Abstract
Alterations in GABAergic neurotransmission are assumed to play a crucial role in the pathophysiology of mood disorders. Glutamic acid decarboxylase (GAD) is the key enzyme in GABA synthesis. This study aimed to differentiate between unipolar and bipolar I depression using quantitative evaluation of GAD-immunoreactive (GAD-ir) neuropil in several brain regions known to be involved in the pathophysiology of mood disorders. Immunohistochemical staining of GAD 65/67 was performed in the orbitofrontal, anterior cingulate and dorsolateral prefrontal cortex (DLPFC), the entorhinal cortex, the hippocampal formation and the medial dorsal and lateral dorsal (LD) thalamic nuclei, with a quantitative densitometric analysis of GAD-ir neuropil. The study was performed on paraffin-embedded brains from 9 unipolar and 12 bipolar I depressed patients (8 and 6 suicidal patients, respectively) and 18 matched controls. In unipolar patients, compared with controls, only the increased relative density of GAD-ir neuropil in the right LD was different from the previous results in depressed suicides from the same cohort (Gos et al. in J Affect Disord 113:45–55, 2009). On the other hand, the left DLPFC was the only area where a significant decrease was observed, specific for bipolar I depression. Significant differences between both diagnostic groups were found in these regions. By revealing abnormalities in the relative density of GAD-ir neuropil in brain structures, our study suggests a diathesis of the GABAergic system in mood disorders, which may differentiate the pathophysiology of unipolar from that of bipolar I depression.
Keywords: GAD 65/67, Postmortem, Depression, Unipolar–bipolar dichotomy
Introduction
Abnormalities in the GABAergic system are assumed to play a crucial yet largely unknown role in the pathogenesis of mood disorders [3, 9, 41]. Gamma-aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the mature brain and plays an important role in synchronised neural network oscillations [17], synaptic plasticity [26] and neurogenesis [25]. The multitude of postmortem, clinical and preclinical studies predominantly suggests a hypothesis of low GABAergic activity in mood disorders (for recent reviews see references [3, 13, 30, 31]).
The quantitative neuropathological differences between major depressive disorder (MDD) and bipolar disorder (BD) have been addressed in neurohistological [7, 12] and molecular studies [15, 37, 40] on the GABAergic system. The pathological changes in markers related to this system, such as decreased parvalbumin mRNA in the dorsolateral prefrontal cortex (DLPFC) [37] and reduced density of glutamic acid decarboxylase 65/67-immunoreactive (GAD 65/67-ir) neurons in DLPFC and temporal cortical areas [7] specific for BD, differentiated this diagnostic entity from MDD. GAD is the rate-limiting enzyme involved in the conversion of glutamate to GABA with two isoforms, GAD 65 and GAD 67. Both of these functionally different isoforms are present in most GABA-containing neurones in the brain, but the transiently activated GAD 65 appears to be restricted to membranes and nerve endings, whereas the constitutively active GAD 67 is more widely distributed in cells [28, 42].
Despite the prevailing similarities, clinical studies accentuate some differences in symptom profiles and severity measures between unipolar and bipolar depression because of their important therapeutic implications (for reviews see references [16, 23, 38]). Similarities between them also predominate in neuroimaging studies (for a review see Ref. [36]) and according to the established consensus, a cross-sectional categorical distinction between unipolar and bipolar depression is currently impossible to make in clinical practice [16]. We hypothesised the presence of differences in the involvement of GABAergic neurotransmission in unipolar and bipolar I depression and have tested this hypothesis performing a quantitative evaluation of GAD 65/67-ir neuropil in several brain regions known to be involved in the pathophysiology of mood disorders.
Materials and methods
Subject characteristics
The demographic and clinical characteristics of the subjects are presented in Tables 1 and 2. The same cohort has previously been analysed according to the impact of suicide [21]. Briefly, postmortem brains from 21 depressed subjects with a clinical diagnosis of MDD (6 women and 3 men, ranging in age from 26 to 68 years) or BD subtype I (7 women and 5 men, ranging in age from 16 to 69 years), according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), were obtained from the Magdeburg Brain Bank. Fourteen of these subjects had died by suicide (8 MDD and 6 BD patients) and the remaining seven had died from natural causes (1 MDD and 6 BD patients). The diagnosis of suicide was made by a forensic pathologist (T.G.). Control brains (C) were collected from 9 women and 9 men with an age range of 33 to 65 years, who had died from natural causes. The postmortem interval (PMI) ranged from 5 to 96 h for patients and from 19 to 72 h for the controls. A toxicology screen of blood and urine for ethanol, other substances of abuse, multiple antidepressant and antipsychotic drugs and their metabolites was performed at each medico-legal autopsy.
