Abstract
CONTEXT:
Tumor protein 53 (tp53) is one of the candidate gene proposed for neural tube defects, which affects central nervous system during early embryonic development, on the basis of mouse models.
AIMS:
The present study is an attempt to unfold the possible role of tp53 G412C polymorphism in the incidence of neural tube defect (NTDs) in humans.
SETTINGS AND DESIGN:
Case-control study was carried out in government hospitals of Delhi, India.
MATERIALS AND METHODS:
Subjects comprised of 100 mothers of NTD children and 100 matched control mothers. Information on some environmental exposures was collected along with blood samples. After DNA extraction, the genotyping of tp53 G412C polymorphism was carried out by PCR-RFLP method.
Statistical Analysys:
Fisher Exact or Chi square test, binary logistic model, and odds ratio (95% confidence interval) calculations were used to evaluate effect of risk factors on NTDs using SPSS v17.0.
RESULTS:
The ‘CC’ genotype of tp53 G412C showed protective effect towards the development of anencephaly and/or encephalocele (OR: 0.44; 95% CI: 0.19-1.00); however, no significant difference among overall NTD cases and controls was observed (P>0.05). Further segregation of all subjects based on 2 different communities, Hindus and Muslims, the association of ‘CC’ genotype of the polymorphism with reduced NTD risk was observed among Hindu community (OR: 0.33; 95% CI: 0.13-0.79).
CONCLUSION:
The study highlights the selective advantage provided by maternal ‘CC’ genotype, thereby reducing risk of cephalic NTDs, probably due to the lower apoptotic activity of the protein, however, more specifically in the presence of community-specific microenvironment.
Keywords: R72P, cephalic NTDs, Hindus, dietary habits
Introduction
Since its discovery in 1979, tumor protein 53 (tp53) has been known to play a key role in cell cycle growth check points and apoptosis. In normal cells, p53 is quiescent and present at basal level, but it responds to genotoxic stresses such as DNA damage and hypoxia by activating pathways that can lead to cell cycle arrest, DNA repair, cellular senescence, differentiation and apoptosis, largely through the transcriptional regulation of p53 target genes.[1,2] Loss of p53 function contributes to the various tumorgenic pathologies; however, excessive protein function has also been implicated in embryonic lethality, neural degeneration, and neural tube defects.[3] Neural Tube Defects (NTDs) refers to the group of developmental defects affecting brain (such as anencephaly and encephalocele) and spinal cord (spina bifida) formation, during early embryogenesis, and have a complex etiology involving environmental and genetic parameters. With exciting roles in wide range of processes, p53 is also crucial for proper embryogenesis and development of neural tube defects. Mouse model studies suggested suppression of p53 mediated apoptotic activity rescues NTDs,[4–6] but also, p53 deficient mice exhibit exencephaly.[7]
The human tp53 gene (located on 17q) comprises 11 exons encoding a 393 amino-acid protein. In more than half of human cancers, the tp53 protein is mutated or is functionally inactivated. Among the all known variations, G412C (rs1042522, R72P) polymorphism, a missense mutation in exon 4, is the most extensively studied variation, showing sharp ethnic difference in allele frequency and its effect.[8] The protein with R72 (412G) is reported to be more efficient in inducing apoptosis than the one with the P72 (412C) variant,[9] which causes increased susceptibility for cancer. Therefore, excessive apoptotic activity of the protein may harm the growing embryo negatively and results in neural tube defects; however, the presence of P72 variant could be associated with normal neural tube development.
Based on the mouse model studies, role of tp53 in human NTDs was evaluated in 2 previous studies. One of the studies found significant association between 3 non-coding variants of tp53 gene and the increased NTD risk; however, no role of R72P polymorphism was observed,[10] whereas other did not find any association with intragenic polymorphism of tp53 gene.[11] Both the studies included only spina bifida NTD phenotype, which is a defect of spinal cord, and did not cover cephalic defects (anencephaly and encephalocele).
In light of the available literature, the present study aimed to unfold the specific role of maternal tp53 G412C polymorphism in the risk for NTD-affected pregnancies. The present study was carried out in North India, which is co-populated by 2 major communities, Hindus and Muslims. Both of these communities, although belong to Euro-Asian populations, have distinct cultural practices, dietary habits, and mating patterns; Muslims are mainly non-vegetarian and practice consanguinity, whereas Hindus do not prefer to eat even eggs, and consanguineous marriages are prohibited in the community. Therefore, the 2 communities differ from each other with respect to their genotypic-phenotypic interactions, and therefore, are also studied separately.
