Abstract
We administered zinc supplementation therapy over three years to patients with chronic hepatitis C and reported and that the aspartate aminotransferase (AST) and alanine aminotaransferase (ALT) levels decreased, and platelet counts increased, significantly in the group with increased serum zinc concentrations. We are continuing this treatment to clarify the long-term consequences and report here the changes in serum zinc concentrations over seven years and compare the cumulative incidence of hepatocellular carcinoma (HCC). We administered polaprezinc to 32 patients, randomly selected for zinc therapy (treatment group), while another 30 formed the control group. We measured the serum zinc and albumin concentrations and conducted a prospective study to determine long-term outcomes. The changes and rates of change of serum zinc concentrations after seven years were 76.7 ± 18.2 µg/dl and +0.302 ± 0.30% in the treatment group and 56.7 ± 12.4 µg/dl and +0.033 ± 0.21% in the control group and had increased significantly (p = 0.0002, p = 0.0036). Progression of liver disease seemed to vary, depending on serum albumin concentrations. In the group with baseline serum albumin concentrations of 4.0 g/dl or more, the change and rate of change of serum zinc concentrations increased significantly, and the cumulative incidence of HCC tended to decrease, in the treated group. According to multivariate analysis, the factors that contribute to a reduction in the incidence of HCC are zinc therapy (risk ratio: 0.113, 95% CI: 0.015–0.870, p = 0.0362), and platelet counts (0.766, 0.594–0.989, 0.0409). Zinc supplementation therapy seems to improve liver pathology and reduce the incidence of HCC.
Keywords: zinc supplementation therapy, chronic hepatitis C, serum zinc concentrations, cumulative incidence of HCC
Introduction
Zinc is an essential component of the diet and is required for the synthesis of enzymes involved in nucleic acid and protein metabolism, including DNA polymerase, RNA polymerase, alcohol dehydrogenase, carbonic anhydrase and alkaline phosphatase.(1,2) It is well known that zinc deficiency may result in diseases such as skin dermatitis(3) and lead to taste disorders. However, the association of zinc deficiency with the pathogenesis of liver disease(4,5) is less well understood. We investigated previously the effects of zinc supplementation therapy in 62 patients with chronic hepatitis C, randomly assigning 32 patients to the treatment group with administration of 300 mg polaprezinc daily, the remaining 30 forming the control group without administration of polaprezinc. Over three years, we measured the serum zinc concentrations, aspartate aminotransferase (AST) levels, alanine aminotaransferase (ALT) levels, and platelet counts and determined the cumulative incidence of hepatocellular carcinoma (HCC). We reported that, in the group with increased serum zinc concentrations, the AST and ALT levels and the cumulative incidence of HCC decreased significantly and the platelet counts increased significantly.(6) We are continuing the treatment in order to determine the long-term outcome of zinc supplementation therapy. The observation period is now an average of 7.7 years and the number of cases of HCC has increased. The changes in serum zinc concentration over seven years and the cumulative incidence of HCC may now be compared between the treatment and control groups to clarify the value of polaprezinc administration.
Materials and Methods
Patients and methods
A total of 62 Japanese patients with chronic hepatitis (CH) or liver cirrhosis (LC) attributable to persistent hepatitis C virus infection and who were examined at Nihon University Itabashi Hospital from September 1999 through January 2001, gave informed consent to their participation in this study. All of the patients were positive for serum HCV RNA (Amplicor HCV Monitor, Roche Diagnostic K.K., Tokyo, Japan) and negative for serum hepatitis B surface antigen (HBsAg, enzyme-linked immunosorbent assay, EIA, Dinabot, Tokyo, Japan), LE cells, anti-smooth muscle antibody (fluorescence antibody method, FA), and anti-mitochondria antibody (FA). No heavy drinkers (more than 30 g ethanol intake per day) and no patients undergoing interferon therapy were included in the study.
We randomly assigned 32 patients to the treatment group (150 mg polaprezinc administered orally per day) and the remaining 30 (not administered polaprezinc) constituted the control group. We measured the serum zinc concentrations and serum albumin concentrations every year and conducted a prospective study to determine the long-term outcome of the treatment. We compared the changes in serum zinc concentrations and serum albumin concentrations over seven and six years, and the cumulative incidence of HCC, between the treatment and control groups. We compared the long-term outcome of patients with CH or LC in terms of the cumulative probability of incidence of HCC.
