Abstract
BACKGROUND:
Primary amenorrhea is defined as the absence of menstruation and secondary sexual characteristics in phenotypic women aged 14 years or older. Hormonal disorders are main causes of primary amenorrhea. Common hormonal cause of primary amenorrhea includes pituitary dysfunction and absent ovarian function. The aim of this study was to estimate the incidence and types of chromosomal abnormalities in patients with primary amenorrhea in Egypt.
MATERIALS AND METHODS:
Chromosomal analysis and hormonal assay were carried out on 223 patients with primary amenorrhea that were referred from different parts of Egypt to Cytogenetic laboratory of Genetic Unit, Children Hospital Mansoura University, from July 2008 to December 2010. FISH technique was carried out in some of cases to more evaluation.
RESULTS:
The frequency of chromosomal abnormalities was 46 (20.63%) in primary amenorrhea patients. The chromosomal abnormalities can be classified into four main types. (1) The numerical abnormalities of the X chromosome were detected in 23 (50 %). (2) Structural abnormalities of the X chromosome were detected in 11 (23.91%). (3) Mosaicism of X chromosome was found in 10 (21.74%). (4) Male karyotype 46, XY was presented in 2 (4.35%).
CONCLUSION:
The present study showed that karyotype and FISH are necessary to detect the causes of primary amenorrhea. This study also revealed the incidence of chromosomal abnormalities in women with primary amenorrhea in Egypt is similar to that reported in previous literatures.
Keywords: Cytogenetic study, primary amenorrhea, karyotype, Fluorescence in situ hybridization
Introduction
Primary amenorrhea is the absence of menstruation and secondary sexual characteristics in phenotypic women aged 14 years or older.[1] Hormonal disorders are main causes of primary amenorrhea. Common hormonal cause of primary amenorrhea includes pituitary dysfunction, chronic systemic disease, and absent ovarian function.[2] Hyperprolactinemia is associated with decreased estradiol concentrations and amenorrhea. Prolactin concentrations are high in women with amenorrhea.[3] Gonadal failure in genetically XX individuals is ovarian failure; when this occurs at any time before onset of sexual maturation, there will be primary amenorrhea and incomplete breast development. XY individuals with gonadal failure will have female genitalia because Mullerian inhibiting factor and testosterone will not be produced. Gonadal tumors occur in up to 25% of women with a Y chromosome; unlike complete androgen insensitivity, these gonads do not secrete hormones and should be removed at the time of diagnosis.[4] Cytogenetic investigation has shown the importance of chromosomal abnormalities as a cause of amenorrhea.[5] The incidence of the chromosomal abnormality (CA) in live births is around 90 per 10,000. Included in the incidence are the numerical (monosomy/trisomy/mosaicism) as well as the structural (translocation/isochromosome/deletion/duplication/ring) CA.[6] The reported incidence of CA in primary amenorrhea is 20-40%.[7]
Materials and Methods
233 women with primary amenorrhea who were referred to Cytogenetic Unit, Children Hospital, Mansoura University, Egypt from July 2008 to December 2010. Their ages ranged from 14 to 30 years. For all patients, steroidal hormonal assay were setup according to ELISA technique,[8–10] and chromosomal cultures were setup according to G- banding,[11] about 1 ml of blood was mixed with 5 ml of RPMI medium, 1 ml of fetal bovine serum, 0.1 μg/ml of Phytohemagglutinin (PHA) and was incubated at 37°C. After 72 hour of incubation, the Colcimid (1 mg/ml) was added and incubated for another 1.5 hour. The cells were then harvested by hypotonic treatment (1.5 hour with 0.075M KCl at 37°C), fixed and washed thrice with fixative solution (methanol and acetic acid in a ratio of 3:1), and then metaphases were spread and stained using standard G-banding technique. For each case, 50 spread metaphases were analyzed with cytovision system.
Fluorescence in situ hybridization (FISH) technique was carried out in some of cases to more evaluation according to technique of.[11] 10 μl of the X,Y centromeric probe were applied to the target area, and the cover slip was applied to the slide immediately. The cover slip was sealed with rubber cement, and the slide was placed on hot plate at 72°C for 2 minutes. The slide was placed in a warmed humidified box, and the box was placed at 37°C in an incubator overnight. The cover slip was removed from slide, and it was immersed in 70 ml of the 0.4 x SSC/0.3% NP-40 in a coplin jar at a 73±1°C water bath for 2 minutes. Then, the slide was immersed in 70 ml of the 2 x SSC/0.1% NP-40 in coplin jar at 25°C for 2 minutes. The slide was air-dried in the dark, and 10 μl of DAPI II counter stain were applied to the target area of slide, and the cover slip was applied to the each slide. The slide was viewed using a suitable filter set on a fluorescence microscope.
