Abstract
Background:
Many environmental and genetic factors are known as factors that increase the susceptibility to periodontitis. As IL-8 is a chemokine that mediate the inflammatory process in periodontal disease, we decided to evaluate the effect of its polymorphism on chronic and aggressive periodontitis.
Materials and Methods:
In this cross-sectional study DNA was isolated from the whole blood of 107 periodontitis patients and 199 healthy individuals. All samples were genotyped for the IL-8 polymorphisms using the polymerase chain reaction with sequence specific primers. The distribution of the interleukin-8 genotype and allele frequencies in control and disease groups was analyzed by the Chi-square test and a P-value of < 0.05 was considered statistically significant.
Results:
The findings revealed that the allele and genotype frequencies of A2767T, T11722T2, and C781T polymorphism of IL-8 gene were not significantly differed between controls and patients. However, there was a significant difference in the genotype frequencies of IL-8 A251T (P < 0.0001), G396T (P < 0.0001), and C1633T (P < 0.0001) gene polymorphism between the patient and the control groups. Additionally, there was a significant difference in the genotype frequencies of C1633T (P < 0.05) polymorphism of IL-8 gene between the aggressive and chronic periodontitis.
Conclusion:
IL-8 gene polymorphism may be protective against periodontitis.
Keywords: Aggressive periodontitis, chronic periodontitis, genetic polymorphism, interleukin-8
INTRODUCTION
Neutrophil infiltration into the inflammation site is one of the hallmarks of acute inflammation. Local chemotactic factors are presumed to mediate the sequence of events leading to the leukocyte infiltration at inflammatory sites.[1]
Accumulating evidence indicates that several leukocyte chemotactic factors and vasodilating mediators are produced at the site of injury and that these mediators induce vasodilation and recruitment of leukocytes, thereby establishing inflammatory reactions. Interleukin-8 (a member of the chemokine family) has been identified as a neutrophil chemotactic factor[2,3] and is produced by various types of cells upon stimulation with inflammatory stimuli. This chemokine exerts a variety of functions on leukocytes, particularly, neutrophils, and exhibits chemotactic activities against T lymphocytes and basophils.[4,5] Besides chemotactic activities, IL-8 induces neutrophils to release lysosomal enzymes, to increase expression of Mac-i and CR-I, and to adhere to unstimulated endothelial cells.[6–8]
Elevated IL-8 levels have been documented in many conditions such as rheumatoid arthritis,[9] gouty arthritis,[10] psoriatic scale,[11] adult respiratory distress syndrome,[12] and chronic and aggressive periodontitis.[13]
Periodontitis is a chronic inflammatory and multifactorial disease for which several susceptibility and risk factors are proposed[14] and despite the traditionally belief of its environmental origin, now it is thought to be determined in part by genetics.[15]
Polymorphisms in a range of human cytokine genes have been correlated with different levels of protein production and some polymorphisms have been associated with inflammatory conditions such as periodontal disease. As interleukin-8 seems to play a role in periodontal inflammation and disease, the investigation of genetic polymorphisms that affect its transcriptional activity may provide important information on its function in periodontal disease.
MATERIALS AND METHODS
Study population
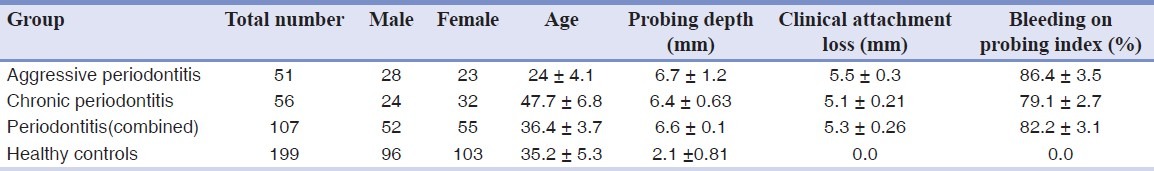
In this cross-sectional study a total of 306 subjects were recruited. A total of 107 patients (55 women and 52 men; aged 17–59 years), consisting of 56 patients with chronic periodontitis and, 51 patients with aggressive periodontitis referred to the periodontology department of Hamedan faculty of dentistry were enrolled. Periodontitis was diagnosed on the basis of clinical parameters including probing depth, clinical attachment loss, and bleeding on probing index and radiographic findings.[16] None of the patients had a history of current manifestation of systemic diseases. Patients with severe medical disorders (diabetes mellitus, immunological disorders, hepatitis, human immunodeficiency virus infection, and cardiovascular involvement), use of orthodontic appliances, need for premedication for dental treatment, chronic usage of anti-inflammatory drugs, smokers, present acute necrotizing ulcerative gingivitis, and women who were pregnant or lactating, were excluded from the study. In addition, 199 healthy nonsmoking dental students or resident volunteers (103 women and 96 men; aged 17–48 years), without any periodontal disease, systemic inflammatory disease, surgical treatment in the past 4 weeks were considered as controls. The absence of periodontal disease was determined according to the following criteria: (a) No sites with bleeding on probing; (b) No sites with a probing depth of >3 mm; and (c) Absence of clinical attachment loss or radiographic bone loss. None of the control subjects had a history of periodontitis or tooth loss because of pathogenic tooth mobility. All subjects signed an informed consent form before enrollment into the study.