Table 1.
Characteristics of subjects
| No./Sex/Age (years) | Psychiatric diagnosis (DSM-IV) | Cause of death | PMI (h) | |
|---|---|---|---|---|
| Control group | 1/M/50 | MI | 72 | |
| 2/M/47 | CF | 24 | ||
| 3/F/52 | Ovarian carcinoma | 24 | ||
| 4/M/47 | Respiratory insufficiency | 24 | ||
| 5/F/64 | Sepsis | 24 | ||
| 6/F/33 | CF | 72 | ||
| 7/M/38 | CF | 19 | ||
| 8/F/50 | Dissecting aortic aneurysm | 72 | ||
| 9/M/64 | HF | 35 | ||
| 10/F/48 | PE | 26 | ||
| 11/M/56 | HF | 24 | ||
| 12/F/65 | HF | 24 | ||
| 13/F/30 | PE | 48 | ||
| 14/M/63 | HF | 48 | ||
| 15/F/64 | HF | 24 | ||
| 16/F/38 | PE | 24 | ||
| 17/M/54 | RHF | 24 | ||
| 18/M/46 | Lymphoma | 24 | ||
| MDD group | 1/F/63 | MDD (296.34) | PE | 17 |
| 2/M/42 | MDD (296.33) | Hanging | 5 | |
| 3/F/46 | MDD (296.24) | Hanging | 48 | |
| 4/F/53 | MDD (296.33) | Hanging | 46 | |
| 5/F/26 | MDD (296.33) | Fall from the height | 22 | |
| 6/F/68 | MDD (296.33) | Hanging | 96 | |
| 7/M/35 | MDD (296.33) | Incision of radial artery | 15 | |
| 8/M/36 | MDD (296.34) | Hanging | 42 | |
| 9/F/39 | MDD (296.23) | Overdose of medication | 48 | |
|
BD group
|
10/F/62 | BD-D (296.54) | PE | 72 |
| 11/M/39 | BD-D (296.54) | CF | 56 | |
| 12/M/69 | BD-D (296.54) | PE | 48 | |
| 13/F/52 | BD-D (296.54) | PE | 24 | |
| 14/F/65 | BD-D (296.54) | HF | 52 | |
| 15/M/44 | BD-D (296.54) | HF | 96 | |
| 16/F/53 | BD-D (296.53) | Electric shock | 47 | |
| 17/M/47 | BD-D (296.53) | Stab wound | 24 | |
| 18/F/16 | BD-D (296.54) | Hanging | 48 | |
| 19/F/46 | BD-D (296.54) | Overdose of medication | 4 | |
| 20/M/42 | BD-D (296.54) | Hanging | 17 | |
| 21/F/59 | BD-D (296.54) | Overdose of medication | 72 |
M male, F female, PMI postmortem interval, MDD major depressive disorder, BD-D bipolar I disorder depressed, CF coronary failure, HF heart failure, MI myocardial infarction, PE pulmonary embolism, RHF right heart failure
Table 2.
Mean daily doses of psychotropic medication in the last 90 days of lifetime (benzodiazepines—in the last 28 days of lifetime)
| Patient no. | Antidepressants (amitriptyline equivalents) mg |
Neuroleptics (chlorpromazine equivalents) mg |
Benzodiazepines (diazepam equivalents) mg |
Lithium mg |
|---|---|---|---|---|
| 1 | 50 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 124 | 109 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 |
| 9 | 93 | 0 | 3.1 | 560 |
| 10 | 0 | 110 | 17.6 | 0 |
| 11 | 0 | 221 | 0.8 | 740 |
| 12 | 0 | 0 | 6.8 | 0 |
| 13 | 0 | 0 | 0 | 0 |
| 14 | 93 | 117 | 3.9 | 0 |
| 15 | 0 | 0 | 0 | 0 |
| 16 | 67 | 0 | 0 | 0 |
| 17 | 20 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 |
| 19 | 133 | 327 | 3.3 | 558 |
| 20 | 95 | 47 | 18.3 | 565 |
| 21 | 112 | 140 | 10 | 0 |
The study was approved by the local ethics committee of the University of Magdeburg as being compliant with the Declaration of Helsinki of 1989 and the applicable EU and German laws. In accordance with the German autopsy laws, informed consent was obtained from the relatives of the deceased for autopsy and dissection of the brains and for use of clinical information for research purposes.