However, other treatments for hepatitis have been provided and various patients have been treated by injections of Stronger neo-minophagen c (Monoammonium glycyrrhizinate, Glycine, L-Cysteine hydrochloride hydrate), oral treatment with ursodeoxycholic acid and amino acid supplementation. Therefore, the effect of administration of zinc preparations alone on improvement in liver function cannot be evaluated in these patients.
Zinc absorption in vivo is due largely to albumin and albumin concentrations also are important for assessing the progression of liver disease. This means that individuals with reduced serum albumin concentrations may have decreased absorption of zinc, leading to the progression of liver disease and an increased risk of HCC. Despite a decrease in serum albumin concentrations, increases in serum zinc concentrations likely were attributable to the zinc supplementation therapy. Therefore, we investigated the patients on the basis of their serum albumin concentrations and divided them into groups with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more. We investigated the cumulative incidence of HCC as the long-term outcome and sought to determine the long-term outcome of the zinc therapy in this prospective study.
Zinc supplementation therapy/Polaprezinc administered therapy/Zinc therapy and observation
1.0 g Promac® (ZERIA Pharmaceutical Co. Ltd., Tokyo, Japan), containing 150 mg polaprezinc, was administered orally twice daily, after breakfast and after dinner. 150 mg polaprezinc contains about 33.3 mg zinc. The follow-up schedule was as follows: the patients underwent abdominal ultrasonography every 3 or 6 months, and abdominal CT examination every 6 to 12 months, for the detection of HCC. When space occupying lesions (SOL) were detected in the livers of the patients by dynamic CT, enhancement of SOL was observed at the early phase of dynamic CT and the disappearance of SOL staining was observed at the late phase. A precise diagnosis was made by abdominal angiography. When SOL in the liver was not enhanced in the early phase of dynamic CT, or if a precise diagnosis could not be made by abdominal angiography, tumor biopsy was carried out and a precise diagnosis was made on the basis of the pathological findings. The end point of the observation period was reached when a patient was treated with interferon, or diagnosed with HCC, or stopped attending for medical examination.
Measurement of serum zinc concentrations
Serum zinc concentration may vary with the diurnal rhythm (high in the morning and low in the afternoon) and the effects of diet and drugs. Measurement of serum zinc concentrations was carried out by a direct colorimetric method,(7) the reference value being 65–110 µg/dl.
Statistical analysis
The gender was compared using the chi-square test for independence. The remaining parameters are indicated by the mean ± SD and were compared using the Mann-Whitney U test. Changes in serum zinc concentrations and serum albumin concentrations were compared between the treatment and the control groups. Serum zinc concentrations and their rate of change were compared over seven years using the Mann-Whitney U test. Cumulative incidence curves were determined with the Kaplan-Meier method and the differences between groups were assessed using the log-rank test. Analysis of factors for the risk of occurrence HCC was made using the Cox proportional hazard model and these were compared by multivariate analysis. Independent factors were identified using SPSS11.0 software (SPSS Inc., Chicago, IL). A p value of less than 0.05 was considered significant.
Results
Comparison of clinical background factors of the study patients
Comparisons of the baseline clinical characteristics between the patients with and without polaprezinc administration are shown in Table 1. We investigated the mean serum AST levels (U/l), the mean serum ALT levels (U/l), the mean serum Platelet count (×104/µl), the serum iron levels (µg/dl), the serum ferritin levels (ng/ml), the mean serum albumin concentrations (g/dl), the mean serum zinc concentrations (µg/dl), and the mean observation period (year) in the treatment and control groups. There were significant difference in serum ALT levels (p = 0.0048) and serum zinc concentrations (p = 0.0338).
Table 1.
