Abstract
Transition metal-catalyzed acyloxy migration of propargylic esters offers versatile entries to allene and vinyl carbene intermediates for various fascinating subsequent transformations. Most π-acidic metals (e.g. gold and platinum) are capable of facilitating these acyloxy migration events. However, very few of these processes involve redox chemistry, which are well-known for most other transition metals such as rhodium. The coupling of acyloxy migration of propargylic esters with oxidative addition, migratory insertion, and reductive elimination may lead to ample new opportunities for the design of new reactions. This tutorial review summarizes recent developments in Rh-catalyzed 1,3- and 1,2-acyloxy migration of propargylic esters in a number of cycloaddition reactions. Related Au- and Pt-catalyzed cycloadditions involving acyloxy migration are also discussed.
1. Introduction
The first ten years in the 21st century is the golden decade for Au-and related π-acidic transition metal catalysis. Over 50 reviews have been published on homogeneous gold catalysis since the first comprehensive review by Hashmi in 2004.1 Numerous new reaction modes and novel reactivities have been discovered. Among them, Au- and related π-acidic metal-catalyzed acyloxy migration of propargylic esters to form allene or vinyl carbene intermediates is one of the most important transformations.
Cycloaddition is an efficient way to construct carbo- and heterocycles by forming at least two bonds in one operation. The scope of classical cycloaddition, however, is often limited to substrates with matched electronic properties. Transition metal complexes can often promote cycloadditions that are not feasible under thermal or photolytic conditions.2, 3 Prior to the recent “gold rush”, most transition metal-catalyzed cycloadditions involved the use of rhodium, ruthenium, nickel or palladium complexes. However, in recent years, more and more Au- and Pt-catalyzed cycloadditions have been developed.4, 5 These new discoveries then fed back in to the loop and inspired the development of recent Rh-catalyzed cycloadditions in this article.
This tutorial review will first discuss early discoveries and recent mechanistic studies on 1,3- and 1,2-acyloxy migration of propargylic esters. Applications of these two isomerizations in transition metal-catalyzed cycloadditions will then be presented.
2. Historical discovery of 1,3- and 1,2-acyloxy migration of propargylic esters in the 20th century
2.1 Transition metal-catalyzed 1,3-acyloxy migration of propargylic esters
In 1959, Saucy and Marbet at Hoffmann-La Roche found, by chance, that diacetates were prepared from propargylic acetates in the presence of catalytic amount of copper powder, copper oxide, copper and silver salts (Scheme 1).6 The reaction could be scaled up to 100g scale. Some of the allenylic acetate intermediates were isolated and characterized by IR. The distribution of propargylic acetate a, allenylic acetate b, and diacetate c was studied over the course of the reaction. Both allenylic acetates and diacetates were also hydrolyzed to their corresponding α,β-unsaturated aldehydes. This is different from the original Meyer-Schuster rearrangement7 and the Rupe rearrangement,8 where propargylic alcohols were isomerized to α,β-unsaturated carbonyl compounds through a propargylic cation intermediate.9
Scheme 1.
Initial discovery of 1,3-acyloxy migration of propargylic esters
The mechanism of silver-catalyzed Saucy-Marbet rearrangement was studied in detail using optically active propargylic esters as well as 14C and 18O-labelled substrates.10 No mixed product was observed in a crossing experiment. The rearrangement of optically active propargylic esters led to a mixture of racemic allenylic esters. Interestingly, allenylic esters erythro-b and threo-b epimerized rapidly in the presence of a silver catalyst (Scheme 2). The rate of epimerization is faster than the rearrangement. The 18O-carbonyl label in the reactant was found exclusively in the alkoxy part of the allene product and the 18O-label was not randomized during the rearrangement and epimerization. Complexation of the silver ion to substrates was found to be the rate-determining step. The rearrangement became slower after the addition of cyclohexene or when the reaction was run in solvents that could coordinate to silver. Based on the above findings, a [3,3]-sigmatropic rearrangement facilitated by silver catalyst was proposed.
Scheme 2.
Mechanistic study of Saucy-Marbet rearrangement
2.2 Transition metal-catalyzed 1,2-acyloxy migration of propargylic esters
In 1976, Ohloff at Firmenich described the cycloisomerization of an enyne to 2-acetoxy-2-carene and carvenone using ZnCl2 (Scheme 3).11 Ohloff proposed that the cycloisomerization was initiated by complexation of zinc chloride to the olefin moiety of the enyne, which is different from current view as discussed in later sections. Cyclization and 1,2-acyloxy migration generated an acetoxonium ion, which could undergo cyclopropanation to form 2-acetoxy-2-carene or deprotonation to form a diene intermediate. Protonation followed by hydrolysis of the enol acetate in the diene intermediate afforded major product carvenone. This serendipitous discovery was mediated by stoichiometric amount of typical Lewis acid and its scope remained unexplored.
Scheme 3.
Initial discovery of 1,2-acyloxy migration of propargylic esters
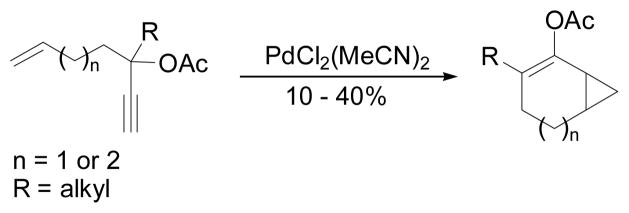
In 1984, Rautenstrauch also from Firmenich reported that 3-acyloxy-1,4-enynes could undergo cycloisomerization to form cyclopentenones and release acetic anhydride in the presence of a palladium catalyst (Scheme 4).12 Among the examined metals, PtCl2(MeCN)2 also catalyzed this transformation. Various alkyl groups were tolerated for R1 and R2 substituents but not other places on the alkene or the alkyne. The proposed cyclopentadiene intermediate could be trapped by N-phenylmaleimide to form Diels-Alder adduct as shown in Scheme 4. Using the same Pd(II) catalyst, cyclopropanation of a tethered alkene to from bicyclic compounds was also reported, albeit in low yield (Scheme 5).12
Scheme 4.
Discovery of Pd-catalyzed 1,2-acyloxy migration of propargylic esters
Scheme 5.
