Abstract
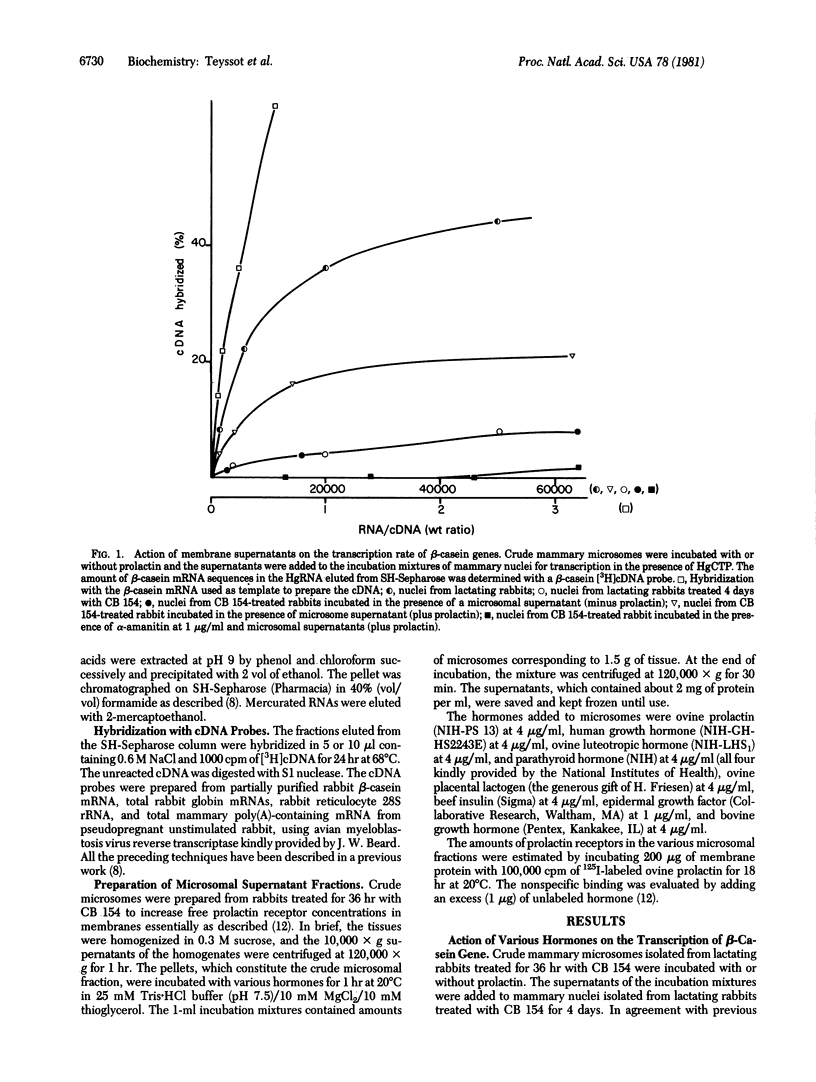
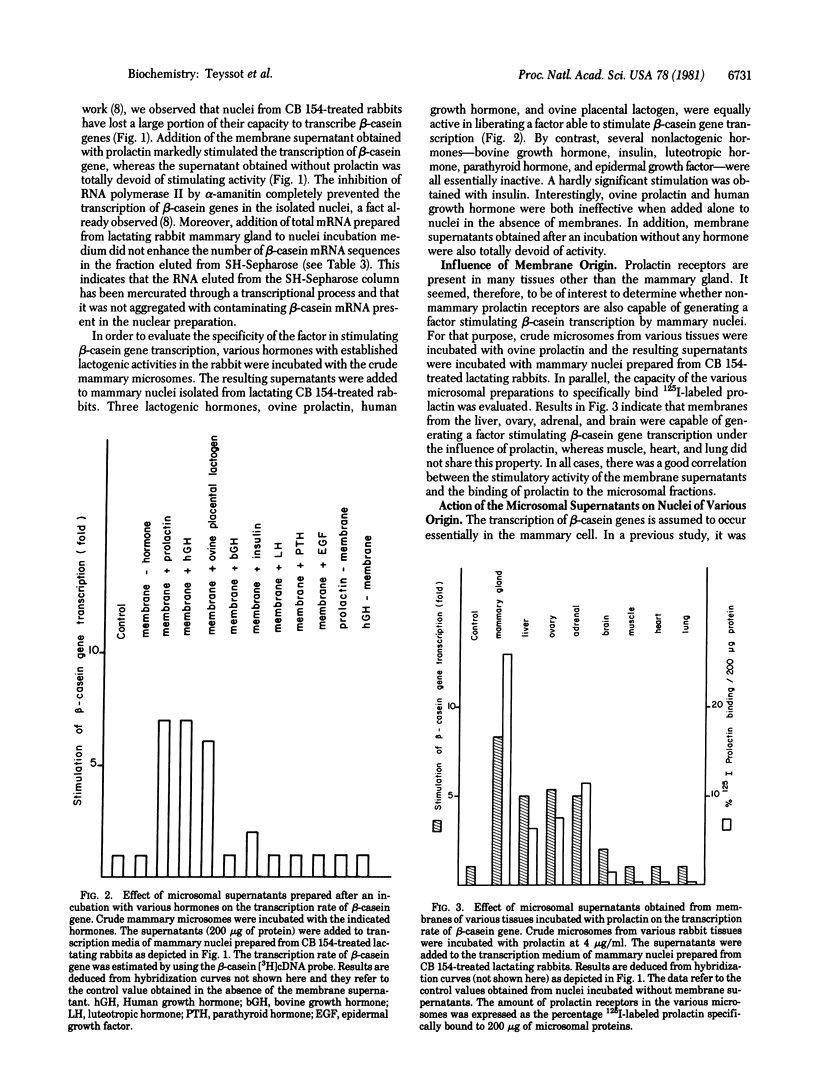
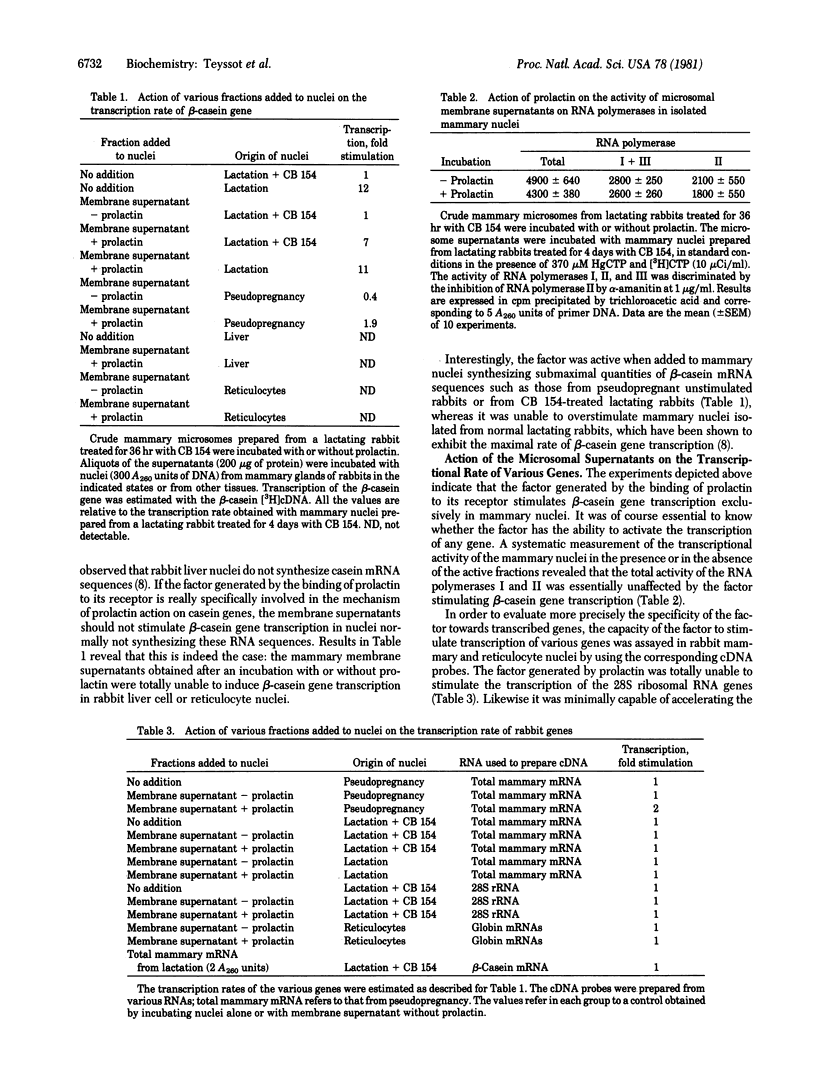
Crude microsomes from lactating rabbit mammary gland were incubated with prolactin. The incubation mixture was centrifuged and the supernatant was incubated with isolated mammary cell nuclei from lactating rabbits treated for 4 days by bromocryptin to antagonize prolactin and to deinduce casein gene transcription. Nuclei were incubated with HgCTP, and the newly synthesized mercurated RNA was isolated on SH-Sepharose columns. The content of β-casein mRNA sequences in the fraction eluted with 2-mercaptoethanol was estimated with a [3H]cDNA probe obtained from partially purified β-casein mRNA. The supernatant markedly stimulated β-casein gene transcription but not 28S rRNA transcription. The same effect was obtained with other lactogenic