Antemortem DSM-IV diagnoses were obtained from psychological autopsies involving a careful review of clinical records and the performance of structured interviews with the physicians involved in the treatment and at least one person who either had lived with or had frequent contact with the subject before death. The DSM-IV Axis I diagnosis of MDD and BD subtype I was established in a consensus meeting by two independent psychiatrists (H.B. and J.S.). The same procedure was followed to exclude psychiatric disorders in controls. All BD patients were in a depressive episode at the moment of death. There was no current or lifetime psychoactive substance disorder history (abuse or dependence according to DSM-IV) in any of the subjects.
The mean doses of psychotropic medication in the last 90 days of life were established according to the clinical records, taking into account the equivalents of psychotropic medication present in the references [8, 10, 32, 33]. The majority of patients (12 of 21) had received psychotropic medication in this period. However, the medicated patients prevailed in the BD group (9 out of 12) compared with the MDD group (3 of 9).
Qualitative neuropathological changes due to neurodegenerative disorders (such as Alzheimer’s disease, Parkinson’s disease and Pick’s disease), tumours, inflammatory, vascular (microangiopathy, infarctions, lacunar infarctions and Binswanger’s disease) or traumatic processes were ruled out by an experienced neuropathologist (C.M.). The alterations suggestive of neurodegenerative disorders were excluded by immunostaining for beta-amyloid, hyperphosphorylated tau-protein and ubiquitin as well as by Gallyas silver stain.
Tissue collection and preparation
After removal, brains were fixed in toto in 8 % phosphate-buffered formaldehyde for at least 2 months (pH = 7.0, t = 15–20 °C). Subsequently, after separation of the brainstem with the cerebellum, the hemispheres were divided by coronal cuts into three bi-hemispherical coronal blocks comprising the frontal lobe anterior to the genu of the corpus callosum (‘anterior’ block), the fronto-temporo-parietal lobe extending over the whole length of the corpus callosum (‘middle’ block) and the occipital lobe (‘posterior’ block). The ‘middle’ block included, in addition to the cortical areas, the thalamus and the hippocampal formation. The shrinkage of brain tissue during paraffin embedding was evaluated for each brain by calculating the ratio of identical distances between the opposite cortical gyri on the most rostral and the most caudal sections of the ‘middle’ block before and after embedding. The individual volume shrinkage factors (VSF) were calculated from the measured linear shrinkage factor (LSF) using the following formula: VSF = (LSF)3. The mean shrinkage factor was 2.25 for control brains, 2.27 for MDD patients and 2.13 for BD patients (a non-significant Kruskal–Wallis test with the three groups as independent variable). Two randomly selected coronal sections with a thickness of 20 μm per above-described ‘anterior’ block (at the level of the pregenual part of the anterior cingulate cortex) and two per ‘middle’ block (at the level of pes hippocampi) were immunohistochemically stained for GAD. Samples from all subject groups and all brain regions were included in every batch together with standard normalisation samples.
Immunohistochemistry
In order to immunolocalise GAD, a monoclonal antibody to GAD 65 and 67 in mice was used (Medical & Biological Laboratories Co., Wobrun, USA). The specificity of the antiserum was confirmed by the supplier by Western blotting and immunocytochemistry. After preincubation of the sections with methanol/H2O2 to depress endogenous peroxidases and repeated washing with phosphate-buffered saline, the primary GAD antibody was used at a 1:100 dilution for 72 h at 4 °C. Sections were then incubated with a biotinylated anti-mouse secondary antibody (Amersham Biosciences UK, Ltd., Little Chalford, UK) for the application of the avidin–biotin technique (Amersham). The chromogen 3,3′-diaminobenzidine (DAB) and ammonium nickel sulphate hexahydrate were used to visualise the reaction product. For the purposes of control, the primary antiserum was replaced either by a buffer or by normal serum and did not show any immunostaining in the investigated regions. Figure 1 shows the GAD-immunostaining pattern in the DLPFC and the lateral dorsal thalamic nucleus (LD).
