Abstract
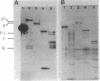
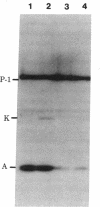
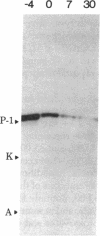
Using a technique developed recently to detect DNA polymerase activity in situ after NaDodSO4 gel electrophoresis (Spanos, A., Sedgwick, S. G., Yarranton, g. T., Hübscher, U. & Banks, G. R. (1981) Nucleic Acids Res. 9, 1825-1839), we present evidence that a high Mr (greater than or equal to 125,000) polypeptide is responsible for chromosomal DNA replication in prokaryotes, lower eukaryotes and high eukaryotes. Not only extracts from Escherichia coli, Ustilago maydis, Drosophila melanogaster, rat neurones, calf thymus, human fibroblast, and HeLa cells possess such high Mr activities, but also highly purified E. coli DNA polymerase III core enzyme, U. maydis DNA polymerase, and D. melanogaster embryo and calf thymus DNA alpha polymerases. The evidence that these activities are responsible for chromosomal DNA replication is genetical (E. coli, U. maydis, and D. melanogaster); also, the high Mr activity disappears from rat neurones during differentiation from an actively dividing precursor cell to a postmitotically mature neurone. Furthermore, when limited proteolysis is allowed to occur, a defined and remarkably similar pattern of intermediate Mr activities is generated in lower eukaryotic and high eukaryotic extracts and, to some extent, in prokaryotic extracts. In higher eukaryotic extracts, a low Mr activity of approximately 35,000 is also generated. Protease inhibitors can retard formation of these catalytically active proteolytic fragments. We propose that the replicative DNA polymerase complex of both prokaryotes and eukaryotes contains a high Mr polypeptide responsible for chain elongation which might be conserved during evolution and which is extremely sensitive to proteolytic cleavage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Banks G. R., Holloman W. K., Kairis M. V., Spanos A., Yarranton G. T. A DNA polymerase from Ustilago maydis. 1. Purification and properties of the polymerase activity. Eur J Biochem. 1976 Feb 2;62(1):131–142. doi: 10.1111/j.1432-1033.1976.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Kornberg A., Sakakibara Y. The dnaN gene codes for the beta subunit of DNA polymerase III holoenzyme of escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5391–5395. doi: 10.1073/pnas.78.9.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Immunological reagents for comparisons of DNA polymerase-alpha and DNA polymerase-beta. J Biol Chem. 1981 Jan 10;256(1):494–498. [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Waltl G., Jantzen H. M., Hamprecht K., Huebscher U., Kuenzle C. C. Diadenosine 5',5'''-P1,P4-tetraphosphate, a ligand of the 57-kilodalton subunit of DNA polymerase alpha. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6081–6085. doi: 10.1073/pnas.76.12.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockensmith J. W., Bambara R. A. Kinetic characteristics which distinguish two forms of calf thymus DNA polymerase alpha. Biochemistry. 1981 Jan 6;20(1):227–232. doi: 10.1021/bi00504a038. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kornberg A. The delta subunit of Escherichia coli DNA polymerase III holoenzyme is the dnaX gene product. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6284–6288. doi: 10.1073/pnas.76.12.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Kornberg A. The dnaZ protein, the gamma subunit of DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980 Dec 25;255(24):11698–11703. [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Limacher W., Scherrer P., Spadari S. Functions of DNA polymerases alpha, beta, and gamma in neurons during development. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):625–629. doi: 10.1101/sqb.1979.043.01.069. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Banks G. R. DNA polymerase of Ustilago maydis: partial characterization of the enzyme and a pol 1 mutation. Mol Gen Genet. 1975 Dec 30;142(3):209–224. doi: 10.1007/BF00425646. [DOI] [PubMed] [Google Scholar]
- Johanson K. O., McHenry C. S. Purification and characterization of the beta subunit of the DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10984–10990. [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Structural and functional properties of calf thymus DNA polymerase delta. Prog Nucleic Acid Res Mol Biol. 1981;26:83–96. doi: 10.1016/s0079-6603(08)60396-7. [DOI] [PubMed] [Google Scholar]
- Low R. L., Arai K., Kornberg A. Conservation of the primosome in successive stages of phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1436–1440. doi: 10.1073/pnas.78.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Murray V., Holliday R. Increased error frequency of DNA polymerases from senescent human fibroblasts. J Mol Biol. 1981 Feb 15;146(1):55–76. doi: 10.1016/0022-2836(81)90366-1. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C., Baril E. F. HeLa cell DNA polymerase alpha is tightly associated with tryptophanyl-tRNA synthetase and diadenosine 5',5"'-P1,P4-tetraphosphate binding activities. Proc Natl Acad Sci U S A. 1981 Feb;78(2):838–842. doi: 10.1073/pnas.78.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. K., Morris C. F., Alberts B. M. Efficient in vitro replication of double-stranded DNA templates by a purified T4 bacteriophage replication system. J Biol Chem. 1980 May 10;255(9):4290–4293. [PubMed] [Google Scholar]
- Spanos A., Sedgwick S. G., Yarranton G. T., Hübscher U., Banks G. R. Detection of the catalytic activities of DNA polymerases and their associated exonucleases following SDS-polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Apr 24;9(8):1825–1839. doi: 10.1093/nar/9.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Nakayama K. DNA polymerase alpha mutants from a Drosophila melanogaster cell line. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7049–7053. doi: 10.1073/pnas.77.12.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G., Sauer B., Lehman I. R. DNA polymerase alpha from Drosophila melanogaster embryos. Subunit structure. J Biol Chem. 1980 Oct 10;255(19):9479–9483. [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]