hormones such as human growth hormone and ovine placental lactogen but was not observed with bovine growth hormone, insulin, parathyroid hormone, luteotropic hormone, or epidermal growth factor. Prolactin and human growth hormone were totally inactive when added directly to nuclei. The factor stimulating β-casein gene transcription was also generated by membranes containing prolactin receptors such as those from liver, ovary, adrenals, and brain but not by membranes from heart, lung, and muscle, which do not bind prolactin. The factor stimulated β-casein transcription when added to mammary nuclei from pseudopregnant or bromocryptin-treated lactating rabbits, in which the transcription rate is submaximal, but was ineffective on mammary nuclei prepared from untreated fully lactating rabbits. The factor was unable to induce β-casein gene transcription in nuclei isolated from rabbit liver and reticulocytes. The factor did not stimulate the transcription of globin genes in nuclei isolated from reticulocytes or the transcription of mammary “housekeeping” genes evaluated by a cDNA probe prepared from total mRNA isolated from an unstimulated mammary gland. The transcription of β-casein genes was abolished by adding α-amanitin to the medium in the presence or in the absence of the factor, indicating that the generation of mercurated β-casein mRNA sequences depended upon the transcriptional activity of RNA polymerase II. The addition of the factor to the incubation mixture did not enhance total and α-amanitin-sensitive RNA synthesis. These data suggest that the binding of prolactin to its receptor in vitro induces the formation of a second messager, which specifically stimulates the transcription of prolactin-sensitive genes.