Fig. 1.
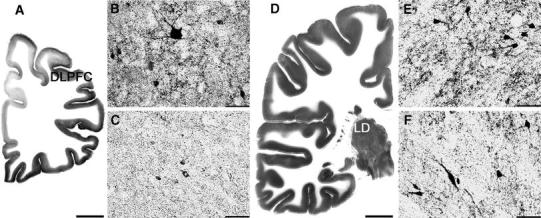
GAD 65/67 immunoreactivity in the left DLPFC (dorsolateral prefrontal cortex) and the right LD (lateral dorsal thalamic nucleus) of unipolar (b and e, respectively) and bipolar I (c and f, respectively) depressed patients; scale bars 50 μm. These regions of interest are shown at low magnification in pictures of GAD-immunostaining of a control case (a and d; scale bars 10 mm)
Quantification
A quantitative morphological analysis was performed in each of the selected sections as reported previously [21]. The relative density of GAD-ir neuropil (the quotient of the area of fibres and/or synaptic endings and total measuring field area, see below) of depressed patients and controls was measured in neocortical areas: prefrontal [DLPFC, orbitofrontal (OFC) and pregenual anterior cingulate] and temporal [parahippocampal gyrus containing the entorhinal cortex (EC)], in the hippocampal formation [dentate gyrus (DG) and the CA1 field of the hippocampus (CA1)] and in thalamic nuclei (medial dorsal [MD] and LD). For each neocortical area, three boxes in layer III and further three in layer V were randomly selected and scanned bilaterally by a video-equipped system (Olympus BX60 microscope equipped with a ColorView Soft Imaging System digital camera) onto the computer using a 20× objective. For the hippocampal complex, the same procedure was carried out in the pyramidal layer of CA1 and in the granular layer of DG. For LD and MD, three boxes were approached bilaterally. The number of evaluated boxes was established by the statistical analysis of preliminary data in which ten boxes per structure were evaluated bilaterally.
The relative GAD-ir neuropil area of the regions mentioned previously was determined using a computer application for densitometric image analysis, AnalySIS® Auto Version 3.2 (Soft Imaging System GmbH, Münster, Germany). For the purpose of measuring, the area of immunostained structures the immunoreactive neuropil was visualised via adjustments of minimum and maximum grey levels of the nickel-enhanced DAB precipitate under visual control. The area of the marked immunoreactive fibres and/or endings was calculated and divided by the total area of the measuring field and thus demonstrated as the relative area of immunoreactive neuropil. The aim was to detect the difference in GABAergic innervation among the analysed groups according to the method described previously [21] rather than obtaining absolute values of the stained neuropil density.
The measurements were performed by one of the authors (K.G.) blinded to the diagnosis. In order to establish inter-rater (K.G., T.G.) and test–retest reliability, repeated measurements for 5 brains were carried out. Intraclass correlation analyses yielded correlation coefficients ranging from 0.90 to 1.00 in both inter-rater and test–retest reliability evaluation.
Data analysis
As normal distribution (i.e. significant results of the Kolmogorov–Smirnoff test) was not given in all the analysed regions, non-parametric statistical procedures were used. Firstly, a Kruskal–Wallis analysis of the variance of ranks (H test) was performed using the diagnostic group as a 3-level independent variable (unipolar vs bipolar I depressed patients vs controls) and GAD-ir neuropil density, respectively. Secondly, the unadjusted two-way post hoc comparisons with the Mann–Whitney U test were carried out to detect between-group differences.
The same statistical procedures were employed to detect the possible differences between the groups according to the age at death, brain weight, postmortem delay and the time of fixation. The χ2-test was employed to detect the possible differences between them with respect to sex. The unadjusted two-way comparisons with the U test were employed to detect the possible differences between MDD and BD patients according to the age at the onset, duration of both the illness and depressive episode and psychotropic medication.
