Abstract
Helicobacter pylori (H. pylori) eradication therapy alone is insufficient to ensure healing of large ulcers with H. pylori-positive gastric ulcer (GU). The question of what is the optimum antiulcer treatment following H. pylori eradication therapy has not been fully elucidated. Furthermore, the ulcer healing effects of eradication therapy itself with H. pylori-positive duodenal ulcer (DU) have not been investigated. In GU study, the eradication therapy + proton pump inhibitor (PPI) group (group A) were administered eradication therapy followed by 7 weeks of a PPI, and the eradication therapy + gastroprotective drug (GP) group (group B) eradication therapy followed by 7 weeks of a GP. In DU study, the eradication therapy + PPI group (group C) were administered eradication therapy followed by 5 weeks of a PPI, and the eradication therapy only group (group D) was eradication therapy alone. In GU study, healing rates for ulcer of ≥15 mm in diameter were significant greater in the group A. In DU study, high healing rates were seen both the group C and D. In conclusion, a PPI could significantly heal GU than a GP after eradication therapy in GU. Meanwhile, the eradication alone is sufficient for DU.
Keywords: Helicobacter pylori eradication therapy, peptic ulcer, proton pump inhibitor, gastroprotective drug, ulcer healing
Introduction
In Caucasian patients with Helicobacter pylori (H. pylori) positive gastric ulcers (GU)(1–4) and duodenal ulcers (DU),(5–9) ulcer healing rates with eradication therapy alone are in no way inferior to those achieved with proton pump inhibitor (PPI) monotherapy, and a consensus has been reached that additional treatment with a PPI following eradication therapy is unnecessary. However, it is evident to heal only about half the number of Japanese patients with GU by H. pylori eradication therapy alone.(10) In addition, these patients also need the additional medication therapy such as PPI, the histamine H2-receptor antagonist (H2RA), or the gastroprotective drug (GP) after H. pylori eradication therapy.(11–14) Among these three kinds of drugs, we expect that each power of healing may be different even if after H. pylori eradication therapy.
Teprenone is one of GPs and is used at the second high frequency in Japan. However, teprenone-related manuscripts have never previously published after H. pylori eradication therapy. PPI is ranked as the standard antiulcer treatment following H. pylori eradication therapy in Japan. In general, PPIs are extremely useful drugs that heal ulcers through powerful suppression of gastric acid secretion, but they are also expensive. In addition, because there are recent reports of an increased risk of community-acquired pneumonia with suppression of gastric acid secretion,(15,16) an increased risks of bone fracture with long-term PPI use,(17) and a decreased antiplatelet effect of clopidogrel due to concomitant therapy with PPI.(18) We may need to consider the use of the treatment other than PPIs. There is no report to compare healing rates of ulcers between PPI and GP following H. pylori eradication therapy in Japanese patients with H. pylori positive GU. In the present study, we conducted a comparative study of the PPI rabeprazole and the GP teprenone as the treatment for GU following the eradication therapy.
On the other hand, it is unclear whether one-week H. pylori eradication therapy is insufficient to heal active DU like GU in Japanese patients since there is no report which examined healing rates by H. pylori eradication therapy alone in the Japanese patients with H. pylori positive DU. We also conducted the comparative study for Japanese patients with H. pylori positive DU between H. pylori eradication therapy alone and H. pylori eradication therapy + PPI.
Patients and Methods
Patients
The subjects of this study were 174 H. pylori positive patients with active peptic ulcers who presented to the Osaka Medical College Hospital and its affiliated hospitals between April 2008 and April 2009 with symptoms of epigastric discomfort or pain. GU or DU was diagnosed at esophagogastroduodenoscopy (EGD), and H. pylori positive status was confirmed using the rapid urease test (RUT: Pyloritech, Sakura Finetek, Tokyo, Japan). Both GU and DU were defined as open ulcers of ⩾5 mm in diameter with a clear demarcation between the lesion and normal mucosa. Ulcer sizes were measured using an endoscopic ruler (M2-4K, Olympus, Tokyo, Japan).