Pd-catalyzed 1,2-acyloxy migration and intramolecular cyclopropanation
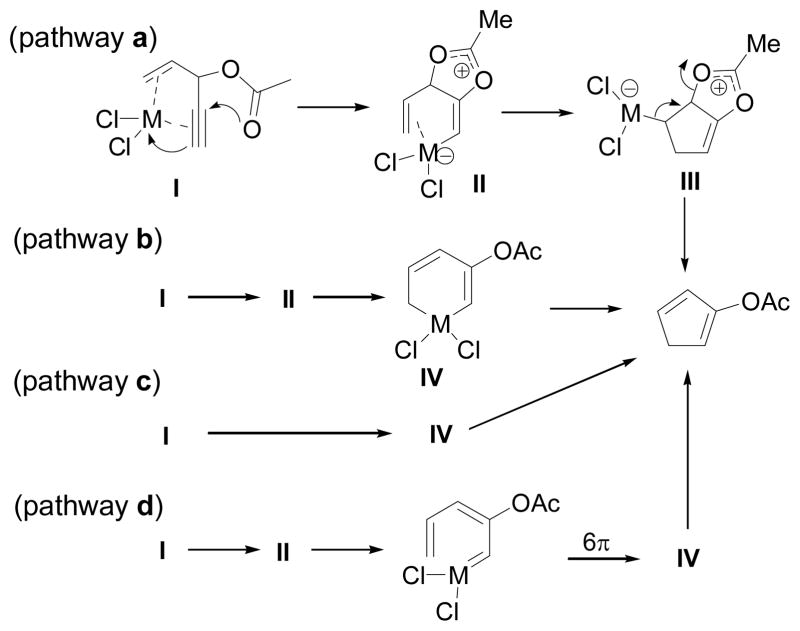
Rautenstrauch proposed that the cycloisomerization was initiated by complexation of the metal (Pd or Pt) to both the alkene and the alkyne parts of 3-acyloxy-1,4-enynes (Scheme 6). Four potential pathways were outlined for the conversion of metal complex I to cyclopentadiene product. Acetoxonium ion II was proposed to be involved as an intermediate in most pathways. In pathway a, insertion of an olefin to the carbon-metal bond in acetoxonium ion II gave bicyclic intermediate III, which underwent elimination to afford diene product. In pathways b, c and d, cyclopentadiene was formed by reductive elimination of metallacyclohexadiene IV, which could be derived from metal complex I in three pathways: a cyclization of acetoxonium ion II (pathway b), a concerted oxidative cyclization of complex I combined with 1,2-acyloxy migration (pathway c), or a 6-π electrocyclization of a carbene intermediate (pathway d). The isolation of cyclopropanation products (Scheme 5) is consistent with the formation of palladium carbene intermediates from 1,2-acyloxy migration of propargylic esters.
Scheme 6.
Mechanism of 1,2-acyloxy migration of propargylic esters proposed by Rautenstrauch
Rautenstrauch’s discovery represents the first example of late transition metal-catalyzed 1,2-acyloxy migration of propargylic esters. Some of the proposed mechanisms are also consistent with recent experimental and computational evidences as discussed in later sections.
3. Recent mechanistic studies on transition metal-catalyzed 1,3- and 1,2-acyloxy migration of propargylic esters
Although transition metal-catalyzed 1,3- and 1,2-acyloxy migration of propargylic esters were discovered several decades ago, their synthetic potential were not realized until recently. In 2007, Marion and Nolan briefly reviewed both 1,2- and 1,3-acyloxy migration of propargylic esters in gold catalysis.13 In the same year, Marco-Contelles and Soriano reviewed the metal-catalyzed 1,2-acyloxy migration process in more details.14 In 2010, Zhang reviewed Au-catalyzed 1,3-acyloxy migration of propargylic esters.15
The selectivity for 1,3- and 1,2-acyloxy migration depends on the substitution pattern of the propargyl moiety and the metal catalyst. The 1,3-acyloxy migration is generally favored for propargylic esters with internal alkynes, while the 1,2-acyloxy migration is usually limited to propargylic esters derived from tertiary or benzylic alcohols containing terminal alkynes (R3 = H, Scheme 7). Calculation by Soriano and Marco-Contelles suggested that the change of regioselectivity was due to electronic effect in Au- and Pt-catalyzed reactions.16 They found that 1,2-acyloxy migration was considerably kinetically favored for terminal alkynes. The R3 alkyl substituent in the internal alkyne enhances the electrophilicity of that acetylenic atom, and allows a faster 1,3-acyloxy migration. The 1,3-acyloxy migration was composed of a first rate-limiting 6-endo-dig cyclization to form a six-membered heterocycle followed by ring opening.
Scheme 7.
Mechanism of 1,3 and 1,2-acyloxy migration of propargylic esters
The mechanism for 1,3- and 1,2-acyloxy migration of propargylic esters, however, is controversial. In 2008, a calculation study suggested that double 1,2-shift through the circled intermediate in Scheme 7 was a preferred pathway for Au-catalyzed 1,3-acyloxy migration.17 It was also noted that the preference was not particularly strong and the intermediates might be in rapid equilibrium. An apparent 1,2-acyloxy migration might also be the result of a 1,3-shift followed by a retro 1,2-migration. In 2009, 18O labelling study showed the allene intermediate was derived from a direct 1,3-shift in Au-catalyzed reactions, which was in accordance with results from previous studies shown in Scheme 2.18 In the same year, labelling studies suggested that a direct 1,3-acyloxy migration occurred in Ag-catalyzed reactions, while both direct 1,3- and double 1,2-acyloxy migration occurred randomly in the case of Au(I) and Au(III)-catalyzed reactions.19 In earlier studies, double 1,2-acyloxy migration was also proposed as a possible pathway in a tandem reaction for the formation of furans.20
Propargylic esters with a chiral cyclopropyl probe were also investigated for the ring expansion reaction.18, 21 Results from these studies indicated that 1,3-acyloxy migration propargylic esters was reversible and chirality transfer was not complete. Racemic propargylic esters could be converted to chiral chromenes in a dynamic kinetic asymmetric transformation.22
Recently, Cho investigated factors controlling the equilibrium of Pt-catalyzed 1,3- and 1,2-acyloxy migration of propargylic esters.23 It was found that the equilibrium was dependent on reaction temperature, substitution pattern of alkynes, and catalysts. It has been shown previously by Sarpong and Zhang that 1,2-acyloxy migration becomes favored when R3 (Scheme 7) is an electron-withdrawing ester or halogen substituent in Pt and Au-catalyzed reactions.24, 25
4. Cycloadditions involving Au-catalyzed 1,3-acyloxy migration of propargylic esters
4.1 [2+2] cycloaddition
In 2005, Zhang reported a Au(I)-catalyzed 1,3-acyloxy migration [2+2] cycloaddition to form tetracyclic dihydroindole derivatives (Scheme 8).26 An allenylic ester was proposed as the initial intermediate after Au-catalyzed 1,3-acyloxy migration. Activation of the allene to form oxonium intermediate followed by nucleophilic attack and trapping of the iminium ion by alkenyl gold furnished the [2+2] cycloaddition product. The allenylic ester was observed when the reaction was stopped at shorter time. Independently prepared allenylic ester also underwent [2+2] cycloaddition smoothly.
Scheme 8.
Au(I)-catalyzed [2+2] cycloaddition with indoles
Recently, Chan demonstrated that the allenylic ester could also undergo [2+2] cycloaddition with terminal alkenes or styrenes in the presence of a gold catalyst (Scheme 9).27
Scheme 9.