Materials and Methods
Personal interviews with the 100 NTD case mothers and 100 control mothers were conducted in 4 government hospitals of Delhi, India (after obtaining formal ethical clearances), during January 2008 and August 2011. This study was conducted according to the guidelines laid down in the Declaration of Helsinki (2000), and all procedures involving human subjects/patients were approved by the Institutional Ethical Committee, following the ethical guidelines of Indian Council of Medical Research (ICMR).
The diagnosis of NTD child was done by doctors of the hospitals, from live births, still births/terminations, and ultrasonography during ante-natal checkups. The inclusion criteria for the cases were the mothers of NTD child, and the control group comprised of the mothers (matched for community and age group) with no previous history of unsuccessful pregnancy or abnormal conceptions.
The pretested interview schedules were used to collect information regarding community background, maternal age, chronic illness (like obesity and diabetes), exposure to hyperthermia (fever or external heat), dietary habits (vegetarian or non-vegetarian), and folic acid supplementation during peri-conceptional period. Intravenous blood samples were also collected from the subjects after an informed written consent. The DNA extraction was done by salting-out method,[12] and genotyping of tp53 G412C polymorphism was carried out by PCR amplification followed by restriction digestion with BstU1 and analysis on 2% agarose gel.[13]
Hardy-Weinberg equilibrium was tested in both case and control groups by Chi-square method. Fisher Exact probability or Chi-square test was applied to analyze the difference in the frequency distribution among case and control group. Binary logistic model and odds ratio (95% confidence interval) calculations were used to evaluate effect of risk factors on the incidence of NTDs. The calculations were done using SPSS v.17.0 package, considering P≤0.05 as statistically significant.
To see the effect on different NTD phenotypes, the cases were segregated into 2 groups: Cephalic NTDs (affecting brain development like anencephaly or encephalocele) and Spinal NTDs (affecting spine-like spina bifida), and were compared with control mothers; the case mothers of multiple NTDs were excluded for this part of analysis. The risk assessment was further refined by dividing the subjects based on 2 communities: Hindus and Muslims, to address the effect of population stratification.
Results
General characteristics of the cases in the present study are represented in Table 1. Majority of affected cases had anencephaly (53%); 27% had spina bifida, and 11% had encephalocele; 9% of cases had multiple NTDs. Of all cases, 15% were live births, and remaining were still births or terminated cases. Other congenital defects were observed among 12% cases. The sex ratio (F/M) of NTD was found to be 1.33. 59% of the cases were Hindus, and 41% were Muslims. The age of case mothers at the time of conception of NTD child ranges between 17 and 35 years, with mean age of 23.02±3.45 years.
Table 1.
General characteristics of case group

Cases and controls significantly differ with respect to chronic illness, hyperthermia, dietary habits, and consumption of folic acid during peri-conceptional period [Table 2]. Odds ratio analysis suggested 2.2-fold risk with the presence of chronic illness (CI: 1.0-4.7), 4.1-fold risk with the incidence of hyperthermia (CI: 2.0-8.4), 2.2-fold risk with vegetarian dietary habits (CI: 1.2-4.2) and 50% reduced risk with consumption of folic acid tablets during peri-conceptional period (CI: 0.3-0.9).
Table 2.
Environmental risk factors
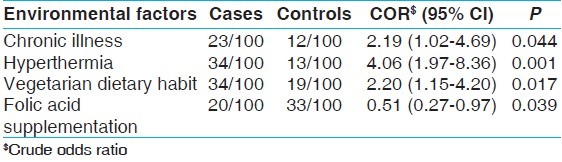
After segregating (data not shown here), the increased risk for cephalic NTDs was likely to be associated with incidence of hyperthermia (3.4; CI: 1.5-7.9), vegetarian dietary habits (2.8; CI: 1.4-6.1), and non-consumption of folic acid (0.4; CI: 0.2-0.9, with folic acid consumption), whereas 3.6-fold risk for spinal NTDs was found with the presence of chronic illness (CI: 1.3-10.0). Among the Hindu community, vegetarian dietary habit was likely to increase the NTD risk by 3.7-fold (CI: 1.5-9.2), whereas among Muslims, folic acid supplementation showed protection against NTD conceptions (0.2; CI: 0.1-0.8) [Table 3].
Table 3.
