Abstract
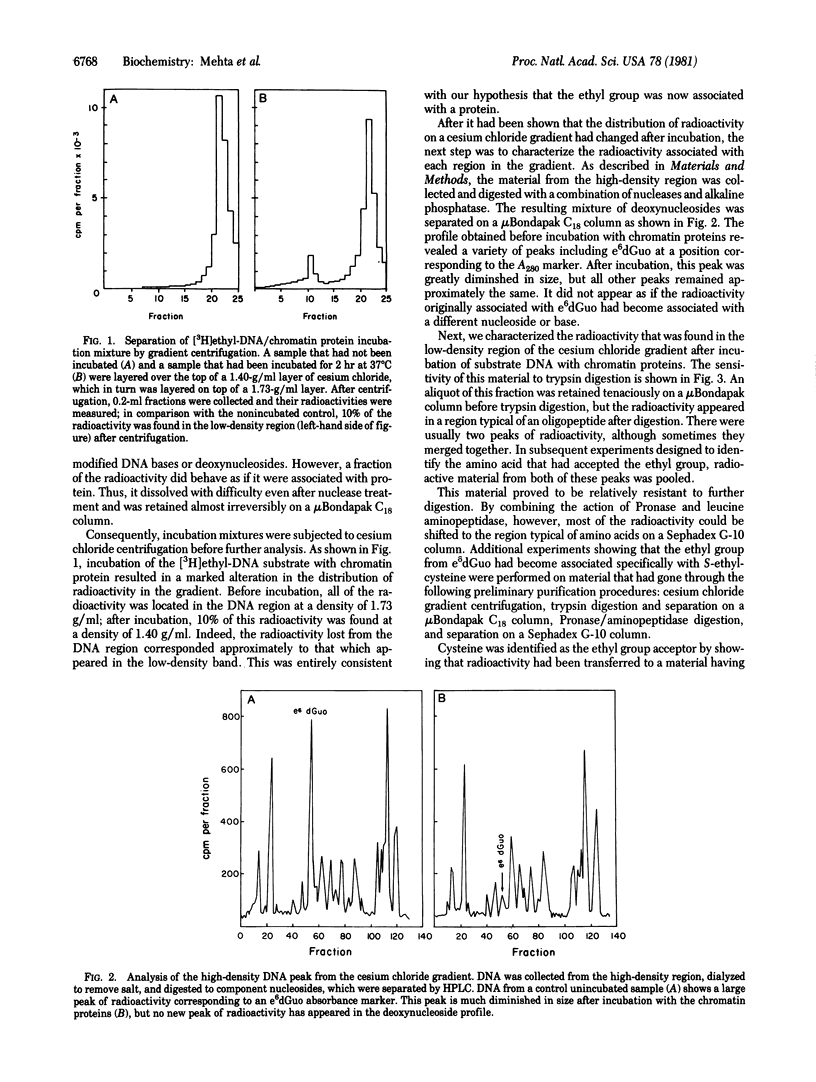
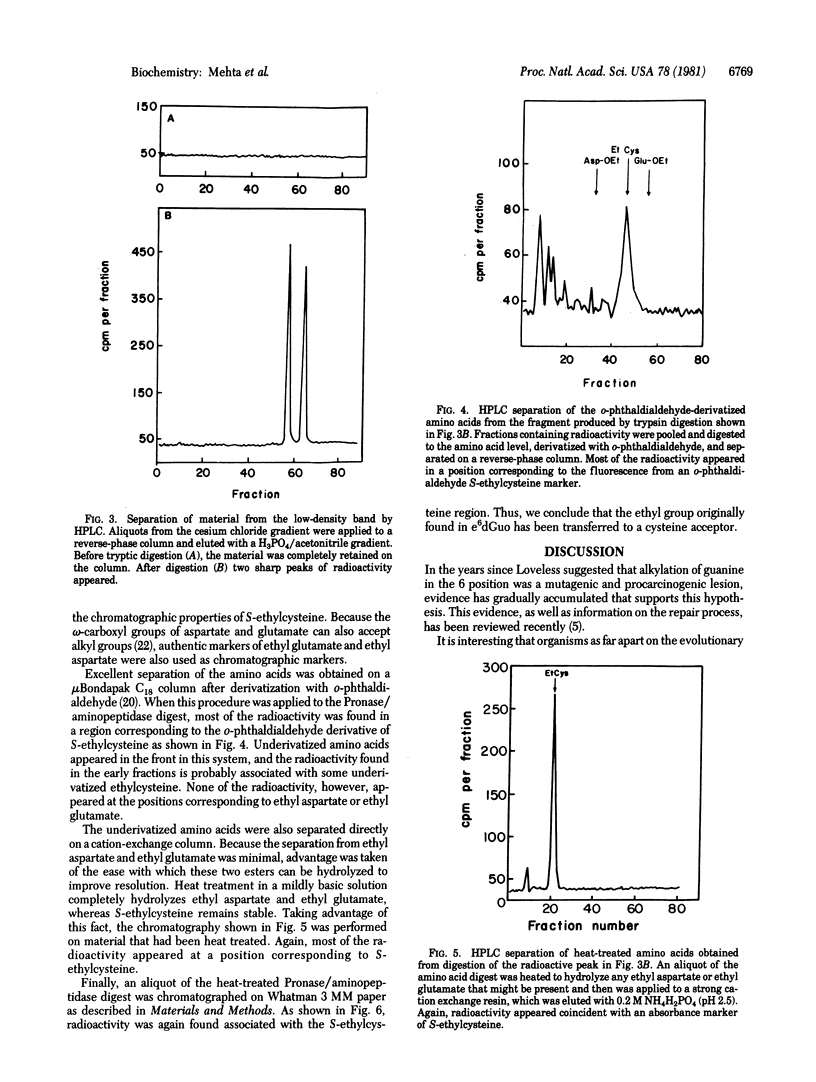
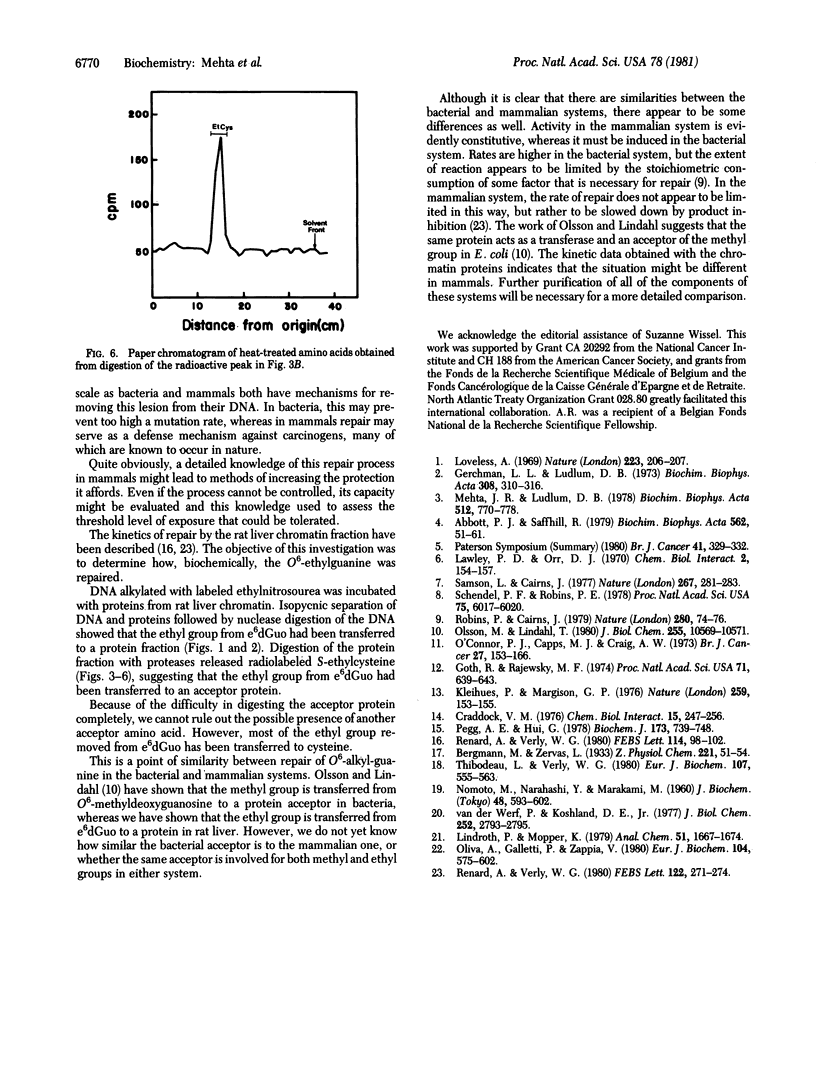
Incubation of O6-[3H]ethylguanine-containing DNA with a rat liver chromatin fraction resulted in a decrease in the O6-ethylguanine content of the DNA. Analysis of the products of this reaction showed that the ethyl group had been transferred from the O6-ethylguanine to a protein acceptor. When the incubation mixture was separated on a cesium chloride gradient, the radioactivity removed from O6-ethylguanine appeared in a low-density band. This material has been isolated and subjected to trypsin digestion and high-pressure liquid chromatography analysis; it was sensitive to trypsin and the digest contained new high-pressure liquid chromatography peaks characteristic of oligopeptides. Radioactive peaks from the trypsin digestion have been digested further to the amino acid level and have been shown to contain S-[3H]ethylcysteine. Thus, we conclude that the repair activity in rat liver chromatin removes the ethyl group from O6-ethylguanine and transfers it to a cysteine moiety contained in an acceptor protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Effect