Au(I)-catalyzed [2+2] cycloaddition with alkenes
4.2 [3+2] cycloaddition
In 2006, Gagosz found that 5-en-2-yn-1-yl acetates could undergo 1,3-acyloxy migration and cycloisomerization to form bicyclic[3.1.0]hexenes in the presence of cationic gold catalyst (Scheme 10).28 The postulated allene intermediate could be independently prepared and converted to the bicyclic product under the same condition. This process may be treated as a formal intramolecular [3+2] cycloaddition of allene with alkene.5 The proposed carbocation could be trapped by alcohol nucleophile to form cyclohexenes. The chirality of the propargylic ester (99% ee) could be transferred to the bicyclic product (90% ee).
Scheme 10.
Au(I)-catalyzed [3+2] cycloaddition with alkenes
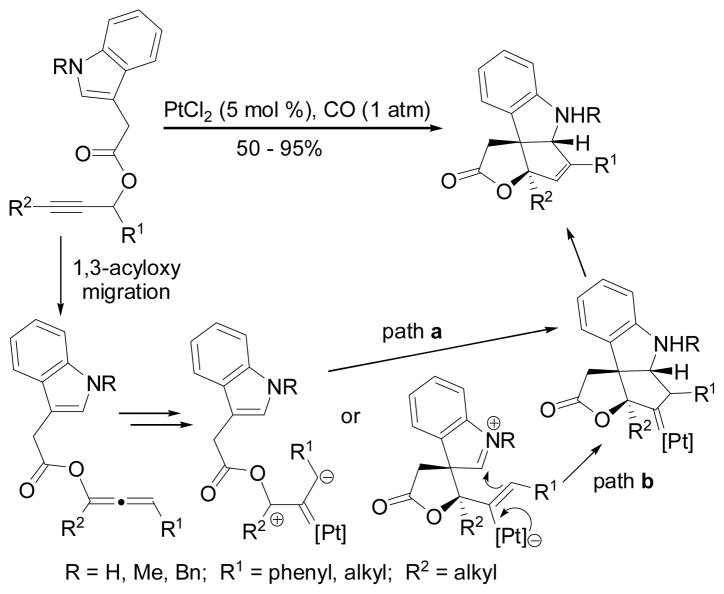
By changing the catalyst from gold to platinum, Zhang found that indoline-fused cyclopentenes could be obtained from [3+2] cycloaddition of allenylic ester and the tethered indole (Scheme 11).29 The reaction may proceed via a concerted dipolar cycloaddition (a) or a stepwise cyclization (b). The comparison between Schemes 8 and 11 suggests substantial difference in the reactivities of alkenylplatinum and alkenylgold intermediates.
Scheme 11.
Pt-catalyzed [3+2] cycloaddition with indoles
Computational studies suggested that a dipole intermediate might be involved in the equilibrium of acyloxy migration as shown in Scheme 7.17 She’s group showed that this intermediate could be trapped by a tethered terminal alkene in a Pt-catalyzed [3+2] cycloaddition (Scheme 12).30 Up to 58% yield could be obtained using gold catalysts. Substrates with a 1,1-disubstituted alkene and substrates with different tethers also worked well. Nitrogen tethers were also examined later.31
Scheme 12.
Pt-catalyzed dipolar [3+2] cycloaddition with alkenes
In a Au-catalyzed tandem reaction involving [3+2] cycloaddition, hydrolytic Michael addition, and retro-Aldol reaction, Wang found that the same dipole intermediate could be trapped by a tethered cyclohexadienones.32
4.3 [4+2] cycloaddition
Liang reported a Pt-catalyzed 1,3-acyloxy migration [4+2] cycloaddition (Scheme 13).33 Low yields were obtained with Au(I) or Au(III) catalysts (8–11%). Aromatic products were isolated in all cases. Only terminal alkynes were examined for the 2C-component and no reaction occurred for propargylic esters with a terminal alkyne. A Pt-mediated enyne cycloisomerization mechanism involving a cyclopropyl Pt-carbene intermediate was proposed.
Scheme 13.
Pt-catalyzed [4+2] cycloaddition with alkynes
Liu reported a 1,3-acyloxy migration [4+2] cycloaddition sequence for the synthesis of bicyclic compounds fused with a benzene (Scheme 14).34 A benzopyrilium intermediate was proposed after Au-catalyzed nucleophilic attack of the carbonyl oxygen to allene.
Scheme 14.
Au(I)-catalyzed [4+2] cycloaddition with alkenes
5. Cycloadditions involving Au-catalyzed 1,2-acyloxy migration of propargylic esters
5.1 [2+1] Cycloaddition (cyclopropanation)
The intramolecular cyclopropanation of alkenes using propargylic esters as the vinylcarbene precursors (the Ohloff process) was further studied using transition metal catalysts such as Pt, Au, and Cu. Better yields were generally obtained than earlier results shown in Schemes 3 and 5. Several sesquiterpene natural products such as cubebol, cubebene, sesquicarene, sesquisabinene, and sesquithujene were synthesized using this method.35, 36 These studies have been reviewed several times and won’t be discussed in details here.13, 14, 37 Computational studies were also conducted for this process.16, 19 Three main mechanistic pathways have been proposed (Scheme 15). The first two involve 1,2-acyloxy migration-cyclopropanation sequence while the last one involves cyclopropanation-1,2-acyloxy migration sequence. Carbene intermediates were proposed in pathways a and c. In all of them, the reaction was initiated by coordination of the metal to alkyne, which is similar to what Rautenstrauch proposed (Scheme 6) but different from original Ohloff’s proposal (Scheme 3).
Scheme 15.
Three pathways proposed for the Ohloff process
In 2003, Ohe and Uemura investigated several transition metal catalysts including Ru(II), Rh(II), Au(III), Pt(II) and Ir(I) for intermolecular cyclopropanation of alkenes using propargylic esters as vinylcarbene precursors.38 The ratio for cis/trans cyclopropanes ranged from 36:64 to 94:6 using [RuCl2(CO)3]2 catalyst. Toste demonstrated that chiral gold complexes could promote highly diastereoselective and enantioselective cyclopropanation of alkenes favoring the cis-isomer (Scheme 16).39 Enantioselective intramolecular cyclopropanation of alkenes to form medium-sized rings was also realized using gold catalysts.40
Scheme 16.
Au-catalyzed intermolecular cyclopropanation
In 2006, an interesting CpRuCl(PPh3)2-catalyzed cyclopropanation of bicyclic alkenes was reported by Tenaglia (Scheme 17).41 A cycloaddition isomerization mechanism involving allene intermediate was proposed.
Scheme 17.
Ru-catalyzed cyclopropanation of bicyclic alkenes
The intermolecular cyclopropanation could also be coupled with other reactions. For example, ring expansion of vinylcyclopropanes may afford cyclopentenes (a formal stepwise [3+2] cycloaddition)42 and Cope rearrangement of divinylcyclopropanes may yield cycloheptadienes (a formal stepwise [4+3] cycloaddition).38, 42 Gung proposed that a concerted [4+3] cycloaddition occurred between dienes and gold-stabilized vinylcarbenes derived from propargylic esters.43 Formal stepwise [4+3] and [4+2] cycloadditions were realized by Toste for the synthesis of benzonorcaradienes,44 fluorenes, and styrenes45 from functionalized cyclopropane intermediates.