Genotype distribution of Tp53 gene G412C polymorphism
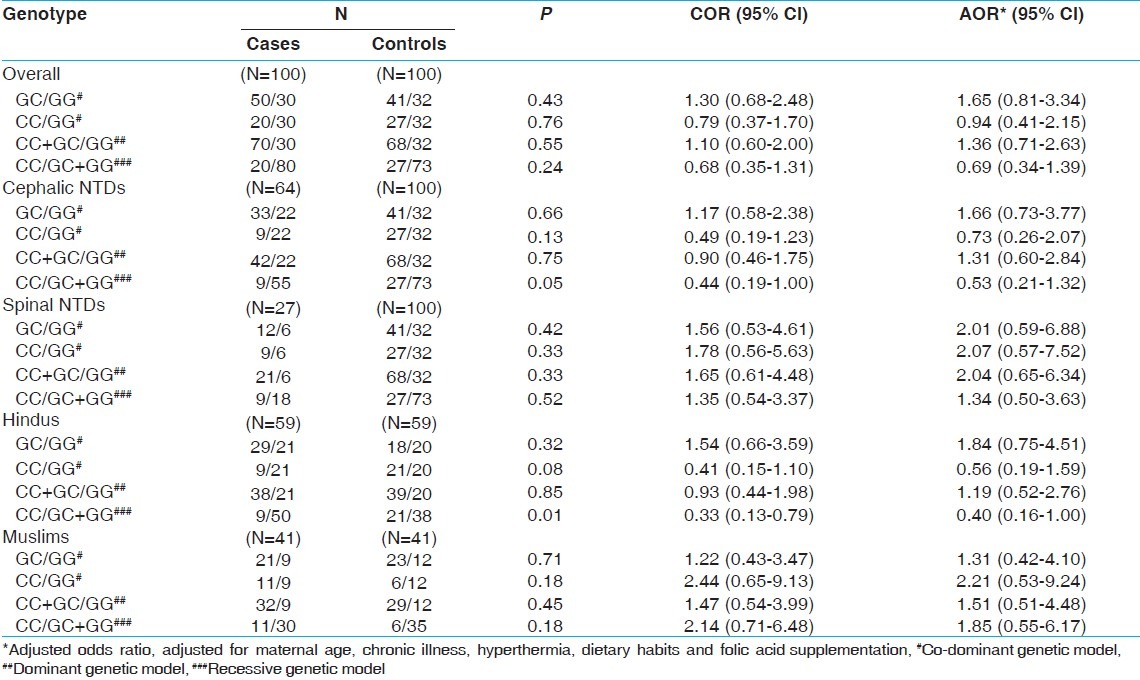
Both, case and control groups, were found to be under Hardy–Weinberg equilibrium (P>0.05, for both). As represented in Figure 1, the homozygous genotypes ‘GG’ and ‘CC’ were found to be more among control group (32% and 27%, respectively) as compared to cases (30% and 20%, respectively), whereas frequency of heterozygotes (GC) was higher among cases (50%) than among the counterpart (41%). The frequency of ‘C’ allele was found to be slightly higher among control group (0.47) against 0.45 among cases, and the ‘G’ allele frequency was 0.55 and 0.53 among cases and controls, respectively. The genotypic and allelic distribution showed no significant difference between cases and controls on applying Chi-square test (P>0.05, for both).
Figure 1.
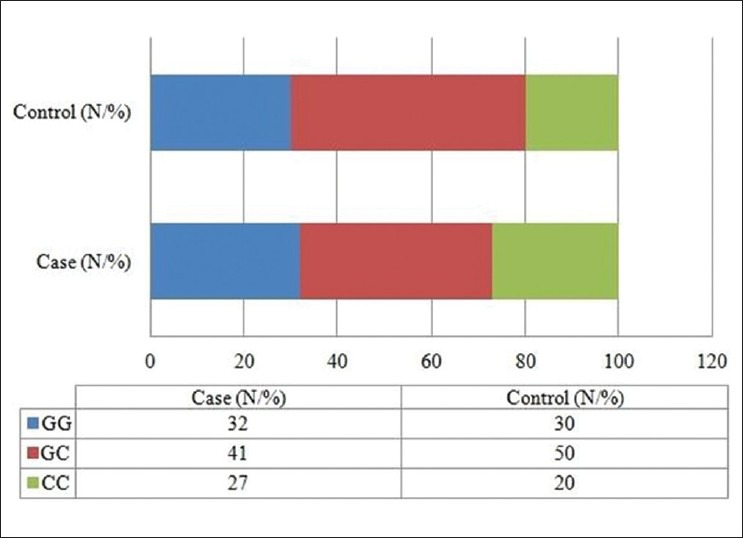
Genotype distribution of Tp53 G412C polymorphism among case and control mothers
The odds ratio suggested no role of tp53 G412 polymorphism in incidence of NTDs. However, segregating the cases based on the NTD phenotype, significantly higher frequency of ‘CC’ genotype was observed among controls (27%) as compared to the cephalic NTD case mothers (14%), resulting in lower risk for cephalic NTDs (COR: 0.4; CI: 0.2-1.0) when compared with the combined effect of GG and GC genotypes (under recessive genetic model). Among the 2 communities, more Hindu controls were found to have ‘CC’ genotypes than respective cases (15% and 36%, respectively). The univariate analysis showed protective effect of the maternal genotype towards the risk of NTDs among Hindus (COR: 0.3; CI: 0.1-0.8), which was further found to be consistent (AOR: 0.4; CI: 0.2-1.0) after adjusting for probable confounding (demographic or environmental) factors. No significant protective effect of the genotype was observed among spinal NTDs or Muslim subgroup.