Comparison of clinical background factors of the study patients
| Parameter | Treatment group | Control group | p value* |
|---|---|---|---|
| Number | 30 | 32 | |
| Gender (male) | 15 (51.7%) | 19 (57.5%) | 0.6441 |
| Age | 56.4 ± 13.1 | 59.8 ± 9.9 | 0.253 |
| BMI | 23.2 ± 3.4 | 22.9 ± 2.6 | 0.7337 |
| AST (U/L) | 63.4 ± 25.4 | 58.6 ± 24.0 | 0.4756 |
| ALT (U/L) | 90.2 ± 39.9 | 62.5 ± 34.6 | 0.0048 |
| Platelet count (×104/µL) | 15.2 ± 4.4 | 15.6 ± 5.3 | 0.7443 |
| Iron (µg/dl) | 125.1 ± 37.2 | 122.3 ± 41.2 | 0.7914 |
| Ferritin (ng/ml) | 124.2 ± 124.1 | 104.0 ± 73.7 | 0.46 |
| Zinc concentration (µg/dl) | 59.8 ± 9.0 | 54.5 ± 10.0 | 0.0338 |
| Albumin (g/dl) | 4.1 ± 0.2 | 4.1 ± 0.3 | 0.3262 |
| Observation period (year) | 8.5 ± 2.3 | 7.1 ± 3.1 | 0.1003 |
*p<0.005. Mann-Whitney U test, chi-square test. AST: Aspartate aminotransferase; the upper limit of the normal range is 38 U/L. ALT: Alanine aminotaransferase; the upper limit of the normal range is 44 U/L. Iron: Reference value being 80–160 µg/dl. Ferritin: Reference value being 20–280 ng/ml. Treatment group: patients with polaprezinc administration. Control group: patients without polaprezinc administration.
Comparison of the change and rates of change of serum zinc concentrations between patients with and without polaprezinc administration
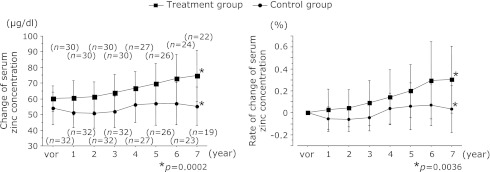
Comparisons between the treatment and control groups in terms of the change and rates of change of serum zinc concentrations are shown in Fig. 1. The observation period was seven years. The change and rates of change of serum zinc concentrations in the treatment group were 59.8 ± 9.1 µg/dl and 0% at baseline (n = 30), 60.8 ± 10.9 µg/dl and +0.029 ± 0.18% after 1 year (n = 30), 61.5 ± 9.5 µg/dl and +0.041 ± 0.16% after 2 years (n = 30), 64.3 ± 11.4 µg/dl and +0.089 ± 0.20% after 3 years (n = 30), 67.4 ± 10.6 µg/dl and +0.142 ± 0.25% after 4 years (n = 27), 70.5 ± 12.8 µg/dl and +0.198 ± 0.24% after 5 years (n = 26), 75.7 ± 19.4 µg/dl and +0.296 ± 0.35% after 6 years (n = 24), 76.7 ± 18.2 µg/dl and +0.302 ± 0.30% after 7 years (n = 22). All rate of change (%) was not expressed in value × 100 (for example, 0.302 was 30.2%). The values for the control group were 54.8 ± 10.0 µg/dl and 0% at baseline (n = 32), 51.1 ± 9.3 µg/dl and –0.057 ± 0.11% after 1 year (n = 32), 50.8 ± 9.2 µg/dl and –0.062 ± 0.10% after 2 years (n = 32), 51.8 ± 9.9 µg/dl and –0.046 ± 0.11% after 3 years (n = 32), 56.5 ± 10.7 µg/dl and +0.038 ± 0.14% after 4 years (n = 27), 57.0 ± 13.4 µg/dl and +0.060 ± 0.21% after 5 years (n = 26), 58.2 ± 13.3 µg/dl and +0.072 ± 0.18% after 6 years (n = 23), 56.7 ± 12.4 µg/dl and +0.033 ± 0.21% after 7 years (n = 19). After seven years, the serum zinc concentrations and rates of change of serum zinc concentrations were 76.7 ± 18.2 µg/dl and +0.302 ± 0.30% for the treatment group and 56.7 ± 12.4 µg/dl and +0.033 ± 0.21% for the control group and the serum concentrations of the treatment group had increased significantly (p = 0.0002, p = 0.0036).
Fig. 1.
Comparisons between the treatment and control groups in terms of the change and rates of change of serum zinc concentrations. After seven years, the serum zinc concentrations and rates of change of serum zinc concentration were 76.7 ± 18.2 µg/dl and +0.302 ± 0.30% for the treatment group and 56.7 ± 12.4 µg/dl and +0.033 ± 0.21% for the control group and the serum concentrations of the treatment group had increased significantly (p = 0.0002, p = 0.0036).
