Abstract
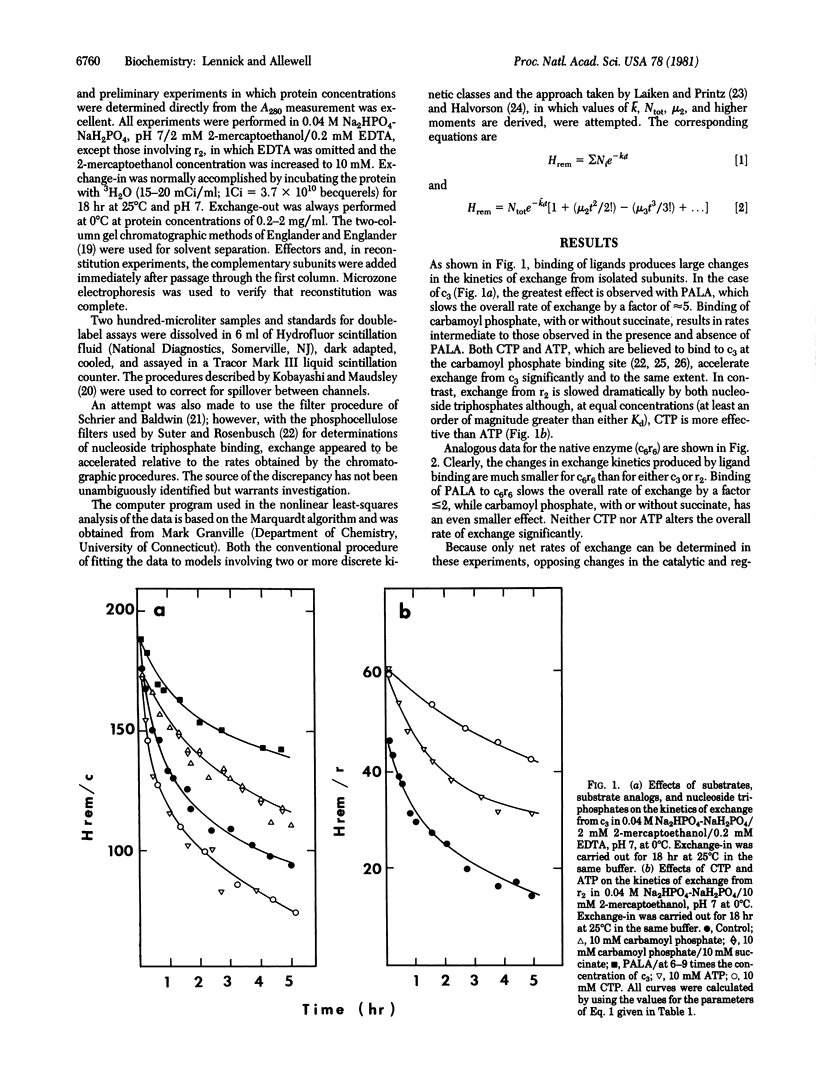
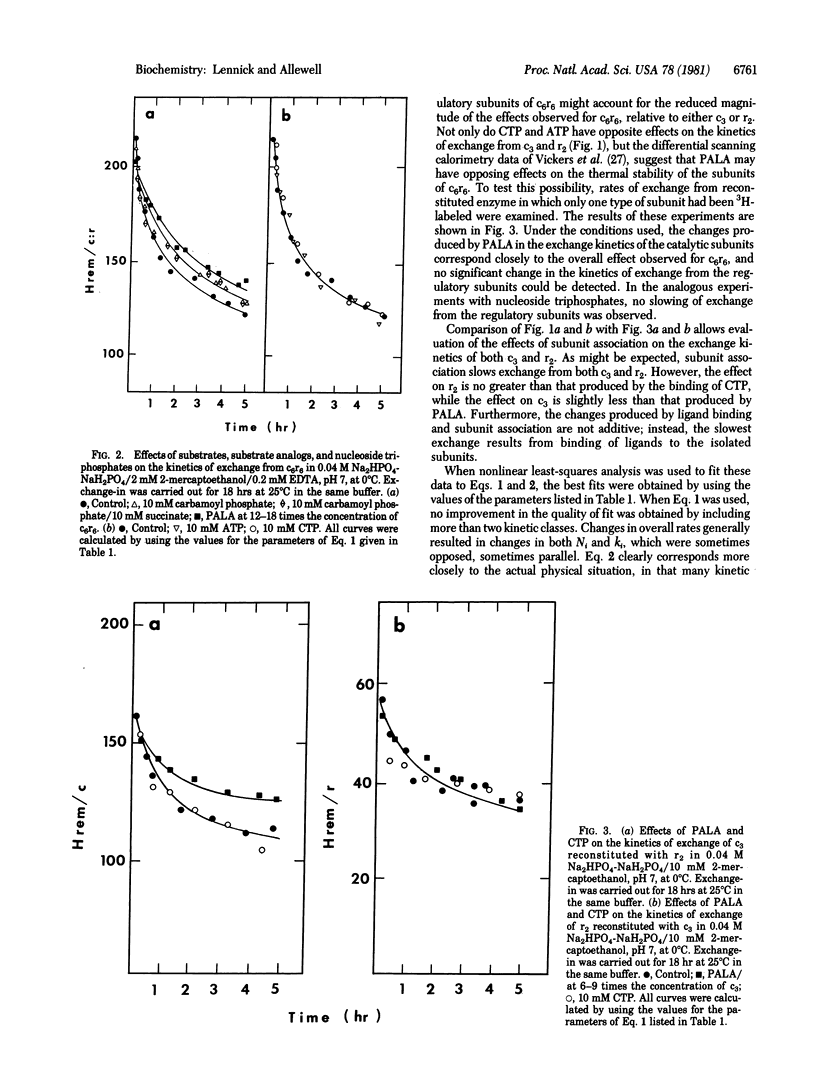
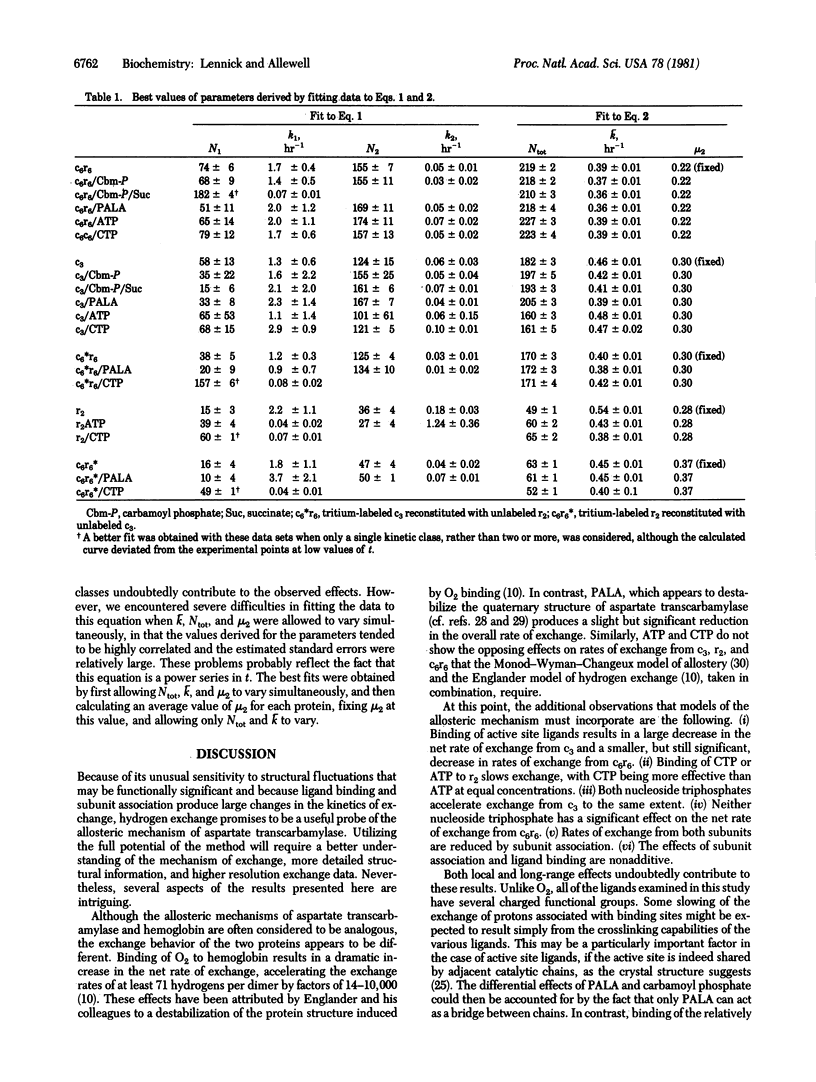
Large changes in solvent accessibility to aspartate transcarbamylase (aspartate carbamoyltransferase, carbamoylphosphate:L-aspartate carbamoyltransferase, EC 2.1.3.2), as monitored by tritium exchange, result from binding of substrates and substrate analogs to the catalytic subunit (c3), binding of nucleoside triphosphates to the regulatory subunit (r2), and subunit association. Rates of exchange are reduced in each of these cases, although to different degrees. Succinate, in the presence of carbamoyl phosphate, retards exchange from c3 no more than carbamoyl phosphate alone, and less than N-phosphonacetyl-L-aspartate, a bisubstrate analog. Larger changes in rates of exchange from r2 are produced by CTP than by ATP; however, both CTP and ATP accelerate exchange from c3 to the same extent. The changes in the kinetics of exchange that result from binding of both substrate analogs and nucleoside triphosphates to the native enzyme (c6r6) are much smaller. Carbamoyl phosphate, with or without succinate, retards exchange only slightly, while the bisubstrate analog has a somewhat larger effect. Experiments with reconstituted enzyme, in which only c3 is tritium labeled, indicate that changes in solvent accessibility produced by active site ligands are largely confined to c3. Neither CTP nor ATP alters the overall rate of exchange from c6r6 significantly. The possibility of opposing changes in the two types of subunits was ruled out in experiments in which only one subunit was labeled. The nonadditive effects of ligation and subunit association imply a set of responsive protons common to both processes and suggest that they are linked not only thermodynamically and functionally but also dynamically.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Halvorson H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4312–4316. doi: 10.1073/pnas.71.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen-tritium exchange. Methods Enzymol. 1972;26:406–413. doi: 10.1016/s0076-6879(72)26021-9. [DOI] [PubMed] [Google Scholar]