Spearman correlation coefficients were calculated to determine the impact of the above demographic, clinical and methodical variables, which might confound the results of dependent variable.
Generally, P values of <0.05 were accepted as statistically significant. When both the H test and triple post hoc comparisons with the U test were considered in combination, the P values were corrected for multiple comparisons in line with the Bonferroni–Holm–Shaffer procedure.
Results
Density of GAD-ir neuropil
The detailed results of group comparisons are shown in Table 3.
Table 3.
Presentation of significant diagnostic group differences regarding GAD-immunoreactive neuropil relative density
| ROI and group | GAD-ir neuropil density | H test P | Quotient of medians | ||
|---|---|---|---|---|---|
| Median (q1, q3; n) | MDD/C | BD/C | MDD/BD | ||
| CA1 left | |||||
| C | 0.004 (0.001, 0.253; 18) | 0.017 | 309** | 4 | 88* |
| MDD | 1.234 (0.723, 3.337; 9) | ||||
| BD | 0.014 (0.002, 0.916; 12) | ||||
| DLPFC left III | |||||
| C | 1.173 (0.016, 3.491; 18) | 0.041 | 0.598 | 0.006* | 100 |
| MDD | 0.702 (0.048, 2.899; 9) | ||||
| BD | 0.007 (0.003, 0.029; 12) | ||||
| DLPFC left V | |||||
| C | 0.552 (0.014, 5.346; 18) | 0.015 | 2 | 0.013* | 115** |
| MDD | 0.806 (0.215, 3.598; 9) | ||||
| BD | 0.007 (0.002, 0.018; 12) | ||||
| EC left III | |||||
| C | 0.008 (0.003, 0.029; 18) | 0.001 | 64** | 40** | 2 |
| MDD | 0.510 (0.167, 1.735; 9) | ||||
| BD | 0.321 (0.050, 1.777; 12) | ||||
| EC left V | |||||
| C | 0.011 (0.006, 0.035; 18) | 0.016 | 59** | 17 | 4 |
| MDD | 0.649 (0.416, 1.143; 9) | ||||
| BD | 0.184 (0.012, 1.814; 12) | ||||
| EC right III | |||||
| C | 0.012 (0.003, 0.069; 18) | 0.009 | 46* | 13* | 4 |
| MDD | 0.548 (0.330, 1.482; 9) | ||||
| BD | 0.152 (0.015, 1.275; 12) | ||||
| EC right V | |||||
| C | 0.023 (0.005, 0.073; 18) | 0.029 | 29** | 5 | 6 |
| MDD | 0.662 (0.329, 2.895; 9) | ||||
| BD | 0.103 (0.003, 1.303; 12) | ||||
| LD right | |||||
| C | 0.237 (0.086, 0.513; 18) | 0.028 | 4* | 0.802 | 6* |
| MDD | 1.048 (0.489, 1.434; 9) | ||||
| BD | 0.190 (0.072, 0.399; 12) | ||||
ROI region of interest, GAD-ir GAD-immunoreactive, C controls, MDD major depressive disorder, and BD bipolar I disorder depressed patients, q1 and q3 quartile 1 and 3,n number of cases, CA1 CA1 field of hippocampus, DLPFC dorsolateral prefrontal cortex, EC entorhinal cortex, LD lateral dorsal thalamic nucleus; III layer III, and V layer V of pyramidal cells in neocortex; H test P—H test P values; * P < 0.05, ** P < 0.01 (P values corrected for multiple comparisons are related to the two-groups comparisons by post hoc U tests; values of the quotient of medians higher than 1 were rounded off to the whole numbers)
Confounders
Variables that could influence GAD-ir neuropil density, such as PMI, time of fixation, shrinkage factor, sex, age at the time of death, brain weight, duration of both the illness and depressive episode, age at the onset of illness and psychiatric medication, were analysed.
The only significant difference existed in the mean duration of illness, which was significantly higher in BD versus MDD patients (17.3 vs. 4.2). The non-normal distribution of data excluded the implementation of analysis of covariance (ANCOVA). However, the duration of illness did not correlate with GAD-ir neuropil density in those regions of interest where significant differences in the investigated parameter between the compared diagnostic groups were found. A positive correlation between the confounding and dependent variables was found in the left OFC in both of the investigated cortical layers in BD patients only (r = 0.71, P = 0.047).