Comparison of the change and rate of change of serum albumin concentrations between patients with and without polaprezinc administration
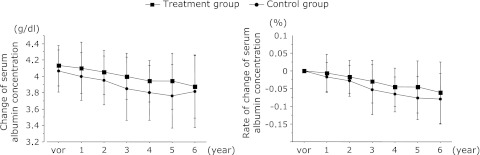
Comparison of the changes and rates of change of serum albumin concentrations between the treatment and control groups are shown in Fig. 2. After 6 years, the serum albumin concentrations were 3.9 ± 0.4 g/dl in the treatment group and 3.8 ± 0.4 g/dl in the control group and had not increased significantly. The serum albumin concentrations showed a gradual decrease in both groups, with longer observation. The serum albumin concentrations decreased with time in both groups and there was no difference in the percentage decrease. This suggests that there is no difference between the two groups in terms of the degree of liver disease progression.
Fig. 2.
Comparisons between the treatment and control groups in terms of the change and rates of change of serum albumin concentrations. After 6 years, the serum zinc concentrations and rates of change of serum zinc concentration were 3.9 ± 0.4 µg/dl in the treatment group and 3.8 ± 0.4 µg/dl in the control group and had increased not significantly.
Comparison of the cumulative incidence of HCC between patients with and without polaprezinc administration
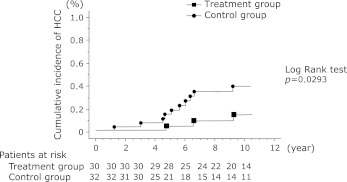
A comparison of the cumulative incidence of HCC between the treatment and control groups is shown in Fig. 3. Hepatocellular carcinoma occurred in 3 of 30 patients (10.0%) in the treatment group and 10 of 32 patients (31.2%) in the control group. The treatment group had a significantly lower incidence of HCC than the control group (p = 0.0355). Furthermore, a comparison of the cumulative incidence of HCC shows that it was significantly lower in the treatment group than the control group (p = 0.0293).
Fig. 3.
A comparison of the cumulative incidence of HCC between the treatment and control groups. Incidence curves were determined using the Kaplan-Meier method and statistical analysis was performed using the long-rank test. A comparison of the cumulative incidence of HCC shows that it was significantly lower in the treatment group than the control group (p = 0.0293).
Comparison of the clinical background factors of the patients, separated according to their baseline serum albumin concentrations
We conducted a prospective study to determine the long-term outcome of the zinc therapy according to the baseline serum albumin concentrations. The study subjects were grouped into those with baseline serum albumin concentrations below 4.0 g/dl in and those with serum albumin concentrations of 4.0 g/dl or more. Comparisons of the clinical characteristics of patients with and without polaprezinc administration are shown in Table 2. There were four patients in the treatment group and 11 in the control group with baseline serum albumin concentrations below 4.0 g/dl and 26 patients in the treatment group and 21 in the control group with baseline serum albumin concentrations of 4.0 g/dl or more. Thus, there were no significant differences in these background factors.
Table 2.
Comparison of the clinical background factors of the patients, separated according to their baseline serum albumin concentrations
| Parameter | Albumin below 4.0 g/dl |
Albumin 4.0 g/dl or more |
|||||
|---|---|---|---|---|---|---|---|
| Treatment group | Control group | p value* | Treatment group | Control group | p value* | ||
| Number | 4 | 11 | 26 | 21 | |||
| Gender (male) | 1 (25.0%) | 6 (54.5%) | 0.5692 | 14 (51.8%) | 13 (48.1%) | 0.8307 | |
| Age | 68.1 ± 1.2 | 63.2 ± 10.9 | 0.4016 | 54.5 ± 13.2 | 58.0 ± 9.1 | 0.2974 | |
| BMI | 23.4 ± 0.2 | 22.5 ± 1.5 | 0.4454 | 23.2 ± 3.5 | 23.1 ± 3.0 | 0.9625 | |
| AST (U/L) | 73.0 ± 17.0 | 66.1 ± 30.2 | 0.7664 | 62.6 ± 26.1 | 54.7 ± 20.0 | 0.2771 | |
| ALT (U/L) | 96.6 ± 65.5 | 56.8 ± 27.3 | 0.1119 | 88.7 ± 38.4 | 65.3 ± 38.0 | 0.0524 | |
| Platelet count (×104/µL) | 10.5 ± 3.0 | 15.2 ± 5.7 | 0.1400 | 15.9 ± 4.1 | 15.8 ± 5.3 | 0.9081 | |
| Zinc concentration (µg/dl) | 55.0 ± 11.4 | 51.7 ± 10.5 | 0.6097 | 60.6 ± 8.7 | 56.0 ± 9.6 | 0.0879 | |
| Albumin (g/dl) | 3.7 ± 0.3 | 3.8 ± 0.1 | 0.3627 | 4.2 ± 0.2 | 4.2 ± 0.2 | 0.8351 | |
| Observation period (year) | 7.4 ± 3.8 | 5.8 ± 2.4 | 0.3437 | 8.7 ± 2.0 | 7.7 ± 3.2 | 0.2266 | |
*p<0.005. Mann-Whitney U test, chi-square test. Treatment group: patients with polaprezinc administration. Control group: patients without polaprezinc administration. The study subjects were grouped into those with baseline serum albumin concentrations below 4.0 g/dl in and those with serum albumin concentrations of 4.0 g/dl or more. Comparison of the clinical characteristics of patients with and without polaprezinc administration.