Keywords: prolactin second messenger
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan L., Means A. R., O'Malley B. W. Steroid hormone regulation of specific gene expression. Vitam Horm. 1978;36:259–295. doi: 10.1016/s0083-6729(08)60986-3. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Fodor E. J., Doty P. The specificity of in vitro chromatin transcription. Nucleic Acids Res. 1979 Jan;6(1):371–383. doi: 10.1093/nar/6.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Effects of prolactin and progesterone on expression of casein genes. Titration of casein mRNA by hybridization with complementary DNA. Eur J Biochem. 1976 Sep;68(1):219–225. doi: 10.1111/j.1432-1033.1976.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Ingram V. M. Is there specific transcription from isolated chromatin? Nucleic Acids Res. 1978 Apr;5(4):1237–1252. doi: 10.1093/nar/5.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Ingram V. M. RNA aggregation during sulfhydryl-agarose chromatography of mercurated RNA. Nucleic Acids Res. 1977 Jun;4(6):1979–1988. doi: 10.1093/nar/4.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Lee D. C. Regulation of gene transcription by estrogen and progesterone. Lack of hormonal effects on transcription by Escherichia coli RNA polymerase. J Biol Chem. 1980 Oct 25;255(20):9693–9698. [PubMed] [Google Scholar]
- Popp D. A., Kiechle F. L., Kotagal N., Jarett L. Insulin stimulation of pyruvate dehydrogenase in an isolated plasma membrane-mitochondrial mixture occurs by activation of pyruvate dehydrogenase phosphatase. J Biol Chem. 1980 Aug 25;255(16):7540–7543. [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Evidence that insulin activates an intrinsic plasma membrane protease in generating a secondary chemical mediator. J Biol Chem. 1980 Jul 25;255(14):6529–6531. [PubMed] [Google Scholar]
- Seals J. R., Jarett L. Activation of pyruvate dehydrogenase by direct addition of insulin to an isolated plasma membrane/mitochondria mixture: evidence for generated of insulin's second messenger in a subcellular system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):77–81. doi: 10.1073/pnas.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Jarett L. Insulin effect on protein phosphorylation of plasma membranes and mitochondria in a subcellular system from rat adipocytes. I. Identification of insulin-sensitive phosphoproteins. J Biol Chem. 1979 Aug 10;254(15):6991–6996. [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Jarett L. Insulin effect on protein phosphorylation of plasma membranes and mitochondria in a subcellular system from rat adipocytes. II. Characterization of insulin-sensitive phosphoproteins and conditions for observation of the insulin effect. J Biol Chem. 1979 Aug 10;254(15):6997–7001. [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Parks W. P., Scolnick E. M. In vitro transcription of Moloney leukemia virus genes in infected cell nuclei and chromatin: elongation of chromatin associated ribonucleic acid by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1977 May 3;16(9):1795–1801. doi: 10.1021/bi00628a005. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Effects of colchicine on the transcription rate of beta-casein and 28 S-ribosomal RNA genes in the rabbit mammary gland. Biochem Biophys Res Commun. 1980 Nov 28;97(2):463–473. doi: 10.1016/0006-291x(80)90286-7. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of progesterone and glucocorticoids in the transcription of the beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1981 Mar;114(3):597–608. doi: 10.1111/j.1432-1033.1981.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of progesterone and glucocorticoids in the transcription of the beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1981 Mar;114(3):597–608. doi: 10.1111/j.1432-1033.1981.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of prolactin in the transcription of beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1980 Sep;110(1):263–272. doi: 10.1111/j.1432-1033.1980.tb04864.x. [DOI] [PubMed] [Google Scholar]
- Ueno K., Sekimizu K., Obinata M., Mizuno D., Natori S. Stimulation of messenger ribonucleic acid synthesis in isolated nuclei by a protein that stimulates RNA polymerase II. Biochemistry. 1981 Feb 3;20(3):634–640. doi: 10.1021/bi00506a029. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Analysis of in vitro transcription of duck reticulocyte chromatin using mercury-substituted ribonucleoside triphosphates. Biochemistry. 1977 Nov 15;16(23):5135–5145. doi: 10.1021/bi00642a029. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Use of mercury-substituted ribonucleoside triphosphates can lead to artefacts in the analysis of in vitro chromatin transcrits. Biochem Biophys Res Commun. 1977 Apr 11;75(3):598–603. doi: 10.1016/0006-291x(77)91514-5. [DOI] [PubMed] [Google Scholar]