In a study for Au-catalyzed cyclopropanation between propargylic esters with vinyl derivatives, it was found that [3+2] cycloaddition products could be obtained when certain electron-rich alkenes were employed (Scheme 18).46 Most vinyl derivatives afforded the cyclopropanation products. In one case, both cyclopropane and cyclopentene products were observed and the cyclopropane could not be converted to the corresponding cyclopentene. A direct [3+2] cycloaddition mechanism was therefore proposed.
Scheme 18.
Au-catalyzed [3+2] cycloaddition
5.3 [3+3] Cycloaddition
Toste demonstrated that the carbene intermediates generated from Au-catalyzed 1,2-acyloxy migration could be trapped by azomethine imides for [3+3] cycloadditions (Scheme 19).47 The reaction also worked with secondary propargylic esters. As the author pointed out, the reaction of alkenyl Fischer carbene with 1,3-dipoles typically proceeds via [3+2] cycloaddition mode. In contrast, the Au-stabilized vinyl carbene served as three carbon component in reactions with 1,3-diploes.
Scheme 19.
Au-catalyzed [3+3] dipolar cycloaddition
5.4 [4+3] Cycloaddition
When the carbene intermediates generated from Au-catalyzed 1,2-acyloxy migration is trapped by α,β-unsaturated imines, a [4+3] cycloaddition was realized by Toste for the synthesis of azepines (Scheme 20).48 This is analogy to [4+3] cycloadditions of rhodium and Fischer carbenes with α,β-unsaturated imines as the author pointed out. The stereochemistry of the Au-catalyzed [4+3] cycloaddition, however, is different from [4+3] cycloadditions involving Fischer carbenes.
Scheme 20.
Au-catalyzed [4+3] cycloaddition
6. Cycloadditions involving Rh-catalyzed 1,3-acyloxy migration of propargylic esters
During a search for more stable alternative carbene precursors,49 Tang’s group found that [Rh(CO)2Cl]2 was able to catalyze the 1,3-acyloxy migration of propargylic esters.50 The resulting allenylic esters were trapped in a [5+1] cycloaddition with CO catalyzed by the same metal complex. Prior to this study, Tanaka’s group reported that [Rh(COD)2]SbF6 catalyzed the 1,3-acyloxy migration of a propargylic ester to yield a mixture of allenylic acetate and its hydrolyzed ketone (Scheme 21).51 Only one example was shown and this transformation was described as a side reaction in their study.
Scheme 21.
Rh(I)-catalyzed 1,3-acyloxy migration of propargylic esters
Rh(I) complexes are very different from previously described typical π-acidic metals (e.g. Au) that are capable of catalyzing 1,3-acyloxy migration of propargylic esters. For example, Au(I) is very reluctant to undergo redox chemistry. Rh(I) complexes, on the other hand, are very facile to undergo oxidative addition, migratory insertion, and reductive elimination. A number of tandem reactions were then realized through the combination of [Rh(CO)2Cl]2-catalyzed Saucy-Marbet 1,3-acyloxy migration and subsequent elaboration of the allenylic acetate intermediates via Rh(I)/Rh(III) cycles.
The selectivity for Rh(I)-catalyzed 1,3- or 1,2-acyloxy migration is similar to the trend observed in Au- and Pt-catalyzed reactions. Propargylic esters with internal alkynes tend to undergo 1,3-acyloxy migration, while 1,2-acyloxy migration is favored for propargylic esters with terminal alkynes or electron deficient internal alkynes.
6.1 [4+1] cycloaddition
The Rh-catalyzed [4+1] cycloaddition of vinylallene with CO was first studied by Murakami and Ito (Scheme 22).52 A mixture of isomers was often observed. When the vinylallene was treated with stoichiometric amount of Wilkinson complex (Scheme 23), the corresponding metallacycle could be isolated as an orange crystal and characterized by x-ray diffraction.53 This metal complex could react with CO and yielded the isomerized cyclopentenone. Enantioselective [4+1] cycloaddition of vinylallene and CO was also realized using chiral phosphine ligands.54 Over 90% ee could be achieved when R1 = R2 = Me, R3 = ester group and R4 = Ph.
Scheme 22.
Rh-catalyzed [4+1] cycloaddition of vinylallene and CO
Scheme 23.
Isolation and characterization of rhodacyclopentene
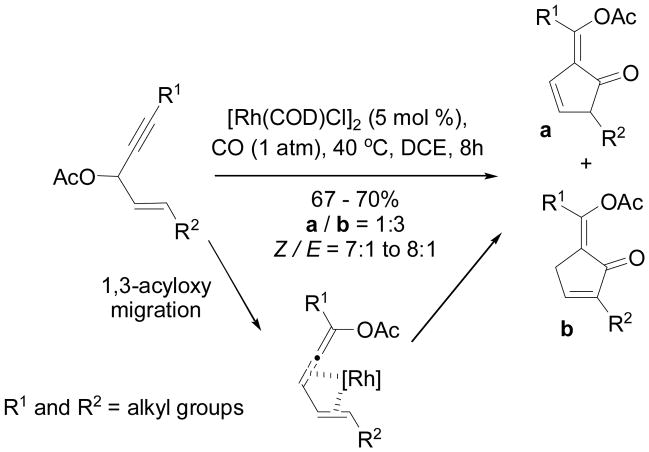
In 2012, Tang’s group reported a Rh-catalyzed 1,3-acyloxy migration of propargylic esters and [4+1] cycloaddition of the resulting allenylic esters with CO.55 A mixture of isomeric alkylidene cyclopentenones was obtained for substrates with a disubstituted alkene (Scheme 24). The ratio of these two isomers did not change significantly by the treatment of bases. A mixture of two products was also obtained from substrates with a tri-substituted alkene. Interestingly, one isomer could be obtained exclusively after the treatment of Et3N (Scheme 25). Hydrolysis of the enol esters yielded 1,3-diketones. The 3-acyloxy-1,4-enynes in Schemes 24 and 25 were conveniently prepared from addition of terminal alkynes to α,β-unsaturated aldehydes followed by esterification.
Scheme 24.
Rh-catalyzed 1,3-acyloxy migration [4+1] cycloaddition for disubstituted alkenes
Scheme 25.
Rh-catalyzed 1,3-acyloxy migration [4+1] cycloaddition for trisubstituted alkenes
Groups of Fukuyama, Ryu, Fensterbank, and Malacria independently reported the tandem 1,3-acyloxy migration [4+1] cycloaddition.56 In their study, a mixture of olefin isomers was generally observed in the presence of 60–80 atm of CO and [Rh(COD)Cl]2 catalyst. In addition to the oxidative cyclization of vinylallene intermediate pathway, these groups also proposed that formation of metallacyclopentene directly from the zwitterionic vinyl rhodium intermediate could be an alternative (Scheme 26).