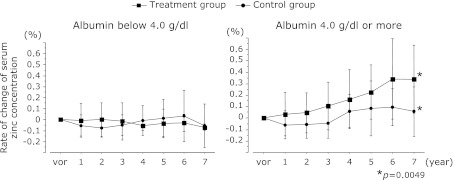
Comparison of the changes and rate of change of serum zinc concentration between the treatment and control groups according to baseline serum albumin concentrations
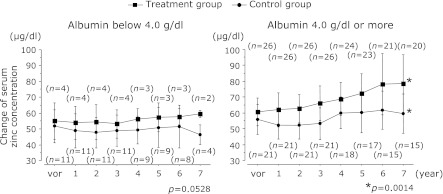
Fig. 4 shows the changes in serum zinc concentrations in the treatment and control groups, and Fig. 5 shows the rates of change of those concentrations, with the patients grouped according to baseline serum albumin concentrations below 4.0 g/dl or 4.0 g/dl or more.
Fig. 4.
The changes in serum zinc concentrations in the treatment and control groups with the patients grouped according to baseline serum albumin concentrations below 4.0 g/dl or 4.0 g/dl or more. After 7 years, the serum zinc concentration was 78.4 ± 18.2 µg/dl in the treatment group with baseline serum albumin concentrations 4.0 g/dl or more and 59.4 ± 12.2 µg/dl in the control group, respectively. The serum zinc concentrations of the treatment group compared to the control group had increased significantly (p = 0.0014). There were no significant differences in the group with baseline serum albumin concentrations below 4.0 g/dl.
Fig. 5.
The rates of change of those concentrations in the treatment and control groups with the patients grouped according to baseline serum albumin concentrations below 4.0 g/dl or 4.0 g/dl or more. After 7 years, the rates of change of serum zinc concentration was +0.339 ± 0.29% in the treatment group with baseline serum albumin concentrations 4.0 g/dl or more and +0.058 ± 0.21% in the control group, respectively. The rate of change of serum zinc concentrations of the treatment group compared to the control group had increased significantly (p = 0.0049). There were no significant differences in the group with baseline serum albumin concentrations below 4.0 g/dl.
The observation period was 7 years. After 7 years, the serum zinc concentration was 78.4 ± 18.2 µg/dl in the treatment group with baseline serum albumin concentrations 4.0 g/dl or more and 59.4 ± 12.2 µg/dl in the control group and the rates of change of serum zinc concentrations were +0.339 ± 0.29% and +0.058 ± 0.21%, respectively. The serum zinc concentrations and rate of change of serum zinc concentrations of the treatment group had increased significantly compared to the control group (p = 0.0014, p = 0.0049). There were no significant differences in the group with baseline serum albumin concentrations below 4.0 g/dl.