Potential subjects were excluded if they were aged <18 years, regular used nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids, had taken antimicrobials within the previous 1 month period, or had a history of gastric surgery or H. pylori eradication therapy. Potential subjects with exposed blood vessels in the base of the ulcer, or clots adherent to the ulcer floor, were also excluded.
Study design
Subjects with GU or DU on EGD, and H. pylori positive by RUT, were assigned to 1 of 3 groups in the GU study or DU study according to ulcer size as measured in the abovementioned manner, i.e. small (5~<10 mm), medium (10~<15 mm), and large (⩾15 mm). Osaka Medical College provided the envelope that filled in the treatment group based on the computer-generated randomization list to each center which conducted the study according to number of target subjects. Number of target subjects in each center was multiples of four. Subjects were randomly assigned in 1:1 proportion, using the computer-generated randomization list received eradication therapy + PPI or eradication therapy + GP for 8 weeks in the GU study, and eradication therapy + PPI or eradication therapy only for 6 weeks in the DU study (Fig. 1 and 2). Both ulcer size (diameter) and depth were considered to be factors that affect healing, but due to difficulties in measuring ulcer depth, we chose ulcer size as the major criterion for classification of lesions in this study as previous reports.(10,13,14) Subjects and treating physicians, but not endoscopists, were informed of the allocated treatment. We explained to subjects the benefits of treatment and the importance of compliance. The situation of taking the eradication therapy, PPI, and GP was filled in on the diary, and collected it. Written consent was obtained from all subjects before enrolment, and approval for this study was obtained from the Osaka Medical College Hospital Ethical Committee.
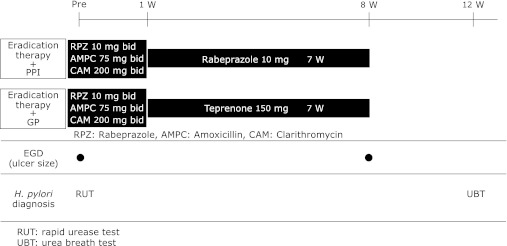
Fig. 1.
Protocol for gastric ulcer arm. Subjects with GU were randomly allocated to an eradication therapy + PPI group, administered eradication therapy commencing the day after EGD, followed by 7 weeks of a PPI, or an eradication therapy + GP group, administered eradication therapy followed by 7 weeks of a GP.
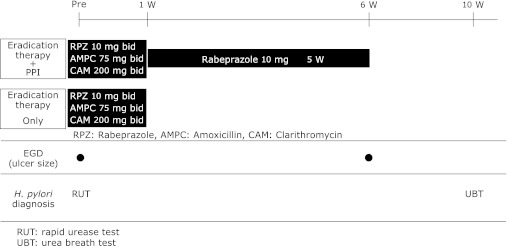
Fig. 2.
Protocol for duodenal ulcer arm. Subjects with DU were randomly allocated to an eradication therapy + PPI group, administered eradication therapy commencing the day after EGD, followed by 5 weeks of a PPI, or an eradication therapy only group administered eradication therapy alone.
GU study
Subjects with GU were randomly allocated to an eradication therapy + PPI group, administered eradication therapy (Rabeprazole 10 mg + Amoxycillin 750 mg + Clarithromycin 200 mg bid for 7 days) commencing the day after EGD, followed by 7 weeks of a PPI (rabeprazole 10 mg/day), or an eradication therapy + GP group, administered eradication therapy followed by 7 weeks of a GP (teprenone 150 mg/day). Ulcer healing rates were evaluated at 8 weeks after study commencement (Fig. 1). Ulcer healing was defined as the status that moved to the scarring stage. We determined the sample size according to the past reports. A two group χ2 test with a 0.05 two-sided significance level will have 90% power to detect the difference between healing rate of H. pylori eradication therapy plus PPI (94.0%)(4) and that of H. pylori eradication therapy plus GP (70.1%)(11) when the sample size in each group is 53. Therefore, we assumed 60 subjects as one group in consideration of the dropout subjects.
DU study
Subjects with DU were randomly allocated to an eradication therapy + PPI group, administered eradication therapy commencing the day after EGD, followed by 5 weeks of a PPI (rabeprazole 10 mg/day), or an eradication therapy only group administered eradication therapy alone. Ulcer healing rates were evaluated at 6 weeks after study commencement (Fig. 2). Ulcer healing was defined as the status that moved to the scarring stage.