Scheme 26.
Alternative mechanism for Rh-catalyzed 1,3-acyloxy migration [4+1] cycloaddition
6.2 [4+2] cycloaddition
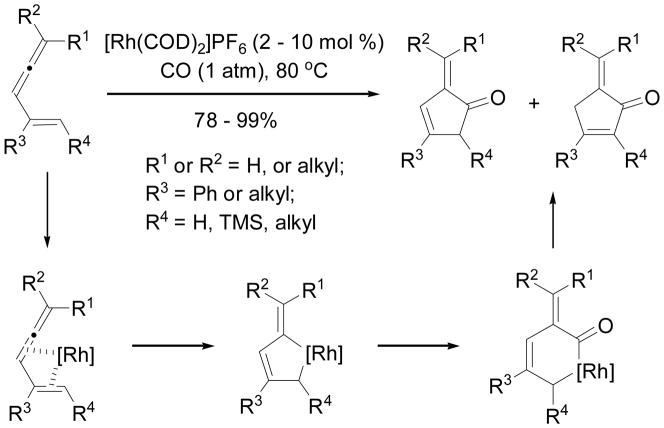
The first Rh-catalyzed intermolecular [4+2] cycloaddition of vinylallene and alkyne was reported by Murakami and Ito (Scheme 27).57 Aromatic benzene derivatives were isolated in good yields. When R4 = H, high regioselectivity was observed for terminal alkynes. Symmetric internal alkynes also participated in the cycloaddition. When R4 = Me, a mixture of 1:1 regioisomers was obtained. When the distal position of the allene is mono- or non-substituted (R1 or R2 = H), the reaction afforded the desired products in low yields (15–24%).
Scheme 27.
Rh-catalyzed intermolecular [4+2] cycloaddition of vinylallene and alkyne
When the propargylic ester in Scheme 28(a) was treated with [Rh(CO)2Cl]2, a tandem 1,3-acyloxy migration and intramolecular [4+2] cycloaddition was realized by Tang’s group.58 Surprisingly, a non-aromatic bicyclic compound with an unusual isotoluene moiety was isolated. An independently prepared allenylic ester could also undergo the [4+2] cycloaddition.
Scheme 28.
Rh-catalyzed 1,3-acyloxy migration intramolecular [4+2] cycloaddition
The enol ester in the product could be hydrolyzed to the corresponding ketone. In two steps, bicyclic compounds with an alkylidene cyclohexenone moiety were prepared as shown in Scheme 28 (b). The rate of reaction dropped significantly for secondary propargylic esters. The addition of electron-poor phosphite ligand [(CF3)2CHO)]3P significantly improved both the rate and the yield (Scheme 28(c)). Interestingly, when the secondary propargylic ester was treated with typical π-acidic metal catalysts, no reaction occurred. This suggests that the equilibrium between propargylic ester and allenylic ester does not always lie on the side of former. Trapping the trace amount of allenylic ester intermediate by the following [4+2] cycloaddition using [Rh(CO)2Cl]2 catalyst is critical for the success of the tandem reaction.
It is important to note that the acyloxy group in the propargylic ester starting material has three essential roles, 1) it eliminates the need for allene preparation; 2) it may inhibit the aromitization process and allow the isolation of isotoluenes; and 3) it differentiated the three olefins in the product for further functionalization.
6.3 [5+1] cycloaddition
A relatively general Rh-catalyzed [5+1] cycloaddition of vinylcyclopropane with CO was recently reported by Yu.59 Historical achievements in [5+1] cycloadditions of vinylcyclopropanes and allenylcyclopropanes with CO were also discussed by Yu. Prior to that, the [5+1] cycloaddition of vinylcyclopropane with CO often requires stoichiometric amount of metals and the scope was often limited.
In a Ir-catalyzed [5+1] cycloaddition of allenylcyclopropane with CO reported by Murakami and Ito, the distal position of allene has to be di-substituted (Scheme 29(a)).60 Only 28% yield of cycloaddition product was obtained when R1 = Ph and R2 = H (Scheme 29 (b) and (c)). The regioselectivity for the cleavage of cyclopropane C-C σ-bonds was only studied with one substrate with a gem-diphenyl substituent. Interestingly, when the allenylcyclopropanes were treated with either neutral or cationic Rh(I) catalysts, simple isomerization to alkylidene cyclopentenes occurred rapidly and no carbonyl product was observed.
Scheme 29.
Ir(I)-catalyzed [5+1] cycloaddition of allenyl cyclopropanes with CO
When cyclopropyl substituted propargylic esters were treated with transition metals such as AuCl3, (Ph3P)AuCl, (Ph3P)AuCl/AgSbF6, PtCl2, PtCl4, AgSbF6, Cu(MeCN)4PF6, PdCl2(MeCN)2, [CpRu(MeCN)]PF6, and Rh2(OAc)4, enones were observed as the main products, presumably derived from hydrolysis of the corresponding allenylic esters (Scheme 30).50
Scheme 30.
1,3-acyloxy migration of cyclopropyl substituted propargylic esters
In the presence of [Rh(CO)2Cl]2 catalyst and CO (1 atm), the allenylic ester intermediate derived from propargylic esters could be trapped in a [5+1] cycloaddition to form alkylidene cyclohexenone products (Scheme 31).50 In contrast to a previous report,60 no five-membered alkylidene cyclopentene product was observed. This suggested that the acyloxy substituent played an important role for the reactivity of the allene towards reductive elimination or CO insertion. The tandem reaction involves an oxidative cyclization to form an alkylidene metallacyclohexene intermediate, CO insertion and reductive elimination. Identical [5+1] cycloaddition products were obtained when independently prepared allenylic esters were treated with [Rh(CO)2Cl]2 catalyst and CO (1 atm). The substituents R1 and R2 can be H, aryl, or alkyl groups. When R1 ≠ R2, a mixture of E/Z isomers was obtained and the ratio ranged from 1:1 to 13:1.
Scheme 31.
Rh(I)-catalyzed 1,3-acyloxy migration [5+1] cycloaddition
The regioselectivity for the cleavage of cyclopropane C-C σ-bond was studied with a series of trans- and cis-substituted cyclopropanes and tri-substituted cyclopropanes (Scheme 32).50 Higher regioselectivity was generally observed in cis-substituted cyclopropanes (ratios = 3.5:1 to >20:1) than the corresponding trans-counterpart (ratios = 1:2.5 to 10:1) when R was an alkyl group. High regioselectivity (16:1 to >20:1) was also observed for all trisubstituted cyclopropanes. Chirality in the cyclopropane starting material could be completely transferred to the product. Highly functionalized decalin rings were also prepared from bicyclic substrates.
Scheme 32.
Regioselective ring expansion of cyclopropanes
Interestingly, cyclopropyl substituted propargylic esters have been studied in Au-catalyzed reactions and completely different products were obtained (Scheme 33). When R1 = alkyl group, 1,3-diketone was formed.61, 62 When R1 = vinyl or phenyl group, alkylidene cyclopentene was observed.18, 21
Scheme 33.