Discussion
The 412‘C’ (P72) variant of tp53 is widely studied in context with lower apoptotic activity or cancer, i.e. cell growth; however, during embryonic development, lower apoptotic activity is required for the proper growth. Complex role of p53 in relation to neural tube defects among mouse models have been demonstrated. Deficiency of XRCC4 (X-ray repair complementing defective repair in Chinese hamster cells 4) or DNA ligase IV, both of which are involved in DNA repair during normal embryogenesis, causes embryonic lethality and massive neuronal apoptosis, and these effects were rescued by p53 deficiency.[4,5] Splotch mice (homozygous for a loss-of-function of Pax3) that results in exencephaly (cephalic defect) and/or spina bifida,[14] can also be rescued by loss of function of p53.[6] On the other hand, around 8-16% of p53-deficient female mice exhibited exencephaly,[15] and the levels of exencephaly increased in double knockouts for p53 and Gadd45a (growth arrest and DNA-damage-inducible protein alpha), which is a one of the downstream effectors induced by p53 to achieve G2-M arrest.[7] Pregnant p53 deficient mice are at higher risk of reduced blastocyst implantation;[16] however, development of neural tube or its defects, condition which is considered in the present study, arises only after the blastocyst implantation of an embryo.
Of the 2 previous studies carried out to identify to role tp53 gene polymorphisms among humans, the first study tested association of unidentified intragenic multiallelic marker with development of lumbosacral myelomeningocele among 78 American Caucasian families, and did not found statistically significant role of the gene.[11] Another study included comprehensive evaluation of genetic variations and haplotypes of tp53 among 549 NTD cases (mainly spina bifida), 532 NTD mothers, 481 NTD fathers, and 999 Irish controls.[10] 3 non-coding variants were found to be associated with increased NTD risk (1 among NTD cases and 2 among NTD mothers); regarding the R72P polymorphism, frequency of ‘CC’ genotype was found to be more among controls than case mothers; however, no significant decreased risk for NTD was observed in the study. In the present study, maternal ‘CC’ genotype was not found to reduce the risk of NTDs, which is found to be consistent with the Pangilinan's with respect to G412C polymorphism.
The mouse model studies suggested role of p53 in mainly exencephaly, which is a precursor for cephalic disorders. Therefore, in the present study, the heterogeneous NTD cases were segregated into cephalic NTDs and spinal NTDs, and it was found that the ‘CC’ genotype, as expected, was associated with reduced maternal risk of having a cephalic NTD-affected pregnancy. This suggested the significant role of the tp53 gene mainly during cephalic development; however, the reduced risk was not found to be consistent after adjustment with probable confounding factors, further pointing towards complex interaction with maternal microenvironment. The 2 culturally distinct communities covered in the study provide different maternal microenvironment, which may result in varied effect of gene polymorphism, and is substantiated in present study. The lower risk for NTD was found among Hindus, whereas no role among Muslims was observed. The reduced risk among Hindus was found to be consistent even after adjustment with studied confounding factors, suggesting the probable effect of some additional unidentified factors, prevalent in the community.
In addition, the higher frequency of ‘CC’ genotype among controls, as compared to cephalic NTD case mothers and Hindu case mothers, were also accompanied by statistically significantly higher frequency of non- vegetarian dietary habit. Conversely, more cephalic NTD cases and Hindu cases were found to be vegetarian than their respective counterparts. Although vegetarian diet is rich in vitamins and micronutrients, which provide antioxidant defense, the other essential micronutrients, which are involved in DNA metabolism and stability, are provided by non-vegetarian diet.[17] In developing countries like India, majority of pregnant women are deficient in micronutrients, which can be provided as supplements during ante-natal care. According to the National Family Health Survey,[18] the exposure to pre or early ante-natal care in India, especially among low socio-economic communities, is very limited. In the absence of additional supplements recommended during ante-natal care, the women of sub-group are expected to be low in essential micronutrients of non-vegetarian diet. Based on the these observations, it can be assumed that non-vegetarian diet in the presence of the maternal ‘CC’ genotype, as observed among control group, possibly provided healthier microenvironment conducive for embryonic growth, protection against excessive apoptotic activity of tp53 protein, and normal neural tube development. On the other hand, the GG and/or GC genotype of tp53 gene in the absence of non-vegetarian micronutrients or other hidden factors, as observed among cephalic NTD and Hindu case mothers, may be deleterious and causes NTDs.