We determined the sample size according to the past reports. A two group χ2 test with a 0.05 two-sided significance level will have 90% power to detect the difference between healing rate of H. pylori eradication therapy plus PPI (92.0%)(9) and that of H. pylori eradication therapy alone (49.0%)(10) when the sample size in each group is 22. Therefore, we assumed 30 subjects as one group in consideration of the dropout subjects.
Subject follow-up
All subjects underwent repeat EGD to assess ulcer healing at 8 weeks after randomization in the GU study, and 6 weeks after randomization in the DU study. Subject compliance with medications was confirmed at each outpatient attendance. The success or failure of eradication therapy was assessed using 13C-urea breath test (UBT) at 12 weeks after study commencement in GU study, and 10 weeks after study commencement in DU study.
Statistical analyses
Comparisons of the proportion of subjects with healed ulcers were made using the χ2 test, with Yates’ correction for continuity (Stat View J-4.5, Macintosh, Abacus Concepts, Inc., Berkeley, CA). Differences with a p value <0.05 were considered significant. Intention-to treat (ITT) analysis was conducted with all enrolled subjects, and per protocol (PP) analysis with subjects who took ⩾80% of the test drugs, and underwent follow-up EGD.
Results
GU study
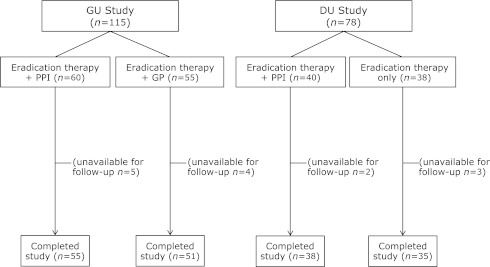
We enrolled 115 H. pylori positive patients with GU. The eradication therapy + PPI group comprised 60 subjects, and 55 (Fig. 3). The number of subjects is not same due to the accidental dispersion of each center in total. Compliance with eradication therapy, PPI, and GP was generally good. We confirmed all subjects except dropping out had taken more than 75% in all medications. No significant differences were seen between groups in terms of age, gender, smoking or alcohol consumption, history of peptic ulcer, or ulcer site or size (Table 1). Five subjects in the eradication therapy + PPI group and 4 in the eradication therapy + GP group were withdrawn due to inability to undergo repeat EGD. No subjects were lost to follow-up because of exacerbation of symptoms. A total of 106 subjects (55 in the eradication therapy + PPI group and 51 in the eradication therapy + GP group) completed this study.
Fig. 3.
Study outline. We enrolled 115 H. pylori positive patients with GU. The eradication therapy + PPI group comprised 60 subjects, and the eradication therapy + GP group 55. We enrolled 78 H. pylori positive patients with DU. The eradication therapy + PPI group comprised 40 subjects, and the eradication therapy only group 38.
Table 1.
Subject characteristics
| GU study |
DU study |
|||||
|---|---|---|---|---|---|---|
| Eradication therapy + PPI (n = 60) | Eradication therapy + GP (n = 55) | p value | Eradication therapy + PPI (n = 40) | Eradication therapy only (n = 38) | p value | |
| Age | 57.4 ± 12.3 | 58.6 ± 13.8 | 0.4023 | 49.9 ± 14.9 | 50.9 ± 16.3 | 0.7042 |
| Gender (M/F) | 41/18 | 36/19 | 0.6920 | 30/10 | 26/12 | 0.9255 |
| Height (cm) | 162.7 ± 8.4 | 164.9 ± 10.0 | 0.2701 | 166.1 ± 8.2 | 163.0 ± 8.9 | 0.2386 |
| Weight (kg) | 58.2 ± 11.5 | 59.5 ± 11.3 | 0.5503 | 61.9 ± 9.5 | 60.9 ± 12.4 | 0.5785 |
| Smoking (%) | 46.7 | 45.5 | 0.9369 | 42.9 | 51.7 | 0.6481 |
| Drinking (%) | 45.0 | 40.0 | 0.9760 | 40.8 | 48.3 | 0.6899 |
| History of ulcer (%) | 35.0 | 34.5 | 0.9716 | 40.8 | 34.5 | 0.4661 |
| Location of ulcer | Antrum, Angulus, Corpus |
0.3076 | Bulbus, Postbulbus |
0.6048 | ||
| 3/31/26 | 7/24/24 | 37/13 | 37/1 | |||
| Ulcer size (mm) | 15.2 ± 7.0 | 13.3 ± 8.1 | 0.0958 | 9.8 ± 4.7 | 9.0 ± 4.1 | 0.5282 |
H. pylori eradication rates were 77% (46/60) in the eradication therapy + PPI group (ITT: 95%CI 64–87%), and 67% (34/55) in the eradication therapy + GP group (ITT: 95%CI 53–79%), with no significant difference seen between groups (p = 0.261) (Table 2).