Results from Au-catalyzed reactions
6.4 [7+1] cycloaddition and ring expansion
When a vinylcyclopropane substituted propargylic ester was treated with [Rh(CO)2Cl]2 catalyst, a mixture of seven- and eight-membered carbocycles was obtained (Scheme 34).63 Both of them are presumably derived from the eight-membered metallacycle via reductive elimination and [7+1] cycloaddition with CO, respectively. A sequence of 1,3-acyloxy migration, oxidative cyclization, and cyclopropane ring expansion may afford the eight-membered metallacycle.
Scheme 34.
Rh-catalyzed 1,3-acyloxy migration ring expansion and [7+1] cycloaddition
The yield of [7+1] cycloaddition product could not be improved by increasing CO pressure. The seven-membered ring expansion product, however, could be obtained in high yield after some optimizations (Scheme 35(a)). Good Z/E selectivity was obtained when R was an aryl group. Only one regioisomer was observed for substituted cyclopropanes. Optical pure product (91% ee) could be obtained from a chiral cyclopropane (92% ee). Bicyclic 5–7 and 6–7 fused rings were prepared in 85% and 91% yields, respectively. Hydrolysis of the enol ester provided a highly functionalized 5–7 fused bicyclic compound (Scheme 35(b)).
Scheme 35.
Rh-catalyzed 1,3-acyloxy migration ring expansion
The seven-membered ring product could be obtained in 62% yield from an allenylvinylcyclopropane, indicating that the acyloxy substituent is not required for the ring expansion (Scheme 36(a)). The acyloxy substituent, however, is important for differentiating the three double bonds in the alkylidene cycloheptadiene product towards selective functionalization as shown in Scheme 35. On the other hand, the allene group is critical for the ring expansion reaction as a dienyl cyclopropane did not yield any desired product as shown in Scheme 36 (b). It is interesting to note that Yu’s group recently reported a Rh-catalyzed [7+1] of cycloaddition of CO and dienyl cyclopropanes with certain substitution patterns.64
Scheme 36.
Rh-catalyzed ring expansion of allenylvinyl- and dienylcyclopropanes
7. Cycloadditions involving Rh-catalyzed 1,2-acyloxy migration of propargylic esters
In 2010, Tanaka’s group first described the Rh(I)-catalyzed 1,2-acyloxy migration of propargylic esters in cyclopropanation and [3+2] cycloadditions.51 About the same time, a Rh-catalyzed [5+1] cycloaddition involving 1,2-acyloxy migration was reported by research groups of Fukuyama, Ryu, Fensterbank, and Malacria.65 Shortly after that, Tang’s group developed a [5+2] cycloaddition involving the same acyloxy migration.66 As discussed in following sections, the Rh(I) vinylcarbenes derived from propargyl esters show various reactivities that are complementary to Au(I) and Pt(II)-carbenes.
7.1 [2+1] and [3+2] cycloaddition
Tanaka’s group found that cationic Rh-complexes could catalyze 1,2-acyloxy migration of propargylic esters and the resulting Rh-carbene reacted with electron-deficient alkynes and alkenes (Scheme 37).51 Secondary propargylic esters participated in the [3+2] cycloaddition with alkynes but not cyclopropanation with alkenes. Low yields (~20%) were obtained when methyl acrylate or styrene was employed as the alkene partner for the cyclopropanation reaction. In previous Au-catalyzed intermolecular cyclopropanations, electron-rich or neutral alkenes are generally preferred substrates.
Scheme 37.
Rh-catalyzed cyclopropanation of alkenes and [3+2] cycloaddition with alkynes
The nucleophilic nature of Rh(I) carbene was rationalized by the coordination of the carboxylate oxygen to the metal (Scheme 38). A metalla Diels-Alder reaction was proposed for the [3+2] cycloaddition with acetylenedicarboxylate. A metathesis followed by reductive elimination pathway may account for the cyclopropanation reaction with acrylamides.
Scheme 38.
Mechanism of Rh-catalyzed cyclopropanation of alkenes and [3+2] cycloaddition with alkynes
7.2 [5+1] cycloaddition
Research groups of Fukuyama, Ryu, Fensterbank, and Malacria developed a Rh-catalyzed tandem 1,2-acyloxy migration [5+1] cycloaddition of 3-acyloxy-1,4-enynes with CO (Scheme 39).56, 65 This method provided access to various functionalized resorcinol derivatives from readily available 3-acyloxy-1,4-enynes.
Scheme 39.
Rh-catalyzed [5+1] cycloaddition
Various transition metals were examined. Gold and platinum salts led to recovered starting materials or cyclopentenones via Rautenstrauch rearrangement. A mixtures of resorcinol derivatives and cyclopentenones were found when Rh2(OAc)4 or [RhCp*Cl2]2 was employed as the catalyst. Other rhodium complexes, including Rh6(CO)16, Rh(acac)(CO)2, and RhCl(PPh3)3 did not catalyze the [5+1] cycloaddition.
Two mechanisms were proposed in the initial report.65 Both of them involve Rh-catalyzed 1,2-acyloxy migration through a zwitterionic vinyl rhodium intermediate. Formation of a vinyl rhodium-carbene intermediate was proposed in pathway a. It was followed by a sequence of CO insertion to form ketene, 6p-electrocyclization, and aromatization. A six-membered metallacycle was proposed in pathway b. It was then followed by CO insertion, reductive elimination, and aromatization. Only pathway a was proposed in a later report as the ketene intermediate could be trapped by methanol.56
7.3 [5+2] cycloaddition
The scope of Rautenstrauch rearrangement shown in Scheme 4 was expanded using gold67 and platinum24 catalysts for the synthesis of functionalized five-membered rings. Optical pure cyclopentenones were also prepared from chiral starting material using gold catalysts.67 Inspired by previous work on using 3-acyloxy-1,4-enynes as 5-carbon synthons for the synthesis of cyclopentenones and resorcinol derivatives, a [5+2] cycloaddition of 3-acyloxy-1,4-enynes with alkynes was developed by Tang’s group.66, 68
Substrates with a 3-acyloxy-1,4-enyne and a tethered alkyne were examined for intramolecular [5+2] cycloaddition in the presence of various metal catalysts. While most Rh(I) metal complexes worked, gold and platinum salts did not yield any seven-membered ring product (Scheme 40).66 When the 2-carbon component was a terminal alkyne, cationic rhodium alone was often sufficient. Addition of electron-poor phosphite ligand was necessary for substrates with an internal alkyne for the 2-carbon component or substitution on the alkene side of 3-acyloxy-1,4-enynes. The alkyne in the 3-acyloxy-1,4-enyne part could be either terminal alkyne or internal alkyne with an electron-withdrawing substituent.
Scheme 40.