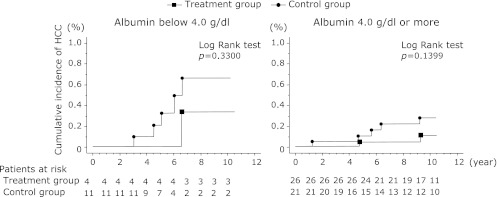
Comparison of the cumulative incidence of HCC between patients with and without poaprezinc administration, grouped according to their baseline serum albumin concentrations
Comparison of the cumulative incidence of HCC between the treatment and control groups, separated into the groups with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more, are shown in Fig. 6. Among the patients with baseline serum albumin concentrations of 4.0 g/dl or more, hepatocellular carcinoma occurred in 2 of 26 (7.7%) in the treatment group and 5 of 21 (23.8%) in the control group (p = 0.2168). Among the patients with baseline serum albumin concentrations below 4.0 g/dl, HCC occurred in 1 of 4 patients (25.0%) in the treatment group and 5 of 11 (45.5%) in the control group (p = 0.6044). Thus, for both categories of baseline serum albumin concentrations, the incidence of HCC tended to be lower in the treatment group than the control group. Furthermore, a comparison of the cumulative incidence of HCC showed that this was lower in the treatment group than the control group.
Fig. 6.
Comparison of the cumulative incidence of HCC between the treatment and control groups, separated into the groups with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more. Incidence curves were determined using the Kaplan-Meier method and statistical analysis was performed using the long-rank test. The cumulative incidence of HCC tended to be lower in the treatment group than the control group.
Factors associated with the incidence of HCC comparing patients with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more using Cox proportional hazard regression analysis
Comparison of the factors associated with the incidence of HCC, comparing the groups with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more are shown in Table 3. In the group with serum albumin concentrations of 4.0 g/dl or more in, it was determined by multivariate analysis of factors that contribute to a lower incidence HCC, polaprezinc administration (risk ratio: 0.113, 95% confidence interval (CI): 0.015–0.870, p = 0.0362) and platelet counts (risk ratio: 0.766, 95% confidence interval (CI): 0.594–0.989, p = 0.0409) were significant. Therefore, zinc supplementation therapy has been shown to be valuable.
Table 3.
Factors associated with the incidence of HCC comparing patients with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more using Cox proportional hazard regression analysis
| Factor | Albumin below 4.0 g/dl |
Albumin 4.0 g/dl or more |
|||||
|---|---|---|---|---|---|---|---|
| Risk Ratio | 95% CI | p value* | Risk Ratio | 95% CI | p value* | ||
| Polaprezinc administration | 0.06 | 0.001–4.975 | 0.2118 | 0.113 | 0.015–0.870 | 0.0362 | |
| Gender (male) | 0.897 | 0.022–36.849 | 0.9544 | 0.541 | 0.063–4.659 | 0.5764 | |
| Age | 1.131 | 0.937–1.365 | 0.1986 | 1.017 | 0.916–1.128 | 0.7568 | |
| AST | 0.989 | 0.952–1.027 | 0.568 | 0.991 | 0.931–1.054 | 0.7667 | |
| ALT | 1.006 | 0.978–1.035 | 0.6735 | 1.017 | 0.985–1.051 | 0.2926 | |
| Platelet count | 0.951 | 0.671–1.348 | 0.7781 | 0.766 | 0.594–0.989 | 0.0409 | |
| Zinc Concentration (baseline) | 1.096 | 0.927–1.296 | 0.2822 | 1.031 | 0.934–1.138 | 0.5405 | |
*p<0.005. Mann-Whitney U test, chi-square test. HCC: hepatocellular carcinoma. CI: confidene interval. Comparison of the factors associated with the incidence of HCC, comparing the groups with baseline serum albumin concentrations below 4.0 g/dl and 4.0 g/dl or more.
Discussion
Zinc in the blood is bound to albumin, α2-macroglobulin and acids. Approximately 30% of zinc is reported to be absorbed through the intestine(8,9) and the rate of absorption is said to be affected by changes in zinc intake.(10–12) Zinc has been reported to antagonize other divalent cations such as iron and copper in the process of absorption.(13,14) Zinc levels may be regulated by impaired absorption of zinc by the intestinal mucosa and the fecal excretion of zinc in the pancreatic juice. Zinc deficiency may lead to various features of liver disease, including a reduced amount of zinc bound to albumin, as albumin concentrations decrease with the progression of liver disease, and an increased amount of zinc bound to amino acids leading to increased excretion in the urine, increased urinary excretion of zinc due to shunting of the portal circulation system, failure to absorb zinc because of changes in the small bowel intestinal mucosa, and a lower zinc content in the liver because of a decrease in effective hepatocyte function. We reported previously that serum zinc concentrations decrease as liver disease progresses. In this study, it is difficult to prove that decreases in AST and ALT levels may be attributed to zinc supplementation therapy alone because the patients received other therapies for liver disease, including conservative treatments with drugs such as ursodeoxycholic acid. Really, there was no significant difference between treatment group and control group, the comparison of the change of the platelets counts and the ALT levels for seven years (Table not shown). Therefore, progression of liver disease seems to vary according to the serum albumin concentrations. We measured the serum albumin concentrations to determine the degree of liver disease progression. This was believed to be lower if the serum zinc concentration led to a decreased the serum albumin concentration. Then, looking at annual trends in the serum albumin concentrations and the decline over time, we found that there was no significant difference between the treatment and control groups. Thus, even in the control and treatment groups, the difference was not considered significant as the progression of liver disease. Considering the natural progression, the serum zinc concentration was believed to have decreased gradually in both groups. If increased serum zinc concentrations were achieved by administration of polaprezinc, this was considered to have been effective zinc therapy.