Table 2.
Helicobacter pylori eradication rates and ulcer healing rates in the gastric ulcer study
| GU |
|||||
|---|---|---|---|---|---|
| Eradication therapy + PPI |
Eradication therapy + GP |
p value | |||
| eradicated, healed/all patients | % | eradicated, healed/all patients | % | ||
| ITT analysis | |||||
| Eradication rate | 46/60 | 77 [64–87] | 37/55 | 67 [53–79] | 0.261 |
| Ulcer healing rate | 48/60 | 80 [68–89] | 34/55 | 62 [48–75] | 0.031 |
| PP analysis | |||||
| Eradication rate | 46/55 | 84 [71–92] | 37/51 | 73 [58–84] | 0.166 |
| Ulcer healing rate | 48/55 | 87 [75–95] | 34/51 | 67 [52–79] | 0.011 |
Numbers in [ ] are 95%CI: lower to upper. ITT = intention-to-treat; PP = per protocol. Ratios were analysed using the χ2 test.
GU healing rates after 8 weeks’ treatment were 80% (48/60) (ITT: 95%CI 68–89%) in the eradication therapy + PPI group, and 62% (34/55) (ITT: 95%CI 48–75%) in the eradication therapy + GP group, with a significant difference between groups (p = 0.031) (Table 2).
Healing rates for small ulcers of 5~<10 mm in diameter were 82% (9/11) (ITT: 95%CI 48–98%) in the eradication therapy + PPI group, and 82% (14/17) (ITT: 95%CI 57–96%) in the eradication therapy + GP group, with no significant difference seen between groups (p = 0.971). Healing rates for medium ulcers of 10~<15 mm in diameter were 69% (11/16) (ITT: 95%CI 41–89%) in the eradication therapy + PPI group, and 67% (10/15) (ITT: 95%CI 38–88%) in the eradication therapy + GP group, with no significant difference seen between groups (p = 0.901).
However, healing rates for large ulcers of ⩾15 mm in diameter were 85% (28/35) (ITT: 95%CI 68–95%) in the eradication therapy + PPI group, significantly greater than that of 43% (10/23) (ITT: 95%CI 23–66%) in the eradication therapy + GP group (p = 0.001) (Table 3).
Table 3.
Healing rates according to ulcer size in the gastric ulcer study
| Ulcer size (mm) | GU |
||||
|---|---|---|---|---|---|
| Eradication therapy + PPI |
Eradication therapy + GP |
p value | |||
| healed/all patients | % | healed / all patients | % | ||
| ITT analysis | |||||
| 5≤diam<10 | 9(1)/11(1) | 82 [48–98] | 14(3)/17(5) | 82 [57–96] | 0.971 |
| 10≤diam<15 | 11(4)/16(4) | 69 [41–89] | 10(2)/15(3) | 67 [38–88] | 0.901 |
| diam≥15 | 28(4)/33(4) | 85 [68–95] | 10(3)/23(6) | 43 [23–66] | 0.001 |
| PP analysis | |||||
| 5≤diam<10 | 9(1)/10(1) | 90 [55–100] | 14(3)/17(5) | 82 [57–96] | 0.589 |
| 10≤diam<15 | 11(4)/13(4) | 85 [55–98] | 10(2)/12(3) | 83 [52–98] | 0.930 |
| diam≥15 | 28(4)/32(4) | 88 [71–96] | 10(3)/22(6) | 45 [24–68] | 0.001 |
Numbers in ( ) denote number of patients in which H. pylori eradication was unsuccessful. Numbers in [ ] are 95%CI: lower to upper. diam: diameter; ITT = intention-to-treat; PP = per protocol. Ratios were analysed using the χ2 test.