Results
233 patients with primary amenorrhea were referred from different parts of Egypt to Cytogenetic laboratory of Genetic Unit, Children Hospital Mansoura University, from July 2008 to December 2010. Their ages ranged from 14 to 30 years. The frequency of chromosomal abnormalities was 46 (20.63%) in primary amenorrhea patients. The chromosomal abnormalities can be classified into four main types. (1) The numerical abnormalities of the X chromosome were detected in 23 (50%). (2) Structural abnormalities of the X chromosome were detected in 11 (23.91%). (3) Mosaicism of X chromosome was found in 10 (21.74%). (4) Male karyotype 46, XY was presented in 2 (4.35%), [Table 1]. The two patients with association of chromosome Y was confirmed by FISH [Figure 1], and the presence of deletion of long arm of chromosome X in 3 patients was confirmed, using FISH [Figure 2].
Table 1.
Classification of chromosomal abnormalities of patients with primary amenorrhea
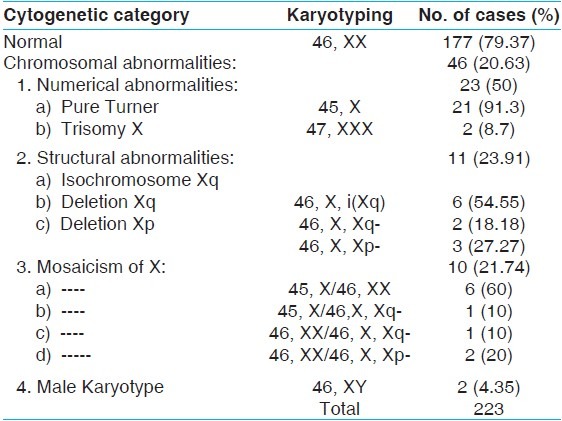
Figure 1.
Interphase and metaphase from FISH analysis showing one X chromosome and one Y chromosome of female confirming the testicular feminization syndrome (XY)
Figure 2.
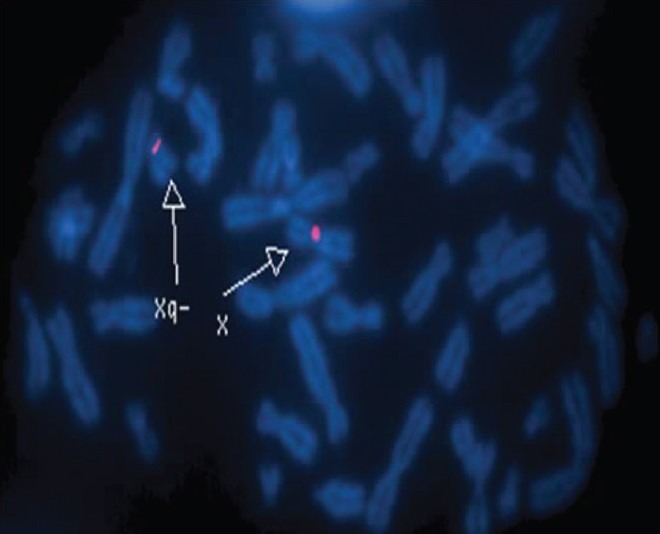
Metaphase from FISH analysis showing one X chromosome and short arm of another X of female confirming the form X, Xq
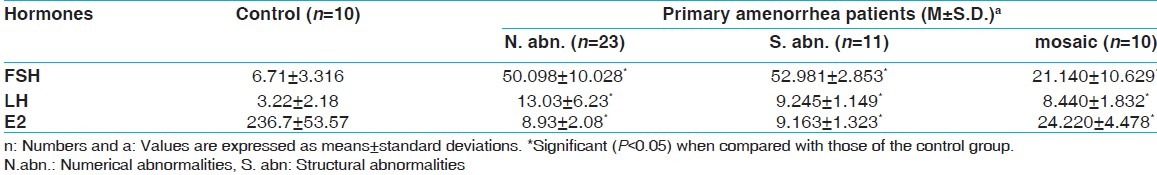
In Table 2, follicle stimulating hormone (FSH) and luteinizing hormone (LH) mean levels in sera of females with primary amenorrhea, whether they have numerical, structural, or mosaicism chromosomal abnormalities, were significantly higher than those of the control group (P<0.05). But, Estradiol (E2) mean levels in sera of them were significantly lower than those of the control group (P<0.05).
Table 2.