In consideration of this, the cumulative incidence of HCC was significantly lower with administration of zinc, which may have an effect on liver disease due to elevated serum zinc concentrations, and such administration seems to be one method to suppress the incidence of hepatocellular carcinoma.
Progression of liver disease seems to vary according to the serum albumin concentrations. For this reason, we divided the study subjects into groups according to their serum albumin concentrations before the start of treatment, those below 4.0 g/dl and those with 4.0 g/dl or more. For patients with baseline serum albumin concentrations of 4.0 g/dl or more, the serum zinc concentrations of the treatment group increased significantly compared to the control group and the cumulative incidence of HCC decreased in the treatment group. In other words, the liver disease was less likely to progress in the group with higher baseline serum albumin concentrations, especially those patients whose serum zinc concentrations increased following zinc supplementation therapy. Conversely, in the group with low baseline concentrations of serum albumin, in whom liver disease was likely to progress, zinc therapy significantly increased the serum zinc concentration but did not tend to lower the cumulative incidence of HCC.
The following is known about the relationship between zinc and liver pathology: a state of zinc deficiency may promote collagen production and activation of hepatic stellate cells and have a negative effect on lipid peroxidation by hepatocytes, resulting in an increase in the phospholipid content of the liver.(15,16) Zinc and copper are antagonistic, zinc may inhibit hepatic fibrosis by reducing the activity of lysyl oxidase but copper promotes hepatocellular fibrosis. Zinc may improve hepatocellular damage and inhibit lipid peroxidation.
Zinc suppresses hepatocellular apoptosis by binding to ferritin, although ferritin also chelates iron and iron levels are reduced by the increase in ferritin.(17) Oxidative stress induced by iron in the liver is reduced with the reduction of iron concentrations and, in parallel, may reduce hepatocellular damage.(18)
Recently, it was reported that iron is related to hepatocellular carcinoma.(19) So, we investigated the serum iron and ferritin levels at the baseline and the end of the observation in the treatment and control groups. At the baseline, the mean serum Iron levels were 125.1 ± 37.2 µg/dl in the treatment group and 122.3 ± 41.2 in the control group (p = 0.7914), the mean serum Ferritin levels were 124.2 ± 124.1 ng/ml and 104.0 ± 73.7 (p = 0.4600). The mean serum Iron levels and Ferritin levels had no significant differences at the baseline in the treatment and the control group (Table 1). At the end of the observation, the mean serum Iron levels were 112.5 ± 58.8 in the treatment group and 114.0 ± 42.5 in the control group (p = 0.9120), the mean serum Ferritin levels were 91.3 ± 76.1 and 97.0 ± 59.8 (p = 0.7521). The serum iron and ferritin levels had no significant differences at the end of the observation between in the treatment and control group, and between in the incidence and non-incidence of HCC (Table not shown). However, the mean serum ferritin levels tended to be lower in treatment group between the baseline (124.2 ± 124.1) and the end of the observation (91.3 ± 76.1). We considered that zinc therapy would reduce the serum ferritin levels.
This study is unique in following the study subjects over seven years and shows that the serum zinc concentration is elevated by zinc therapy. In addition, the side effects of an overdose of zinc are minimal, so that it may be administered safely. Zinc therapy is believed to contribute to improvement of the condition of the liver, including lowering the incidence of HCC, safely and without side effects. Only in the group with low serum albumin concentrations before the start of treatment did zinc therapy fail to achieve an increase in serum zinc concentrations. We assumed that zinc was effective in lowering the incidence of HCC. If the serum zinc concentration is increased, this might reduce the incidence of HCC, so it is desirable to devise some kind of treatment, such as zinc therapy to increase the amount of zinc, or combination therapy with zinc and an amino acid formula.