Healing rates according to success and failure of eradication were 85% (39/46) (PP: 95%CI 71–94%) and 100% (9/9) (PP: 95%CI 66–100%) in the eradication therapy + PPI group, with no significant difference seen between groups (p = 0.210). Healing rates according to success and failure of eradication were 70% (26/37) (PP: 95%CI 53–84%) and 57% (8/14) (PP: 95%CI 29–82%) in the eradication therapy + GP group, with no significant difference seen between groups (p = 0.375). Healing rates according to the ulcer position (antrum, angulus, corpus) were 100% (3/3) (PP: 95%CI 29–100%), 86% (24/28) (PP: 95%CI 67–96%), 88% (21/24) (PP: 95%CI 68–97%) in the eradication therapy + PPI group, and 50% (3/6) (PP: 95%CI 12–88%), 68% (15/22) (PP: 95%CI 45–86%), 70% (16/23) (PP: 95%CI 47–87%) in the eradication therapy + GP group, respectively. Most ulcers were existed in angulus and corpus. There was no significant difference at healing rates according to the ulcer position in the eradication therapy + PPI and the eradication therapy + GP group.
DU study
We enrolled 78 H. pylori positive patients with DU. The eradication therapy + PPI group comprised 40 subjects, and the eradication therapy only group 38 (Fig. 3). The number of subjects is not same due to the accidental dispersion of each center in total. Compliance with eradication therapy and PPI was generally good. We confirmed all subjects except dropping out had taken more than 75% in all medications. No significant differences were seen between groups in terms of age, gender, smoking or alcohol consumption, history of peptic ulcer, or ulcer site or size (Table 1). Two subjects in the eradication therapy + PPI group and 3 in the eradication therapy only group were withdrawn due to inability to undergo repeat EGD. No subjects were lost to follow-up because of exacerbation of symptoms. A total of 73 subjects (38 in the eradication therapy + PPI group and 35 in the eradication therapy only group) completed this study.
H. pylori eradication rates were 68% (27/40) in the eradication therapy + PPI group (ITT: 95%CI 50–81%), and 76% (29/38) in the eradication therapy only group (ITT: 95%CI 60–89%), with no significant difference seen between groups (p = 0.387) (Table 4).
Table 4.
Helicobacter pylori eradication rates and ulcer healing rates in the duodenal ulcer study
| DU |
|||||
|---|---|---|---|---|---|
| Eradication therapy + PPI |
Eradication therapy only |
p value | |||
| eradicated, healed/all patients | % | eradicated, healed/all patients | % | ||
| ITT analysis | |||||
| Eradication rate | 27/40 | 68 [50–81] | 29/38 | 76 [60–89] | 0.387 |
| Ulcer healing rate | 37/40 | 93 [80–98] | 33/38 | 87 [72–96] | 0.41 |
| PP analysis | |||||
| Eradication rate | 27/38 | 71 [54–85] | 29/35 | 83 [66–93] | 0.233 |
| Ulcer healing rate | 37/38 | 97 [86–100] | 33/35 | 94 [81–99] | 0.507 |
Numbers in [ ] are 95%CI: lower to upper. ITT = intention-to-treat; PP = per protocol. Ratios were analysed using the χ2 test.
DU healing rates after 6 weeks’ treatment were 93% (37/40) in the eradication therapy + PPI group (ITT: 95%CI 80–98%), and 87% (33/38) in the eradication therapy only group (ITT: 95%CI 72–96%) (p = 0.410) (Table 4).