Rh-catalyzed intramolecular [5+2] cycloaddition
The mechanism for the intramolecular [5+2] cycloaddition was proposed based on Rautenstrauch hypothesis and previous studies on 3-acyloxy-1,4-enynes (Scheme 41). After the coordination of the rhodium metal to 3-acyloxy-1,4-enyne, a zwitterionic intermediate might be formed after 1,2-acyloxy migration. A metallacyclohexadiene intermediate could be formed in two pathways: direct cyclization or 6p-electrocyclization of a dienyl Rh(I)-carbene intermediate. Insertion of an alkyne followed by reductive elimination then yielded the cycloheptatriene product. The observation of a cyclopropane byproduct derived from cyclopropanation of cyclooctadiene supports the involvement of Rh(I) carbenes.
Scheme 41.
Mechanism of Rh-catalyzed intramolecular [5+2] cycloaddition
The Rh-catalyzed intermolecular [5+2] cycloaddition of 3-acyloxy-1,4-enynes with alkynes accompanied by a 1,2-acyloxy migration was subsequently developed (Scheme 42).68 Interestingly, the optimal cationic rhodium catalysts previously developed were not effective for the intermolecular cycloaddition. Gold, platinum, and palladium salts did not work either. The desired mono-cyclic cycloheptatriene product was observed only when a neutral rhodium catalyst was employed. Wilkinson catalyst worked well in most cases. High regioselectivity was observed when the 2-carbon component had a terminal alkyne. Low conversions were obtained for 3-acyloxy-1,4-enynes with a secondary propargylic ester.
Scheme 42.
Rh-catalyzed intermolecular [5+2] cycloaddition
A metallacyclohexadiene intermediate in Scheme 43 can be generated following a mechanism similar to what is shown in Scheme 41. Four metallacyclooctatriene intermediates can be proposed after the insertion of an alkyne to this metallacyclohexadiene intermediate. The alkyne could insert into either the sp3-carbon-metal bond or sp2-carbon-metal bond. For a terminal alkyne, the R group could be either close or distal to the forming C-C bond. In Houk’s computational studies on Rh-catalyzed reactions involving unsymmetrical alkynes, the bulkier alkyne substituent prefers to be distal to the forming C-C bond.69 If this system follows the same trend, the insertion of alkyne to sp2-carbon-metal bond is then preferred.
Scheme 43.
Mechanism of Rh-catalyzed intermolecular [5+2] cycloaddition
Conclusion
Homogeneous gold catalysis has demonstrated its power and utility in various reactions. In the field of cycloaddition, traditional transition metals (e.g. Rh, Ru, Ni, and Pd) have gained significant successes, which certainly impacted the development of Au-catalyzed cycloadditions. In return, new reaction modes and novel reactivities discovered in gold catalysis, further stimulate the development of new cycloaddition reactions using traditional transition metals. This review uses the acyloxy migration of propargylic esters in cycloaddition as an example to illustrate the interplay between traditional transition metals such as rhodium and π-acidic transition metals such as gold. The 1,3-and 1,2-acyloxy migration of propargylic esters to allenes and vinylcarbenes are just two of many transformations that can be catalyzed by Au and related π-acidic metals. It is expected that more “gold-like” reactivities can be found in Rh(I) complexes with appropriate ligands. The combination of the new reactivity of Rh(I) complexes with their well-known capability to catalyze cycloadditions should offer myriad opportunities for the design of new reactions.
Supplementary Material
Acknowledgments
We thank NIH (R01 GM088285) and the University of Wisconsin for financial support and a Young Investigator Award (to W.T.) from Amgen.
Notes and references
- 1.Hashmi ASK. Gold Bull. 2004;37:51. [Google Scholar]
- 2.Lautens M, Klute W, Tam W. Chem Rev. 1996;96:49. doi: 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]
- 3.Ojima I, Tzamarioudaki M, Li Z, Donovan RJ. Chem Rev. 1996;96:635. doi: 10.1021/cr950065y. [DOI] [PubMed] [Google Scholar]
- 4.Shen HC. Tetrahedron. 2008;64:7847. and references cited therein. [Google Scholar]
- 5.Lopez F, Mascarenas JL. Beilstein J Org Chem. 2011;7:1075. doi: 10.3762/bjoc.7.124. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saucy G, Marbet R, Lindlar H, Isler O. Helv Chim Acta. 1959;42:1945. [Google Scholar]
- 7.Meyer KH, Schuster K. Chem Ber. 1922;55B:819. [Google Scholar]
- 8.Rupe H, Kambli E. Helv Chim Acta. 1926;9:672. [Google Scholar]
- 9.Engel DA, Dudley GB. Org Biomol Chem. 2009;7:4149. doi: 10.1039/b912099h. [DOI] [PubMed] [Google Scholar]
- 10.Schlossa H, Sieber W, Hesse M, Hansen HJ, Schmid H. Helv Chim Acta. 1973;56:875. [Google Scholar]
- 11.Strickler H, Davis JB, Ohloff G. Helv Chim Acta. 1976;59:1328. [Google Scholar]
- 12.Rautenstrauch V. J Org Chem. 1984;49:950. [Google Scholar]
- 13.Marion N, Nolan SP. Angew Chem Int Ed. 2007;46:2750. doi: 10.1002/anie.200604773. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 14.Marco-Contelles J, Soriano E. Chem Eur J. 2007;13:1350. doi: 10.1002/chem.200601522. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Zhang G, Zhang L. Synlett. 2010:692. [Google Scholar]
- 16.Soriano E, Marco-Contelles J. Chem Eur J. 2008;14:6771. doi: 10.1002/chem.200800305. [DOI] [PubMed] [Google Scholar]
- 17.Correa A, Marion N, Fensterbank L, Malacria M, Nolan SP, Cavallo L. Angew Chem Int Ed. 2008;47:718. doi: 10.1002/anie.200703769. [DOI] [PubMed] [Google Scholar]
- 18.Mauleon P, Krinsky JL, Toste FD. J Am Chem Soc. 2009;131:4513. doi: 10.1021/ja900456m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion N, Lemiere G, Correa A, Costabile C, Ramon RS, Moreau X, de Fremont P, Dahmane R, Hours A, Lesage D, Tabet JC, Goddard JP, Gandon V, Cavallo L, Fensterbank L, Malacria M, Nolan SP. Chem Eur J. 2009;15:3243. doi: 10.1002/chem.200801387. [DOI] [PubMed] [Google Scholar]
- 20.Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J Am Chem Soc. 2007;129:9868. doi: 10.1021/ja072446m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garayalde D, Gomez-Bengoa E, Huang XG, Goeke A, Nevado C. J Am Chem Soc. 2010;132:4720. doi: 10.1021/ja909013j. [DOI] [PubMed] [Google Scholar]