In conclusion, zinc supplement administration has been suggested to improve liver pathology and suppress the occurrence of hepatocellular carcinoma. Zinc supplementation therapy may be administered aggressively, safely and without side effects.
Acknowledgments
The authors thank all the members of Liver Group in Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine.
Abbreviations
- ALT
alanine aminotaransferase
- AST
aspartate aminotransferase
- HCC
hepatocellular carcinoma
Conflict of Interest
No conflicts of interest has been declared by the authors.
References
- 1.Dibley MJ. Pres in Nutrition. Eighteen Edition. Tokyo: Kenpakusha; 2002. pp. 344–345. [Google Scholar]
- 2.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130(5S Suppl):1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 3.Okada A, Takagi Y, Itakura T, Satani M, Manabe H. Skin lesions during intravenous hyperalimentation: zinc defi ciency. Surgery. 1976;80:629–635. [PubMed] [Google Scholar]
- 4.Yoshida Y, Higashi T, Nouso K, et al. Effects of zinc deficiency/zinc supplementation on ammonia metabolism in patients with decompensated liver cirrhosis. Acta Med Okayama. 2001;55:349–355. doi: 10.18926/AMO/32003. [DOI] [PubMed] [Google Scholar]
- 5.Moriyama M, Matsumura H, Fukushima A, et al. Clinical significance of evaluation of serum zinc concentrations in C-viral chronic liver disease. Dig Dis Sci. 2006;51:1967–1977. doi: 10.1007/s10620-005-9051-7. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka S, Matsumura H, Nakamura H, et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver Cirrhosis. J Clin Biochem Nutr. 2009;45(3):292–303. doi: 10.3164/jcbn.08-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muranaka H, Kato N. Detection of serum zinc by atomic absorption spectrometry. Rinsho Byori. 1969;17:559–562. (in Japanese) [PubMed] [Google Scholar]
- 8.AMA Department . JAMA. Vol. 241. AMA Department of Foods and Nutrition; 1979. Guidlines for essential trace element preparations for parental use. A statement by an expert panel; pp. 2051–2054. [PubMed] [Google Scholar]
- 9.Evans GW. Zinc absorption and transport. In: Prassd AS, editor. In trace elements in Human Health and Disease, in Zinc and Copper. Vol. 1. New York: Academic Press; 1976. [Google Scholar]
- 10.Hunt JR, Mullen LK, Lykken GI. Zinc retention from an experimental diet based on the U.S. F.D.A. total diet study. Nutr Res. 1992;12:1335–1344. [Google Scholar]
- 11.Lee DY, Prasad AS, Hydrick-Adair C, Brewer G, Johnson PE. Homeostasis of zinc in marginal human zinc deficiency: role of absorption and endogenous excretion of zinc. J Lab Clin Med. 1993;122:549–556. [PubMed] [Google Scholar]
- 12.Taylor CM, Bacon JR, Aggett PJ, Bremner I. Homeostatic regulation of zinc absorption and endogenous losses in zinc-deprived men. Am J Clin Nutr. 1991;53:755–763. doi: 10.1093/ajcn/53.3.755. [DOI] [PubMed] [Google Scholar]
- 13.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 14.Dufner-Beattie J, Wang F, Kuo YM, et al. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 15.Cabré M, Camps J, Paternáin JL, Ferré N, Joven J. Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin Exp Pharmacol Physiol. 2000;27:694–699. doi: 10.1046/j.1440-1681.2000.03322.x. [DOI] [PubMed] [Google Scholar]
- 16.Camps J, Bargallo T, Gimenez A, et al. Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin Sci (Lond) 1992;83:695–700. doi: 10.1042/cs0830695. [DOI] [PubMed] [Google Scholar]
- 17.Yadrick MK, Kenney MA, Winterfeldt EA. Iron, copper, and zinc status: response to supplementation with zinc or zinc and iron in adult females. Am J Clin Nutr. 1989;49:145–150. doi: 10.1093/ajcn/49.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radsic Biol Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki T, Terai S, Sakaida I. Deferoxamine for advanced hepatocellular carcinoma. N Engl J Med. 2011;365:576–578. doi: 10.1056/NEJMc1105726. [DOI] [PubMed] [Google Scholar]