- Englander S. W. Measurement of structural and free energy changes in hemoglobin by hydrogen exchange methods. Ann N Y Acad Sci. 1975 Apr 15;244:10–27. doi: 10.1111/j.1749-6632.1975.tb41518.x. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Hensley P., Schachman H. K. Communication between dissimilar subunits in aspartate transcarbamoylase: effect of inhibitor and activator on the conformation of the catalytic polypeptide chains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3732–3736. doi: 10.1073/pnas.76.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Johnson R. S., Schachman H. K. Propagation of conformational changes in Ni(II)-substituted aspartate transcarbamoylase: effect of active-site ligands on the regulatory chains. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1995–1999. doi: 10.1073/pnas.77.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knier B. L., Allewell N. M. Calorimetric analysis of aspartate transcarbamylase from Escherichia coli. Binding of substrates and substrate analogues to the native enzyme and catalytic subunit. Biochemistry. 1978 Mar 7;17(5):784–790. doi: 10.1021/bi00598a005. [DOI] [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P. Kinetic class analysis of hydrogen-exchange data. Biochemistry. 1970 Mar 31;9(7):1547–1553. doi: 10.1021/bi00809a011. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Calhoun D. B., Englander J. J., Englander S. W. A high energy structure change in hemoglobin studied by difference hydrogen exchange. J Biol Chem. 1980 Nov 25;255(22):10687–10694. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Malin E. L., Englander S. W. The slowest allosterically responsive hydrogens in hemoglobin. Completion of the hydrogen exchange survey. J Biol Chem. 1980 Nov 25;255(22):10695–10701. [PubMed] [Google Scholar]
- Markus G., McClintock D. K., Bussel J. B. Conformational changes in aspartate transcarbamylase. 3. A functional model for allosteric behavior. J Biol Chem. 1971 Feb 10;246(3):762–771. [PubMed] [Google Scholar]