Steroidal hormones of patients with primary amenorrhea and chromosomal abnormalities
Discussion
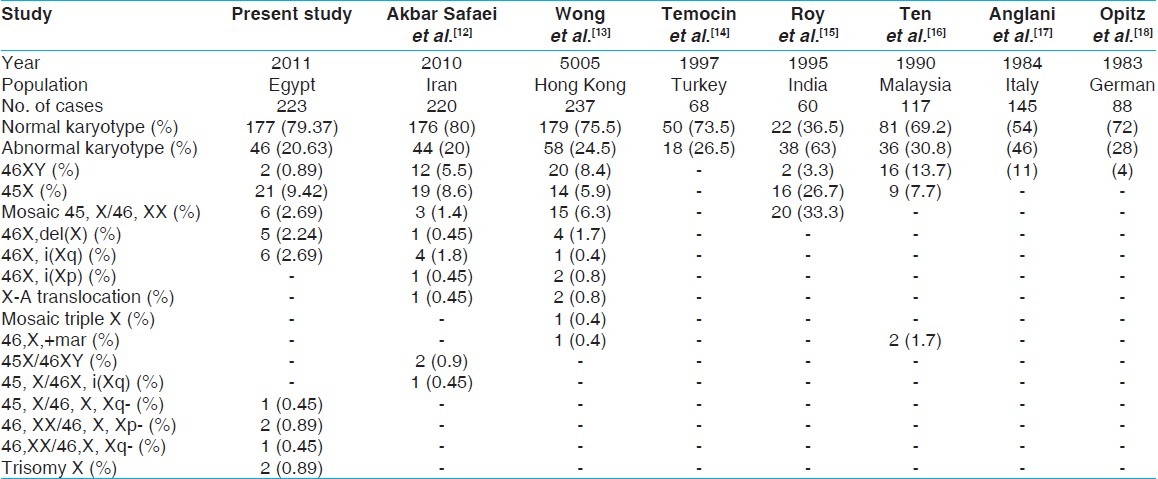
20.63% of chromosomal abnormalities in primary amenorrhea of present study compared with those in various study conducted between 1983 and 2010 [Table 3].
Table 3.
Chromosome abnormalities in primary amenorrhea in various study and compared with present study
Genes essential for gonadal function are located on the proximal part of Xp, the long arm of X proximal to Xq13 and/or the long arm of X distal to Xq26. Large deletion of Xq with breakpoints at or proximal to q13 are expected to produce gonadal dysgenesis with primary amenorrhea, half of the patients with such deletions have Turner`s syndrome.[19] That explained the cause of our two patients with deletion of Xq have primary amenorrhea, but have not feature of Turner syndrome, whereas other three patients who have deletion of Xp have primary amenorrhea and all the features of Turner syndrome. Also, the six patients with isochromosome Xq have all features of Turner syndrome.
Ten et al. found one patient with primary amenorrhea and 47, XXX had mental retardation and the cause of that, an extra X, can slow down embryonic cell development in a special way.[16] Similar to that, there were mental retardation in two of our patients with karyotype of 47, XXX.
XY individuals with gonadal failure will have female genitalia because Mullerian inhibiting factor and testosterone will not be produced. Gonadal tumors occur in up to 25% of women with a Y chromosome; unlike complete androgen insensitivity, these gonads do not secrete hormones and should be removed at the time of diagnosis.[4] In our study, we diagnosed two patients of primary amenorrhea as testicular feminization syndrome (XY) using FISH technique.
The molecular cytogenetic technique, FISH, accurately delineate the nature and origin of marker chromosomes in primary amenorrhea patients, which is difficult by conventional cytogenetics.[20] As similar to that, in present study, the presence of deletion of long arm of chromosome X in 2 patients was confirmed, using FISH, which is very difficult by conventional cytogenetic where short arm of the X chromosome is similar to the Y chromosome.
Ten et al.[16] found that, serum follicle stimulating hormones and luteinizing hormones were always increased in primary amenorrhea patients with chromosomal abnormalities. This is found to be so in all our patients.