DNA isolation
Around 10 mL of venous blood was collected from each subject into tubes containing 50 mmol/L EDTA, and genomic DNA was isolated from anticoagulated peripheral blood Buffy coat using Miller's salting-out method.[17,18] Then, the genomic DNA was stored at -80°C until required for genotyping. The genotyping was performed using the polymerase chain reaction sequence specific primer method.[19] Internal control primers were included as a control for false-negative reactions. The control primers (5′-TGC CAA GTG GAG CAC CCA A-3′ and 5′-GCA TCT TGC TCT GTG CAG AT-3′) were used at 0.2 lmol/L. The interleukin-8 polymorphism at position T11722T2 was identified by the sequence-specific forward primers GTAAAATACAGTGATGAGTGTTACGATAC and GTAAAATACAGTGATGAGTGTTACAATAA, in combination with the consensus reverse primer, GTTGTGTCCATATGAGAATGTGTC, at position A251T was identified by the sequence-specific forward primers CCACAATTTGGTGAATTATCAAT and CCACAATTTGGTGAATTATCAAA, in combination with the consensus reverse primer, TGCCCCTTCACTCTGTTAAC, at position G396T was identified by the sequence-specific forward primers TTTACGTTAAATATATGCATGTTACC and TTTACGTTAAATATATGCATGCTACA, in combination with the consensus reverse primer, AACATGACTTCCAAGCTGGC, at position C781T was identified by the sequence-specific forward primers TCATAACTGACAACATTGAACG and AGTCATAACTGACAACATTGAACA in combination with the consensus reverse primer, TGAGTTGAGCAAGGTAACTCAG, at position C1633T was identified by the sequence-specific forward primers TATGTATGGTCTTTCTGGTCGTG and AACTATGTATGGTCTTTCTGGTCGTA in combination with the consensus reverse primer, GGACTTAGACTTTATGCCTGACTTAAG at position A2767T was identified by the sequence-specific forward primers CCCAGTTAAATTTTCATTTCAGATAT and CCCAGTTAAATTTTCATTTCAGATAA in combination with the consensus reverse primer, GACAAACACTTGATTACTTTGACAACA with an expected product size of 130 bp. Amplification was carried out using a DNA Technology MTC 400 in a total volume of 15 lmol/L that contained 100 ng of genomic DNA, 1 lmol/L of each allele-specific primer pair, 200 lmol/L of dNTPs, 10 lmol/L Tris–HCl (pH 8.3), 50 mmol KCl, 1.5 mmol/L MgCl2, and 0.5 IU of Taq DNA polymerase. The reaction was carried out as follows: initial denaturation at 94°C for 2 min, followed by 10 cycles of amplification at 96°C for 20 s and annealing at 64°C for 50 s, with extraction for 40 s at 72°C, followed by 20 cycles of denaturation at 96°C for 20 s and annealing at 61°C for 50 s, with extraction for 40 s at 72°C. The size of the product was 130 bp.
Data analysis
Allele and genotype frequencies were obtained by direct counting. The distribution of the interleukin-8 genotype and allele frequencies in control and disease groups was analyzed by the Chi-square test. The study groups were tested for Hardy–Weinberg equilibrium comparing the expected with the observed genotype frequencies. The results of the logistic regression model were expressed as odds ratio and 95% confidence interval. All P-values were evaluated in a two-sided model, and a P-value of <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 10.0 software package (SPSS, Chicago, IL, USA).
RESULTS
The clinical and demographic data of patient and control groups are displayed in [Table 1]. The frequencies of these cytokine genotypes in patients and control individuals were found to be in accordance with those expected by the Hardy-Weinberg equilibrium.
Table 1.