- Matsumoto S., Hammes G. G. An equilibrium binding study of the interaction of aspartate transcarbamylase with cytidine 5'-triphosphate and adenosine 5'-triphosphate. Biochemistry. 1973 Mar 27;12(7):1388–1394. doi: 10.1021/bi00731a019. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. C., Browne D. T. Binding of regulatory nucleotides to aspartate transcarbamylase: nuclear magnetic resonance studies of selectively enriched carbon-13 regulatory subunit. Biochemistry. 1980 Dec 9;19(25):5768–5773. doi: 10.1021/bi00566a016. [DOI] [PubMed] [Google Scholar]
- Nabedryk-Viala E., Calvet P., Thiéry J. M., Galmiche J. M., Girault G. Interaction of adenine nucleotides with the coupling factor of spinach chloroplasts. A hydrogen-deuterium exchange study. FEBS Lett. 1977 Jul 1;79(1):139–143. doi: 10.1016/0014-5793(77)80369-4. [DOI] [PubMed] [Google Scholar]
- Printz M. P., Gounaris A. D. Substrate- and inhibitor-induced conformational changes in enzymes measured by tritium-hydrogen exchange. II. Yeast pyruvate decarboxylase. J Biol Chem. 1972 Nov 25;247(22):7109–7115. [PubMed] [Google Scholar]
- Rosa J. H., Richards F. M. Hydrogen exchange from identified regions of the S-protein component of ribonuclease as a function of temperature, pH, and the binding of S-peptide. J Mol Biol. 1981 Feb 5;145(4):835–851. doi: 10.1016/0022-2836(81)90318-1. [DOI] [PubMed] [Google Scholar]
- Rosa J. J., Richards F. M. An experimental procedure for increasing the structural resolution of chemical hydrogen-exchange measurements on proteins: application to ribonuclease S peptide. J Mol Biol. 1979 Sep 25;133(3):399–416. doi: 10.1016/0022-2836(79)90400-5. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Baldwin R. L. Concentration-dependent hydrogen exchange kinetics of 3H-labeled S-peptide in ribonuclease S. J Mol Biol. 1976 Aug 15;105(3):409–426. doi: 10.1016/0022-2836(76)90101-7. [DOI] [PubMed] [Google Scholar]
- Shrake A., Ginsburg A., Schachman H. K. Calorimetric estimate of the enthalpy change for the substrate-promoted conformational transition of aspartate transcarbamoylase from Escherichia coli. J Biol Chem. 1981 May 25;256(10):5005–5015. [PubMed] [Google Scholar]