- 22.Wang YM, Kuzniewski CN, Rauniyar V, Hoong C, Toste FD. J Am Chem Soc. 2011;133:12972. doi: 10.1021/ja205068j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho EJ. Chem Eur J. 2012;18:4495. doi: 10.1002/chem.201103799. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 24.Prasad BAB, Yoshimoto FK, Sarpong R. J Am Chem Soc. 2005;127:12468. doi: 10.1021/ja053192c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Lu B, Zhang L. Chem Commun. 2010;46:9179. doi: 10.1039/c0cc03669b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L. J Am Chem Soc. 2005;127:16804. doi: 10.1021/ja056419c. [DOI] [PubMed] [Google Scholar]
- 27.Rao W, Susanti D, Chan PWH. J Am Chem Soc. 2011;133:15248. doi: 10.1021/ja2052304. [DOI] [PubMed] [Google Scholar]
- 28.Buzas A, Gagosz F. J Am Chem Soc. 2006;128:12614. doi: 10.1021/ja064223m. [DOI] [PubMed] [Google Scholar]
- 29.Zhang G, Catalano VJ, Zhang L. J Am Chem Soc. 2007;129:11358. doi: 10.1021/ja074536x. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Zheng J, Yu B, Chen Q, Wang X, He Y, Yang Z, She X. J Am Chem Soc. 2010;132:1788. doi: 10.1021/ja910346m. [DOI] [PubMed] [Google Scholar]
- 31.Zheng H, Huo X, Zhao C, Jing P, Yang J, Fang B, She X. Org Lett. 2011;13:6448. doi: 10.1021/ol202746s. [DOI] [PubMed] [Google Scholar]
- 32.Cai S, Liu Z, Zhang W, Zhao X, Wang DZ. Angew Chem Int Ed. 2011;50:11133. doi: 10.1002/anie.201104028. [DOI] [PubMed] [Google Scholar]
- 33.Lu L, Liu X, Shu X, Yang K, Ji K, Liang Y. J Org Chem. 2009;74:474. doi: 10.1021/jo802043z. [DOI] [PubMed] [Google Scholar]
- 34.Teng TM, Liu RS. J Am Chem Soc. 2010;132:9298. doi: 10.1021/ja1043837. [DOI] [PubMed] [Google Scholar]
- 35.Fehr C, Winter B, Magpantay I. Chem Eur J. 2009;15:9773. doi: 10.1002/chem.200901292. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 36.Fuerstner A, Schlecker A. Chem Eur J. 2008;14:9181. doi: 10.1002/chem.200801382. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 37.Rudolph M, Hashmi ASK. Chem Soc Rev. 2012;41:2448. doi: 10.1039/c1cs15279c. [DOI] [PubMed] [Google Scholar]
- 38.Miki K, Ohe K, Uemura S. J Org Chem. 2003;68:8505. doi: 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]
- 39.Johansson MJ, Gorin DJ, Staben ST, Toste FD. J Am Chem Soc. 2005;127:18002. doi: 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]
- 40.Watson IDG, Ritter S, Toste FD. J Am Chem Soc. 2009;131:2056. doi: 10.1021/ja8085005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenaglia A, Marc S. J Org Chem. 2006;71:3569. doi: 10.1021/jo060276a. [DOI] [PubMed] [Google Scholar]
- 42.Garayalde D, Kruger K, Nevado C. Angew Chem Int Ed. 2011;50:911. doi: 10.1002/anie.201006105. [DOI] [PubMed] [Google Scholar]
- 43.Gung BW, Bailey LN, Wonser J. Tetrahedron Lett. 2010;51:2251. doi: 10.1016/j.tetlet.2010.02.099. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorin DJ, Dube P, Toste FD. J Am Chem Soc. 2006;128:14480. doi: 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]
- 45.Gorin DJ, Watson IDG, Toste FD. J Am Chem Soc. 2008;130:3736. doi: 10.1021/ja710990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperger CA, Tungen JE, Fiksdahl A. Eur J Org Chem. 2011:3719. [Google Scholar]
- 47.Shapiro ND, Shi Y, Toste FD. J Am Chem Soc. 2009;131:11654. doi: 10.1021/ja903863b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro ND, Toste FD. J Am Chem Soc. 2008;130:9244. doi: 10.1021/ja803890t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew Chem Int Ed. 2008;47:8933. doi: 10.1002/anie.200803910. [DOI] [PubMed] [Google Scholar]
- 50.Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew Chem Int Ed. 2011;50:1346. doi: 10.1002/anie.201006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata Y, Noguchi K, Tanaka K. J Am Chem Soc. 2010;132:7896. doi: 10.1021/ja102418h. [DOI] [PubMed] [Google Scholar]
- 52.Murakami M, Itami K, Ito Y. Angew Chem Int Ed. 1995;34:2691. [Google Scholar]
- 53.Murakami M, Itami K, Ito Y. J Am Chem Soc. 1996;118:11672. [Google Scholar]
- 54.Murakami M, Itami K, Ito Y. J Am Chem Soc. 1999;121:4130. [Google Scholar]
- 55.Li X, Huang S, Schienebeck CM, Shu D, Tang W. Org Lett. 2012;14:1584. doi: 10.1021/ol300330t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuyama T, Ohta Y, Brancour C, Miyagawa K, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Eur J. 2012;18:7243. doi: 10.1002/chem.201200045. [DOI] [PubMed] [Google Scholar]
- 57.Murakami M, Ubukata M, Itami K, Ito Y. Angew Chem Int Ed. 1998;37:2248. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2248::AID-ANIE2248>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Huang S, Li X, Lin CL, Guzei IA, Tang W. Chem Commun. 2012;48:2204. doi: 10.1039/c2cc17406e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang GJ, Fu XF, Li Q, Yu ZX. Org Lett. 2012;14:692. doi: 10.1021/ol2031526. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 60.Murakami M, Itami K, Ubukata M, Tsuji I, Ito Y. J Org Chem. 1998;63:4. doi: 10.1021/jo9718859. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Zhang L. J Am Chem Soc. 2006;128:8414. doi: 10.1021/ja062777j. [DOI] [PubMed] [Google Scholar]
- 62.Barluenga J, Riesgo L, Vicente R, Lopez LA, Tomas M. J Am Chem Soc. 2007;129:7772. doi: 10.1021/ja072864r. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Zhang M, Shu D, Robichaux PJ, Huang S, Tang W. Angew Chem Int Ed. 2011;50:10421. doi: 10.1002/anie.201104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao Z, Li J, Yu ZX. Org Lett. 2011;13:134. doi: 10.1021/ol102700m. [DOI] [PubMed] [Google Scholar]
- 65.Brancour C, Fukuyama T, Ohta Y, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Commun. 2010;46:5470. doi: 10.1039/c0cc00747a. [DOI] [PubMed] [Google Scholar]
- 66.Shu X-z, Huang S, Shu D, Guzei IA, Tang W. Angew Chem Int Ed. 2011;50:8153. doi: 10.1002/anie.201103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]
- 68.Shu X-z, Li X, Shu D, Huang S, Schienebeck CM, Zhou X, Robichaux PJ, Tang W. J Am Chem Soc. 2012;134:5211. doi: 10.1021/ja2109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu P, Houk KN. Inorg Chim Acta. 2011;369:2. and references cited therein. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.