There were no significant differences in the mean doses of psychotropic medication between the compared groups of patients. However, there were significant, mostly negative correlations between GAD-ir neuropil density and the mean doses of psychotropic medication, predominantly in BD patients (Table 4).
Table 4.
The significant correlations found between psychotropic medication and GAD-immunoreactive neuropil relative density in regions of interest of unipolar (MDD) and bipolar I (BD) depressed patients (antidepressants, neuroleptics and lithium—in the last 90 days, benzodiazepines—in the last 28 days of lifetime)
| ROI | AD | NL | BDZ | Lithium | |
|---|---|---|---|---|---|
| MDD | BD | BD | BD | BD | |
| ACC left V | 0.89* | ||||
| DG left | −0.85* | ||||
| EC left III | 0.82* | ||||
| EC right V | −0.94* | ||||
| MD left | −0.89* | −0.76* | |||
| OFC left III | 0.72* | ||||
| OFC right III | −0.73* | ||||
| OFC right V | −0.76* | ||||
ROI region of interest, AD antidepressants, NL neuroleptics, BDZ benzodiazepines, ACC anterior cingulate cortex, DG dentate gyrus, EC entorhinal cortex, MD medial dorsal thalamic nucleus, OFC orbitofrontal cortex, III layer III, and V layer V of pyramidal cells in neocortex; the values of correlation coefficients r are shown; * P < 0.05
Discussion
Diagnostic issues
Compared with healthy controls, the increased density of GAD-ir neuropil prevailed in the evaluated cerebral regions of depressed patients, being accentuated in the EC and the hippocampus, predominantly in MDD (Table 3). MDD patients had increased values of this parameter compared with BD patients and controls in the left CA1 and the right LD. The left DLPFC was the only area where significantly decreased GAD-ir neuropil density was found in BD compared with MDD patients and controls.
We have recently revealed in a series of publications that processes leading to suicide have an outstanding impact on the observed neurohistological abnormalities independent from the main Axis I diagnosis [18–22, 39]. This impact was also accentuated in our previous quantitative study on GAD-ir neuropil in the same cohort [21], where we found an increase in its density specific for depressed suicidal patients from the MDD and BD diagnostic groups. Therefore, the impact of suicide should be considered in the interpretation of our current results.
Because suicidal patients prevailed in the MDD group (8 out of 9), the increased GAD-ir neuropil density observed in this group could be related to suicide in regions where the increase had previously been found [21], such as in the left CA1. The right LD was the only region in MDD patients where the currently observed increase did not overlap with previous findings in the suicidal group. Therefore, only the increased parameter in this structure involved in separate aspects of episodic memory [1, 11, 35] should be considered as being specific for unipolar depression (Fig. 1).
On the other hand, the BD group was balanced in terms of suicidal versus non-suicidal patients. Therefore, the decreased GAD-ir neuropil density currently observed in the left DLPFC seems to be specific for bipolar I depression (Fig. 1) when considering an opposite effect previously found in suicide [21]. A recent immunoblotting study [27] revealed that the amount of GAD 65 specific for axonal endings was unchanged in DLPFC in both medicated and medication-free MDD patients, which is consistent with our interpretation of the present findings (Table 3).
The effect we observed was consistent with other data obtained in the PFC in both BD and schizophrenia [2, 5, 24, 40, 43]. Moreover, in line with the study on GAD 65 and GAD 67 gene expression in elderly schizophrenics [14], we found a positive correlation between the duration of illness and GAD-ir neuropil density in BD patients in one of the investigated PFC regions (namely in the left OFC; r = 0.71, P = 0.047). Therefore, both ours and the cited results for PFC support similarities between schizophrenia and BD.
Influence of psychotropic medication
We demonstrated some significant correlations, predominantly negative, between the relative density of GAD-ir neuropil and the mean doses of psychotropic medication (Table 4). However, the correlations found in the MDD group are not very meaningful because only three MDD patients received antidepressants. On the other hand, in the BD group, correlations prevailed in areas where no significant differences in GAD-ir neuropil density were observed (5 out of 7). Moreover, the number of treated BD patients did not exceed 7 (out of 12) in each of the analysed groups of psychotropic agents (Table 2). Therefore, it cannot be conclusively stated whether medication did in fact confound our results.