Conclusion
The present study showed that karyotype and FISH are necessary to detect the causes of primary amenorrhea. This study also revealed the incidence of chromosomal abnormalities in women with primary amenorrhea in Egypt is similar to that reported in previous literatures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Berek J. Berek and Novak's Gynecology. 14th edition. Philadelphia: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 2.Rosa RF, Dibi RP, Picetti Jdos S, Rosa RC, Zen PR, Graziadio C, et al. Amenorrhea and X chromosome abnormalities. Rev Bras Ginecol Obstet. 2008;30:511–7. doi: 10.1590/s0100-72032008001000006. [DOI] [PubMed] [Google Scholar]
- 3.Touraine P, Plu-Bureau G, Beji C, Mauvais-Jarvis P, Kuttenn F. Long-term follow-up of 246 hyperprolactinemic patints. Acta Obstet Gynecol Scand. 2001;80:162–8. doi: 10.1034/j.1600-0412.2001.080002162.x. [DOI] [PubMed] [Google Scholar]
- 4.Manuel M, Katayama PK, Jones HW., Jr The abe of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol. 1976;124:293–300. doi: 10.1016/0002-9378(76)90160-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Shen GM, Feng Q, Sun XG, Luo Y. Cytogenetic studies of 131 patients with primary amenorrhea (including three novel abnormal karyotypes) Yi Chuan. 2008;30:996–1002. doi: 10.3724/sp.j.1005.2008.00996. [DOI] [PubMed] [Google Scholar]
- 6.Mueller RF, Young ID. Emery's elements of medical genetics. 11th ed. Edinburgh, UK: Churchill Livingstone; 2001. p. 249. [Google Scholar]
- 7.Kobilková J, Chrz R, Bauer J, Málková J, Michalová K, Málková-Silinková E, et al. Cytodiagnostic study of women with primary amenorrhea and karyotype 46XX. Acta Cytol. 1977;21:334–7. [PubMed] [Google Scholar]
- 8.Mehta RR, Valcourt L, Graves J, Green R, Das Gupta TK. Subcellular concentrations of estrone, estradiol, androstenedione and 17 beta-hydroxysteroid dehydrogenase (17-beta-OH-SDH) activity in malignant and non-malignant human breast tissues. Int J Cancer. 1987;40:305–8. doi: 10.1002/ijc.2910400304. [DOI] [PubMed] [Google Scholar]
- 9.Knobil E. The neuroendocrine control of the menstrual cycle. Omega Diagnostics Ltd. 1988;23:566–74. [Google Scholar]
- 10.Arakawa H, Maeda M, Tsuji A. Anal. Biochem 97248. Saronno (Va) Italy: 1979. Direct Immunoenzymatic Determination of Cortisol in Serum, Plasma. Quoted from Equipar srl Via G. Ferrari, 21/N-21047. [Google Scholar]
- 11.Rooney DE, Czepulkowski BH. Human Chromosome Preparation, Esential Techniques Series. London: UK Essex University Press; 1997. pp. 37–38. 102-111. [Google Scholar]
- 12.Safaei Akbar, Vasei Mohammad, Ayatollahi Hossein. Cytogenetic analysis of patients with primary amenorrhea in southwest of Iran. Iranian Journal of Pathology. 2010;5:121–5. [Google Scholar]
- 13.Wong MS, Lam ST. Cytogenetic analysis of patients with primary and secondary amenorrhoea in Hong Kong: retrospective study. Hong Kong Med J. 2005;11:267–72. [PubMed] [Google Scholar]
- 14.Temoçin K, Vardar MA, Süleymanova D, Ozer E, Tanriverdi N, Demirhan O, et al. Results of cytogenetic investigation in adolescent patients with primary or secondary amenorrhea. J Pediatr Adolesc Gynecol. 1997;10:86–8. doi: 10.1016/s1083-3188(97)70057-3. [DOI] [PubMed] [Google Scholar]
- 15.Roy AK, Banerjee D. Cytogenetic study of primary amenorrhoea. J Indian Med Assoc. 1995;93:291–2. [PubMed] [Google Scholar]
- 16.Ten SK, Chin YM, Noor PJ, Hassan K. Cytogenetic studies in women with primary amenorrhea. Singapore Med J. 1990;31:355–9. [PubMed] [Google Scholar]
- 17.Anglani F, Baccichetti C, Artifoni L, Lenzini E, Tenconi R. Frequency of abnormal karyotypes in relation to the ascertainment method in females referred for suspected sex chromosome abnormality. Clin Genet. 1984;24:242–7. doi: 10.1111/j.1399-0004.1984.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 18.Opitz O, Zoll B, Hansmann I, Hinney B. Cytogenetic investigation of 103 patients with primary or secondary amenorrhea. Hum genet. 1983;65:46–7. doi: 10.1007/BF00285026. [DOI] [PubMed] [Google Scholar]
- 19.Fraccaro M, Maraschio P, Pasquali F, Scappaticci S. Women heterozygous for deficiency of the (p21- pter) region of the X chromosome are fertile. Hum Genet. 1977;39:283–92. doi: 10.1007/BF00295421. [DOI] [PubMed] [Google Scholar]
- 20.Vijayalakshmi J, Teena Koshy, Harpreet Kaur, Andrea Mary F, Selvi R, Deepa Parvathi V, et al. Cytogenetic analysis of patients with primary amenorrhea. Int J Hum Genet. 2010;10:71–6. [Google Scholar]