Clinical and demographic data of patients and controls groups
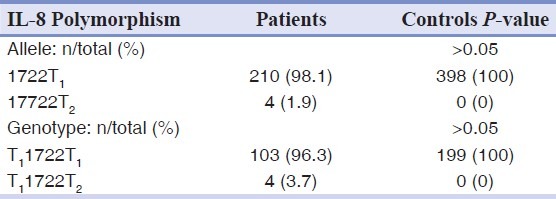
The allele and genotype frequencies of A2767T, T11722T2, and C781T polymorphism of IL-8 gene were not significantly differed between patients and controls [Tables 2–4].
Table 2.
Allele and genotype frequencies of the A2767T polymorphism of IL-8 gene in patients and controls groups
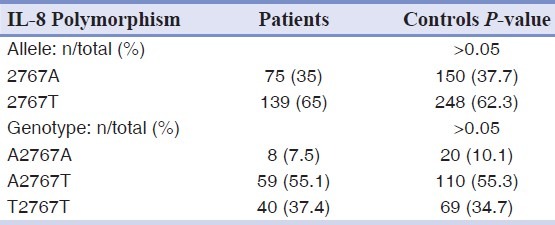
Table 4.
Allele and genotype frequencies of the C781T polymorphism of IL-8 gene in patients and controls groups
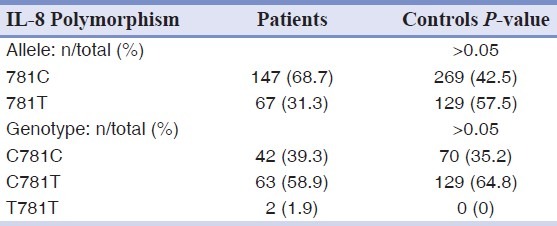
Table 3.
Allele and genotype frequencies of the T11722T2 polymorphism of IL-8 gene in patients and controls groups
However, there was a significant difference in the genotype frequencies of IL-8 A251T, G396T and C1633T gene polymorphism between the patient and control groups [Tables 5–7].
Table 5.
Allele and genotype frequencies of the A251T polymorphism of IL-8 gene in patients and controls groups
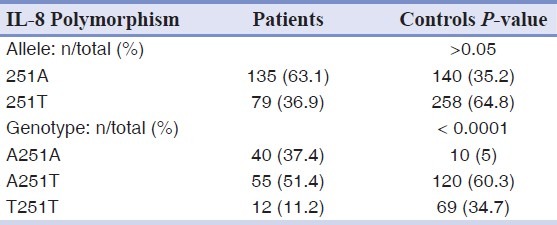
Table 7.
Allele and genotype frequencies of the C1633T polymorphism of IL-8 gene in patients and controls groups
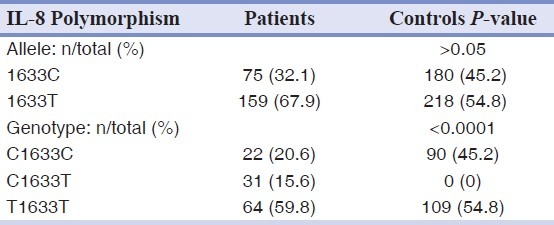
Table 6.
Allele and genotype frequencies of the G396T polymorphism of IL-8 gene in patients and controls groups
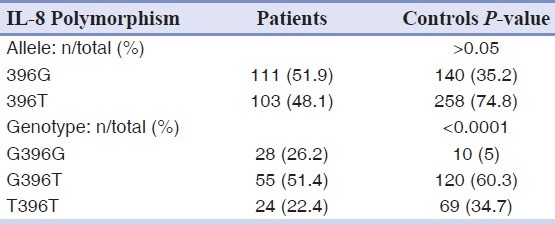
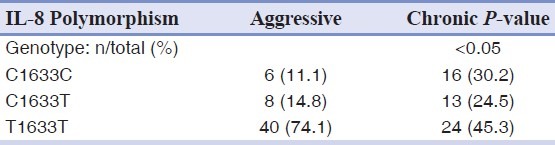
As shown in [Table 8] there was a significant difference in the genotype frequencies of C1633T polymorphism of IL-8 gene between the aggressive and chronic periodontitis (P<0.05).
Table 8.
Allele and genotype frequencies of the C1633T polymorphism of IL-8 gene in chronic and aggressive periodontitis
DISCUSSION
Inflammation is a vital consequence of tissue injuries associated with various causes, such as infection of microorganisms, trauma, and invasion of foreign particles. It has the classical clinical signs of redness, heat, swelling, and pain. These signs are due to extravasation of plasma and infiltration of leukocytes into the site of inflammation produced by leukocyte chemotactic factors and vasodilating mediators.