Other human and concurrent preclinical studies have suggested that antidepressants might up-regulate GAD [6, 7, 27, 34]. In experimental conditions, antidepressants may also down-regulate the level of GABA in cortical areas [29]. Therefore, in the light of both previous [21] and present findings, antidepressant medication might exert an effect that possibly counteracts the abnormality in the GABAergic system. However, the putatively positive effect of pharmacotherapy is regionally differentiated and is absent in the DLPFC and LD (Table 4).
Limitations
A major limitation of our study is the relatively small number of cases. For future studies, the numbers of cases from both diagnostic groups should be increased and subgroups of remitted MDD and BD I patients should be included in order to differentiate between trait and state conditions of the GABAergic system in uni- and bipolar depression. A further limitation of this study is the lack of data on lifetime drug exposure, as we could only collect data on psychotropic medication covering the last 3 months before death. Moreover, especially in studies investigating mood disorders, the heterogeneity of the results may likely be caused by clinical sample variation. The available well-documented clinical files do not suggest that some of our MDD patients were actually BD ones. However, we cannot unequivocally exclude this doubt existing in view of the current criticism on the MDD concept [4]. The implementation of a monoclonal antibody to both isoforms of GAD should be considered as another limiting factor in the interpretation of our results and in the discussion with the overwhelming majority of previous studies which have applied isoform-specific antibodies.
Conclusion
When considering previous results regarding the influence of suicide, an increase in the relative density of GAD-ir neuropil in the right LD seems to be specific for MDD, whereas its decrease observed in the left DLPFC may be pathognomonic of bipolar I depression. By revealing a diagnosis- and brain region-specific pattern of abnormalities in the GAD-ir, our study suggests a diathesis of the GABAergic system, which may differentiate the pathophysiology of unipolar from that of bipolar I depression. The suggested diathesis of the GABAergic system in depression may be partially regulated by psychotropic medication.
Acknowledgments
The research was supported by grants of the Stanley Medical Research Institute to B.B. and J.S. (Grant No. 07R-1832), the Brain Net and the Bundesministerium für Bildung und Forschung to B.B. (BMBF NBL-3/2). The authors would like to thank Renate Stauch and Dieter Krell for their excellent technical assistance.
Conflict of interest
All authors declare no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Tomasz Gos and Johann Steiner contributed equally to this work.
References
- 1.Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Angst J, Gamma A, Bowden CL, Azorin JM, Perugi G, Vieta E, Young AH. Diagnostic criteria for bipolarity based on an international sample of 5,635 patients with DSM-IV major depressive episodes. Eur Arch Psychiatry Clin Neurosci. 2012;262:3–11. doi: 10.1007/s00406-011-0228-0. [DOI] [PubMed] [Google Scholar]
- 5.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/S0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 6.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 7.Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Meyer-Lotz G, Brodhun M, Dobrowolny H, Baumann B, Gos T, Bernstein HG, Bogerts B. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann N Y Acad Sci. 2007;1096:157–169. doi: 10.1196/annals.1397.081. [DOI] [PubMed] [Google Scholar]
- 8.Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 10.Busto U, Sellers EM, Naranjo CA, Cappell H, Sanchez-Craig M, Sykora K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. N Engl J Med. 1986;315:854–859. doi: 10.1056/NEJM198610023151403. [DOI] [PubMed] [Google Scholar]
- 11.Cipolotti L, Husain M, Crinion J, Bird CM, Khan SS, Losseff N, Howard RS, Leff AP. The role of the thalamus in amnesia: a tractography, high-resolution MRI and neuropsychological study. Neuropsychologia. 2008;46:2745–2758. doi: 10.1016/j.neuropsychologia.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377–386. doi: 10.1016/S0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 13.Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35:818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- 15.Fatemi SH, Folsom TD, Thuras PD. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res. 2011;128:37–43. doi: 10.1016/j.schres.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin GM, Anderson I, Arango C, Bowden CL, Henry C, Mitchell PB, Nolen WA, Vieta E, Wittchen HU (2008) ECNP consensus meeting. Bipolar depression. Nice, March 2007. Eur Neuropsychopharmacol 18:535–549 [DOI] [PubMed]
