Journal of Physiology. 2007;50:77–88. [PubMed] [Google Scholar]
- Cho YH, Jeantet Y. Differential involvement of prefrontal cortex, striatum, and hippocampus in DRL performance in mice. Neurobiology of Learning and Memory. 2010;93:85–91. doi: 10.1016/j.nlm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse (New York, N.Y.) 2003;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dandy KL, Gatch MB. The effects of chronic cocaine exposure on impulsivity in rats. Behavioural Pharmacology. 2009;20:400–405. doi: 10.1097/FBP.0b013e328330ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the wisconsin card sorting test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? The European Journal of Neuroscience. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behavioural Brain Research. 2008;189:170–179. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP impairs attentional set shifting: Reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: Reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex (New York, N.Y.: 1991) 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakalic I, Panlilio LV, Quiroz C, Schindler CW. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience. 2010;165:313–324. doi: 10.1016/j.neuroscience.2009.10.051. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSMIV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural Pharmacology. 1998;9:299–308. [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology. 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addictive Behaviors. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Oo H, Olausson P, Taylor JR, Nairn AC. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behavioural Pharmacology. 2009;20:695–704. doi: 10.1097/FBP.0b013e328333a2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. The European Journal of Neuroscience. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Neddens J, Teuchert-Noodt G. Ontogeny of the dopamine innervation in the nucleus accumbens of gerbils. Brain Research. 2005;1066:16–23. doi: 10.1016/j.brainres.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: Effect on d-amphetamine self-administration. Pharmacology, Biochemistry, and Behavior. 1979;11:553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology. 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Kelly H, McCormick CM. Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacology, Biochemistry, and Behavior. 2011;97:640–646. doi: 10.1016/j.pbb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McFadden L, Yamamoto BK, Matuszewich L. Alterations in adult behavioral responses to cocaine and dopamine transporters following juvenile exposure to methamphetamine. Behavioural Brain Research. 2011;216:726–730. doi: 10.1016/j.bbr.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar JD, Harris AH, Moos RH. Predictors of outcome for patients with substance-use disorders five years after treatment dropout. Journal of Studies on Alcohol. 2006;67:685–693. doi: 10.15288/jsa.2006.67.685. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: Comparison with non-drug-using controls. Drug and Alcohol Dependence. 1998;50:181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Research.Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral Neuroscience. 2010;124:470–477. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline AC, O'Malley PM, Schulenberg JE, Bachman JG, Johnston LD. Substance use among adults 35 years of age: Prevalence, adulthood predictors, and impact of adolescent substance use. American Journal of Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Research.Developmental Brain Research. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nalwa V, Rao PS. DRL responding under uncertain reinforcement in rats after medial frontal cortical lesions. Behavioural Brain Research. 1985;17:73–76. doi: 10.1016/0166-4328(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of withdrawal from an escalating dose of amphetamine on conditioned fear and dopamine response in the medial prefrontal cortex. Behavioural Brain Research. 2008;186:12–22. doi: 10.1016/j.bbr.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Impaired DRL 30 performance during amphetamine withdrawal. Behavioural Brain Research. 2003;143:101–108. doi: 10.1016/s0166-4328(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacology, Biochemistry, and Behavior. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Sable HJ, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: Effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicology and Teratology. 2009;31:149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol KE, Richards JB, Layton K, Seiden LS. Amphetamine analogs have differential effects on DRL 36-s schedule performance. Psychopharmacology. 1995;121:57–65. doi: 10.1007/BF02245591. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Key M, Blackman DE. Differential effects of chlordiazepoxide and d-amphetamine on responding maintained by a DRL schedule of reinforcement. Psychopharmacologia. 1974;38:159–171. doi: 10.1007/BF00426110. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicology and Teratology. 2004;26:651–661. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiology & Behavior. 2009;96:493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug and Alcohol Dependence. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Research. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Marquez Avila H, White MD, Gulley JM. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology. 2008;199:539–548. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neuroscience Letters. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of pavlovian approach behavior in rats: Differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine ("ecstasy") Biological Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. The American Journal of Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Wan X, Peoples LL. Amphetamine exposure enhances accumbal responses to reward-predictive stimuli in a pavlovian conditioned approach task. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28:7501–7512. doi: 10.1523/JNEUROSCI.1071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Golden KM. Separation of drug effects on timing and behavioral inhibition by increased stimulus control. Experimental and Clinical Psychopharmacology. 2000;8:451–461. doi: 10.1037//1064-1297.8.4.451. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, Chakravarty S, Self DW, Nestler EJ. Increased impulsivity during withdrawal from cocaine self-administration: Role for DeltaFosB in the orbitofrontal cortex. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: Increased cue-triggered "wanting" for sucrose reward. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]