Healing rates for small ulcers of 5~<10 mm in diameter were 92% (24/26) (ITT: 95%CI 75–99%) in the eradication therapy + PPI group, and 86% (18/21) (ITT: 95%CI 64–97%) in the eradication therapy only group, with no significant difference seen between groups (p = 0.466). Healing rates for medium ulcers of 10~<15 mm in diameter were 100% (8/8) (ITT: 95%CI 63–100%) in the eradication therapy + PPI group, and 91% (10/11) (ITT: 95%CI 59–100%) in the eradication therapy only group, again with no significant difference seen between groups (p = 0.381). Healing rates for large ulcers of ⩾15 mm in diameter were 83% (5/6) (ITT: 95%CI 36–100%) in the eradication therapy + PPI group, and 83% (5/6) (ITT: 95%CI 36–100%) in the eradication therapy + GP group, once again with no significant difference seen between groups (p = 1.000) (Table 5).
Table 5.
Healing rates according to ulcer size in the duodenal ulcer study
| Ulcer size (mm) | DU |
||||
|---|---|---|---|---|---|
| Eradication therapy + PPI |
Eradication therapy only |
p value | |||
| healed/all patients | % | healed/all patients | % | ||
| ITT analysis | |||||
| 5≤diam<10 | 24(7)/26(7) | 92 [75–99] | 18(3)/21(5) | 86 [64–97] | 0.466 |
| 10≤diam<15 | 8(1)/8(1) | 100 [63–100] | 10(1)/11(1) | 91 [59–100] | 0.381 |
| diam≥15 | 5(3)/6(3) | 83 [36–100] | 5(0)/6(0) | 83 [36–100] | 1.000 |
| PP analysis | |||||
| 5≤diam<10 | 24(7)/24(7) | 100 [86–100] | 18(3)/20(5) | 90 [68–99] | 0.133 |
| 10≤diam<15 | 8(1)/8(1) | 100 [63–100] | 10(1)/10(1) | 100 [69–100] | — |
| diam≥15 | 5(3)/6(3) | 83 [36–100] | 5(0)/5(0) | 100 [48–100] | 0.338 |
Numbers in ( ) denote number of patients in which H. pylori eradication was unsuccessful. Numbers in [ ] are 95%CI: lower to upper. diam: diameter; ITT = intention-to-treat; PP = per protocol. Ratios were analysed using the χ2 test.
High DU healing rates were seen for ulcers of all sizes in both eradication therapy + PPI and eradication therapy only groups, with no significant difference seen between groups.
Healing rates according to success and failure of eradication were 96% (26/27) (PP: 95%CI 81–100%) and 100% (11/11) (PP: 95%CI 71–100%) in the eradication therapy + PPI group, with no significant difference seen between groups (p = 0.589). Healing rates according to success and failure of eradication were 100% (29/29) (PP: 95%CI 88–100%) and 67% (4/6) (PP: 95%CI 22–96%) in the eradication therapy only group, with significant difference seen between groups (p = 0.001).
Discussion
This is the first report about a comparative study of a PPI and a GP as the treatment for GU following the eradication therapy, and also about the effect of the eradication therapy on the DU healing in the Japanese patients. In the Japanese patients with H. pylori positive GU, healing rates of eradication therapy + PPI and eradication therapy + GP were similar for ulcers of <15 mm, but eradication therapy + PPI significantly accelerated healing rates than eradication therapy + GP for those of ⩾15 mm. It was clarified that there was difference at healing rates according to the kind of ulcer drug used after 1 week H. pylori eradication therapy. Meanwhile, in the Japanese patients with H. pylori positive DU, healing rates of the eradication therapy only group were as high as the eradication therapy + PPI group. This result was similar to those of Caucasian patients unlike the relationship between Japanese and Caucasian patients with H. pylori positive GU.
There are some reports of different antiulcer therapies after the eradication therapy. Terano et al.(11) and Hiraishi et al.(12) conducted a double-blind, placebo-controlled trial, administering the GP rebamipide or irsogladine maleate and placebo for 7 weeks following the eradication therapy in Japanese patients with GU. In other words, a double-blind, placebo-controlled trial has demonstrated that only about 60% of H. pylori positive GU heal in Japanese patients with the eradication therapy alone, and that not only inhibitors of gastric acid secretion, but also a GP such as rebamipide or irsogladine maleate, can be useful as post-eradication antiulcer therapy.