of a single treatment with the alkylating carcinogens dimethylnitrosamine and methyl methanesulphonate on liver regenerating after partial hepatectomy. III. Effect on DNA synthesis in vivo and on DNA polymerase activity assayed in vitro. Chem Biol Interact. 1976 Nov;15(3):247–256. doi: 10.1016/0009-2797(76)90150-2. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976 Jan 15;259(5539):153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Capps M. J., Craig A. W. Comparative studies of the hepatocarcinogen N,N-dimethylnitrosamine in vivo: reaction sites in rat liver DNA and the significance of their relative stabilities. Br J Cancer. 1973 Feb;27(2):153–166. doi: 10.1038/bjc.1973.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Galletti P., Zappia V., Paik W. K., Kim S. Studies on substrate specificity of S-adenosylmethionine: protein-carboxyl methyltransferase from calf brain. Eur J Biochem. 1980 Mar;104(2):595–602. doi: 10.1111/j.1432-1033.1980.tb04463.x. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Hui G. Formation and subsequent removal of O6-methylguanine from deoxyribonucleic acid in rat liver and kidney after small doses of dimethylnitrosamine. Biochem J. 1978 Sep 1;173(3):739–748. doi: 10.1042/bj1730739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard A., Verly W. G. A chromatin factor in rat liver which destroys O6-ethylguanine in DNA. FEBS Lett. 1980 May 19;114(1):98–102. doi: 10.1016/0014-5793(80)80868-4. [DOI] [PubMed] [Google Scholar]
- Renard A., Verly W. G. Kinetic analysis of O6-ethylguanine disappearance from DNA catalyzed by the chromatin factor of rat liver. FEBS Lett. 1980 Dec 29;122(2):271–274. doi: 10.1016/0014-5793(80)80454-6. [DOI] [PubMed] [Google Scholar]
- Robins P., Cairns J. Quantitation of the adaptive response to alkylating agents. Nature. 1979 Jul 5;280(5717):74–76. doi: 10.1038/280074a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau L., Verly W. G. Cellular localization of the apurinic/apyrimidinic endodeoxyribonucleases in rat liver. Eur J Biochem. 1980 Jun;107(2):555–563. doi: 10.1111/j.1432-1033.1980.tb06063.x. [DOI] [PubMed] [Google Scholar]
- Van Der Werf P., Koshland D. E., Jr Identification of a gamma-glutamyl methyl ester in bacterial membrane protein involved in chemotaxis. J Biol Chem. 1977 Apr 25;252(8):2793–2795. [PubMed] [Google Scholar]