- 17.Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011 doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gos T, Krell D, Brisch R, Bielau H, Trübner K, Bernstein HG, Bogerts B. The changes of AgNOR parameters of anterior cingulate pyramidal neurons are region-specific in suicidal and non-suicidal depressive patients. World J Biol Psychiatry. 2007;8:245–255. doi: 10.1080/15622970601169758. [DOI] [PubMed] [Google Scholar]
- 19.Gos T, Krell D, Bielau H, Brisch R, Trübner K, Steiner J, Bernstein HG, Jankowski Z, Bogerts B. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is elevated in violent suicidal depressive patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:513–520. doi: 10.1007/s00406-008-0825-8. [DOI] [PubMed] [Google Scholar]
- 20.Gos T, Krell D, Brisch R, Bielau H, Trübner K, Steiner J, Bernstein HG, Bogerts B. Demonstration of decreased activity of dorsal raphe nucleus neurons in depressed suicidal patients by the AgNOR staining method. J Affect Disord. 2008;111:251–260. doi: 10.1016/j.jad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Gos T, Günther K, Bielau H, Dobrowolny H, Mawrin C, Trübner K, Brisch R, Steiner J, Bernstein HG, Jankowski Z, Bogerts B. Suicide and depression in the quantitative analysis of glutamic acid decarboxylase-immunoreactive neuropil. J Affect Disord. 2009;113:45–55. doi: 10.1016/j.jad.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Gos T, Krell D, Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Bernstein HG, Jankowski Z, Bogerts B. Demonstration of disturbed activity of the lateral amygdaloid nucleus projection neurons in depressed patients by the AgNOR staining method. J Affect Disord. 2010;126:402–410. doi: 10.1016/j.jad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Möller HJ, Kasper S, WFSBP Task Force On Treatment Guidelines For Bipolar Disorders The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11:81–109. doi: 10.3109/15622970903555881. [DOI] [PubMed] [Google Scholar]
- 24.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 25.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laprade N, Soghomonian JJ. Gene expression of the GAD67 and GAD65 isoforms of glutamate decarboxylase is differentially altered in subpopulations of striatal neurons in adult rats lesioned with 6-OHDA as neonates. Synapse. 1999;33:36–48. doi: 10.1002/(SICI)1098-2396(199907)33:1<36::AID-SYN4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 30.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Ng WX, Lau IY, Graham S, Sim K. Neurobiological evidence for thalamic, hippocampal and related glutamatergic abnormalities in bipolar disorder: A review and synthesis. Neurosci Biobehav Rev. 2009;33:336–354. doi: 10.1016/j.neubiorev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Perry PJ, Alexander B. Sedative/hypnotic dependence: patient stabilization, tolerance testing, and withdrawal. Drug Intell Clin Pharm. 1986;20:532–537. doi: 10.1177/106002808602000702. [DOI] [PubMed] [Google Scholar]
- 33.Rey MJ, Schulz P, Costa C, Dick P, Tissot R. Guidelines for the dosage of neuroleptics. I: Chlorpromazine equivalents of orally administered neuroleptics. Int Clin Psychopharmacol. 1989;4:95–104. doi: 10.1097/00004850-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 35.Saunders RC, Mishkin M, Aggleton JP. Projections from the entorhinal cortex, perirhinal cortex, presubiculum, and parasubiculum to the medial thalamus in macaque monkeys: identifying different pathways using disconnection techniques. Exp Brain Res. 2005;167:1–16. doi: 10.1007/s00221-005-2361-3. [DOI] [PubMed] [Google Scholar]
- 36.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souery D, Zaninotto L, Calati R, Linotte S, Mendlewicz J, Sentissi O, Serretti A. Depression across mood disorders: review and analysis in a clinical sample. Compr Psychiatry. 2012;53:24–38. doi: 10.1016/j.comppsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Wei J, Wu JY. Post-translational regulation of l-glutamic acid decarboxylase in the brain. Neurochem Res. 2008;33:1459–1465. doi: 10.1007/s11064-008-9600-5. [DOI] [PubMed] [Google Scholar]
- 43.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]