Murakami et al.(13) also conducted a study comparing the GP irsogladine maleate and the H2RA famotidine, finding no significant difference in ulcer healing rates (PP: 85.2% (46/54) vs 79.6% (43/54), p = 0.448), and no differences in healing rates stratified for ulcer size. Of great interest is the sub-analysis showing higher healing rates of the irsogladine group than those of the famotidine group in subjects who drink alcohol and smoke. This suggests the possibility that some GPs may possess different ulcer healing properties not present in inhibitors of gastric acid secretion.
We then conducted a trial comparing the GP sofalcone and the H2RA cimetidine as post-eradication antiulcer therapy. We found no significant difference in ulcer healing rates 7 weeks after eradication therapy (PP: 71.9% (23/32) vs 71.0% (22/31), p>0.05).(14) Although this study was of H. pylori positive GU ⩾10 mm in size, similar healing rates were seen with a GP and an H2RA.
The above studies have demonstrated that GPs, although as monotherapy without eradication therapy their healing rates are inferior to those achieved with inhibitors of gastric acid secretion, if therapy follows eradication therapy, similar healing rates can be achieved with certain subtypes of GPs as with inhibitors of gastric acid secretion.
Our results showed that no significant difference was seen in healing rates between the group administered the PPI rabeprazole and the group administered the GP teprenone for 7 weeks for ulcers <15 mm in size following the eradication therapy in patients with GU. However, healing rates were significantly greater in the PPI group than that in the GP group for ulcers ⩾15 mm in size. For ulcers ⩾15 mm in size, there may be differences in healing rates among various GPs. We should confirm this point in future studies.
As for the limitation of GU study, we did not have the eradication therapy only group. It is unknown whether healing rates of PPI and GP groups are significantly high compared with the placebo group. However, it is evident to heal only about half the number of Japanese patients with GU by H. pylori eradication therapy from the past report,(10) and it is ethically a problem to use the placebo. Therefore, we compared the PPI group with the GP group.
As for the limitation of DU study, the healing rate of the eradication therapy only group was higher than that of our expectation. In addition, because healing rates of patients who failed eradication were significantly lower, we need to treat a PPI in consideration of the ulcer healing in patients by eradication therapy alone. In these respects, randomized double blind study with more large sample size would be conducted to compare healing rates of the eradication therapy only group with those of the eradication therapy + PPI group.
In conclusion, in H. pylori positive Japanese patients with GU, as post-eradication antiulcer therapy a PPI rabeprazole and a GP teprenone yield similar healing rates for ulcers <15 mm in size. For GU ⩾15 mm, healing rates are lower with teprenone than with rabeprazole. In H. pylori positive Japanese patients with DU, it is now clear that eradication therapy alone is sufficient to achieve healing. Based on these results, we recommend tailored therapy considering the ulcer site and size, comprising eradication therapy alone for H. pylori positive GU <10 mm in size, and on a case-by-case basis further treatment with PPI, H2RA or GP for GU ⩾10 mm in size, and we would like Japanese primary physicians to consider the treatment option. For H. pylori positive DU, eradication therapy alone should be the standard antiulcer therapy regardless of the ulcer size. However, it is unclear whether or not administer a PPI following eradication therapy in consideration of failure of eradication since healing rates of patients who failed eradication were significantly lower.
Acknowledgments
We would like to thank the investigators who participated in this study. The study was conducted at OMC G & H research group (Hokusetsu General Hospital, Osaka Saiseikai Nakatsu Hospital, Sousei Hospital, Daiichi Towakai Hospital, Ishikiriseiki Hospital, Hirakata City Hospital, Moriguchi Keijinkai Hospital, and Osaka Medical College Hospital).
Abbreviations
- AMPC
Amoxycillin
- bid
twice daily
- CAM
Clarithromycin
- Diam
Diameter
- DU
duodenal ulcers
- EGD
Esophagogastroduodenoscopy
- GP
gastroprotective drug
- GU
gastric ulcers
- H. pylori
Helicobacter pylori
- H2RA
H2-receptor antagonist
- ITT
intention-to-treat
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PP
per-protocol
- PPI
proton pump inhibitor
- RPZ
Rabeprazole
- RUT
rapid urease test
- UBT
urea breath test
Conflict of Interest
This study is no conflict of interest.