The strength of the present study is that it is the first report suggesting role of tp53 G412C polymorphism in lowering the risk of NTDs, giving further directions to researchers to design studies with more comprehensive approach. The study had some limitations; first, the maternal genotype was screened, however, the transmission effect of this polymorphism could not be assessed as the NTD child's samples were not available. Second, the study was designed with heterogeneous group of NTDs, and the samples were later segregated into subgroups, which resulted in smaller sample sizes. Third, the environmental or genetic modifiers of the gene polymorphism specific in the community could not be evaluated in detail. Further studies overcoming the limitations of the present study would provide better insight with respect to the association between tp53 gene polymorphism and NTDs.
Conclusion
In the study, ‘CC’ genotype of tp53 G412C polymorphism, which is known to increase the cancer susceptibility, was found to be associated with lower risk of specific NTD phenotypes (cephalic disorders) and among specific community (Hindus). This is the first study to report significant protective role of maternal tp53 gene in the incidence of neural tube defects. The present study provides future directions to the researchers to design studies with more comprehensive approach to validate the results in different ethnic populations/communities with larger cohort group, which may include parent-child triode and the modifying factors specific in the population.
Acknowledgment
We acknowledge the financial support provided by the Indian Council of Medical Research, New Delhi, India. We are thankful to the doctors and staff of the various hospitals for helping us in data collection. Sincere thanks to all the subjects for giving us the required information and blood samples.
Footnotes
Source of Support: Indian Council of Medical research, New Delhi, India
Conflict of Interest: None declared.
References
- 1.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–72. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 2.Oren M. Decision making by p53: Life, death and cancer. Cell Death Differ. 2003;10:431–42. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 3.Mendrysa SM, Ghassemifar S, Malek R. p53 in the CNS: Perspectives on development, stem cells, and cancer. Genes Cancer. 2011;2:431–42. doi: 10.1177/1947601911409736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, et al. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 6.Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: Implications for Pax-3-dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson AD, Hildesheim J, Fornace AJ, Jr, Hollander MC. Neural tube development requires the cooperation of p53- and Gadd45a-associated pathways. Birth Defects Res A Clin Mol Teratol. 2006;76:129–32. doi: 10.1002/bdra.20217. [DOI] [PubMed] [Google Scholar]
- 8.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont P, Leu JI, Della Pietra AC, 3rd, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–65. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 10.Pangilinan F, Geiler K, Dolle J, Troendle J, Swanson DA, Molloy AM, et al. Construction of a high resolution linkage disequilibrium map to evaluate common genetic variation in TP53 and neural tube defect risk in an Irish population. Am J Med Genet A. 2008;146A:2617–25. doi: 10.1002/ajmg.a.32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melvin EC, George TM, Worley G, Franklin A, Mackey J, Viles K, et al. Genetic studies in neural tube defects.NTD Collaborative Group. Pediatr Neurosurg. 2000;32:1–9. doi: 10.1159/000028889. [DOI] [PubMed] [Google Scholar]
- 12.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamel N, Black MJ, Ghadirian P, Foulkes WD. No association between p53 codon 72 polymorphism and risk of squamous cell carcinoma of the head and neck. Br J Cancer. 2000;82:757–9. doi: 10.1054/bjoc.1999.0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- 15.Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995;10:175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–24. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 17.Kazimírová A, Barancoková M, Krajcovicová-Kudlácková M, Volkovová K, Staruchová M, Valachovicová M, et al. The relationship between micronuclei in human lymphocytes and selected micronutrients in vegetarians and non-vegetarians. Mutat Res. 2006;611:64–70. doi: 10.1016/j.mrgentox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.National Family Health Survey (NFHS-3), 2005-06, India: Key Findings. Mumbai: IIPS; 2007. [Last accessed on 24th July 2012]. International Institute for Population Sciences (IIPS) and Macro International. Available from: http://www.measuredhs.com/pubs/pdf/SR128/SR128.pdf . [Google Scholar]