- Stryker M. H., Parker F. S. A hydrogen--deuterium exchange study of the conformation of bovine liver glutamate dehydrogenase. Arch Biochem Biophys. 1970 Nov;141(1):313–321. doi: 10.1016/0003-9861(70)90137-2. [DOI] [PubMed] [Google Scholar]
- Subramani S., Bothwell M. A., Gibbons I., Yang Y. R., Schachman H. K. Ligand-promoted weakening of intersubunit bonding domains in aspartate transcarbamolylase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3777–3781. doi: 10.1073/pnas.74.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter P., Rosenbusch J. P. Asymmetry of binding and physical assignments of CTP and ATP sites in aspartate transcarbamoylase. J Biol Chem. 1977 Nov 25;252(22):8136–8141. [PubMed] [Google Scholar]
- Thiry L., Hervé G. The stimulation of Escherichia coli aspartate transcarbamylase activity by adenosine triphosphate. Relation with the other regulatory conformational changes; a model. J Mol Biol. 1978 Nov 15;125(4):515–534. doi: 10.1016/0022-2836(78)90314-5. [DOI] [PubMed] [Google Scholar]
- Vickers L. P., Donovan J. W., Schachman H. K. Differential scanning calorimetry of asparate transcarbamoylase and its isolate subunits. J Biol Chem. 1978 Dec 10;253(23):8493–8498. [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Correlation between the amide proton exchange rates and the denaturation temperatures in globular proteins related to the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):31–37. doi: 10.1016/0022-2836(79)90550-3. [DOI] [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Kirschner M. W., Schachman H. K. Aspartate transcarbamoylase (Escherichia coli): preparation of subunits. Methods Enzymol. 1978;51:35–41. doi: 10.1016/s0076-6879(78)51007-0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Schachman H. K. Communication between catalytic subunits in hybrid aspartate transcarbamoylase molecules: effect of ligand binding to active chains on the conformation of unliganded, inactive chains. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5187–5191. doi: 10.1073/pnas.77.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]