References
- 1.Bazzoli F, Zagari RM, Fossi S, et al. Short-term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6:773–777. [Google Scholar]
- 2.Lind T, Veldhuyzen van Zanten S, Unge P, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH 1 Study. Helicobacter. 1996;1:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 3.Zanten SJ, Bradette M, Farley A, et al. The DU-MACH study: eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther. 1999;13:289–295. doi: 10.1046/j.1365-2036.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Bayerdörffer E, Diete U, et al. The GU-MACH study: the effect of 1-week omeprazole triple therapy on Helicobacter pylori infection in patients with gastric ulcer. Aliment Pharmacol Ther. 1999;13:703–712. doi: 10.1046/j.1365-2036.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 5.Goh KL, Navaratnam P, Peh SC, et al. Helicobacter pylori eradication with short-term therapy leads to duodenal ulcer healing without the need for continued acid suppression therapy. Eur J Gastroenterol Hepatol. 1996;8:421–423. [PubMed] [Google Scholar]
- 6.Hosking SW, Ling TK, Chung SC, et al. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomized controlled trial. Lancet. 1994;343:508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- 7.Lam SK, Ching CK, Lai KC, et al. Does treatment of Helicobacter pylori with antibiotics alone heal duodenal ulcer? A randomized double blind placebo controlled study. Gut. 1997;41:43–48. doi: 10.1136/gut.41.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge ZZ, Zhang DZ, Xiao SD, Chen Y, Hu YB. Does eradication of Helicobacter pylori alone heal duodenal ulcers? Aliment Pharmacol Ther. 2000;14:53–58. doi: 10.1046/j.1365-2036.2000.00673.x. [DOI] [PubMed] [Google Scholar]
- 9.Zanten SJ, Bradette M, Farley A, et al. The DU-MACH study: eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther. 1999;13:289–295. doi: 10.1046/j.1365-2036.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi K, Fujiwara Y, Tominaga K, et al. Is eradication sufficient to heal gastric ulcers in patients infected with Helicobacter pylori? A randomized, controlled, prospective study. Aliment Pharmacol Ther. 2003;17:111–117. doi: 10.1046/j.1365-2036.2003.01402.x. [DOI] [PubMed] [Google Scholar]
- 11.Terano A, Arakawa T, Sugiyama T, et al. Rebamipide, a gastro-protective and anti-inflammatory drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori in a Japanese population: a randomized, double-blind, placebo-controlled trial. J Gastroenterol. 2007;42:690–693. doi: 10.1007/s00535-007-2076-2. [DOI] [PubMed] [Google Scholar]
- 12.Hiraishi H, Haruma K, Miwa H, Goto H. Clinical trial: irsogladine maleate, a mucosal protective drug, accelerates gastric ulcer healing after treatment for eradication of Helicobacter pylori infection—the results of a multicenter, double-blind, randomized clinical trial (IMPACT study) Aliment Pharmacol Ther. 2010;31:824–833. doi: 10.1111/j.1365-2036.2010.04250.x. [DOI] [PubMed] [Google Scholar]
- 13.Murakami K, Okimoto T, Kodama M, et al. Comparison of the efficacy of irsogladine maleate and famotidine for the healing of gastric ulcers after Helicobacter pylori eradication therapy: a randomized, controlled, prospective study. Scand J Gastroenterol. 2011;46:287–292. doi: 10.3109/00365521.2010.531485. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi K, Watanabe T, Tanigawa T, Tominaga K, Fujiwara Y, Arakawa T. Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: a randomized controlled comparative trial with cimetidine, an H2-receptor antagonist. J Gastroenterol Hepatol. 2010;25 (Suppl 1):S155–S160. doi: 10.1111/j.1440-1746.2010.06232.x. [DOI] [PubMed] [Google Scholar]
- 15.Laheij RJ, Van Ijzendoorn MC, Janssen MJ, Jansen JB. Gastric acid-suppressive therapy and community-acquired respiratory infections. Aliment Pharmacol Ther. 2003;18:847–851. doi: 10.1046/j.1365-2036.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 17.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 18.Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713–718. doi: 10.1503/cmaj.082001. [DOI] [PMC free article] [PubMed] [Google Scholar]