Several leukocyte chemotactic factors are known, such as C5a, N-formyl peptides derived from bacteria, and leukotriene B4, although these factors exhibit chemotactic activities against any types of leukocytes. At the site of inflammation, specific types of leukocytes infiltrate depending on the types, doses of, and time intervals from the tissue injury, raising the possibility of the presence of cell type-specific leukocyte chemotactic factors. This assumption has been supported by the discovery of a large number of low-molecular weight leukocyte chemotactic cytokines (chemokines) that exhibit cell type-specific leukocyte chemoattraction.[4]
IL-8 is a member of the family of chemokines. It is mainly involved in the initiation and amplification of acute inflammatory reactions and in chronic inflammatory processes. Therefore, it plays an important role in chronic inflammatory diseases in which inflammation is a substantial pathophysiological feature. IL-8 production is not constitutive but occurs ordinarily in the presence of inflammatory stimuli such as LPS, IL-1, and tumor necrosis factor (TNF) in a wide variety of cells including monocytes, T lymphocytes, neutrophils, vascular endothelial cells, dermal fibroblasts, keratinocytes, hepatocytes,[20,21] and human gastric cancer cells.[22] Actually, IL-8 was not detected in normal adult plasma but the intravenous injection of Lipopolysaccharide (LPS) induced massive elevation of plasma IL-8 levels, reaching maximal levels in 2 h after the injection.[23]
Gingival Crevicular Fluide (GCF) IL-8 level in periodontitis patients is evaluated in a study by Domiaty. The study results indicated a negative correlation between IL-8, clinical, and radiographic parameters. These results suggest an inverse relation between IL-8 and chronic periodontitis.[24]
IL8, located on human chromosome 4, exhibits functional polymorphisms which affects IL-8 production. There was some data about the relation of IL-8 polymorphism and periodontitis.
Andia et al. investigated the association of the single nucleotide polymorphism (SNP) rs4073 with chronic periodontitis and concluded that the SNP rs4073 is associated with chronic periodontitis, in nonsmoker Brazilian subjects, since the frequency of A allele is higher in the disease than in the control group and the TA genotype was associated with increased levels of IL8 mRNA transcripts.[25]
Kim et al. investigated whether the -845(T/C) and -738(T/A) SNPs in the IL8 gene, as well as the haplotypes they form together with the previously investigated -353(A/T), are associated with susceptibility to chronic periodontitis. They finally concluded that although none of the investigated SNPs in the IL8 gene was individually associated with periodontitis, some haplotypes showed significant association with susceptibility to, or protection against, chronic periodontitis in a Brazilian population.[26]
In our study allele and genotype frequencies of the A2767T, T11722T2, C781T, A251T, T251T, G396T, T396T, and T1633T polymorphisms of IL-8 gene in patients and controls groups was evaluated. According to our findings there was no association between A2767T, T11722T2, and C781T polymorphism of IL-8 gene and periodontitis. However, A251T, T251T, G396T, T396T, T1633T genotypes were more frequent in controls, which indicates that individuals who have these genotypes may be more protected from periodontitis.
CONCLUSION
In conclusion, IL-8 gene polymorphism may be a protective factor against periodontitis in Iranian people.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Akihisa H, Nobuaki S, Tohru A, Takashi W, Naofumi M, Kouji M. Essential involvement of interleukin-8 in acute inflammation. J Leukoc Biol. 1994;56:559–64. [PubMed] [Google Scholar]
- 2.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, et al. Purification of a human monocyte-derived neutriphil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–7. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–93. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White MV, Yoshimura T, Hook W, Kaliner MA, Leonard EJ. Neutrophil attractant/activation protein-1(NAP-1) causes human basophil histamine release. Immunol Lett. 1989;22:151–4. doi: 10.1016/0165-2478(89)90182-x. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CG, Anderson AO, Apella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein(NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–6. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 6.Walz A, Peveri P, Aschauer H. Purification and amino acid sequencing of NAF, a novel neutrophil activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–61. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- 7.Huber AR, Kunkel SL, Todd RF, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenousinterleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 8.Detmers PA, Lo SK, Olsen EE, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–62. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan FM, Zachariae CO, Chantry D, Larsen CG, Turner M, Maini RN, et al. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production ofvinterleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990;20:2141–4. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- 10.Terkeltaub R, Zachariae C, Santoro D, Martin J, Peveri P, Matsushima K. Monocyte-derived neutrophil chemotactic factor/interleukin-8 is a potential mediator of crystal induced inflammation. Arthritis Rheum. 1991;34:894–903. doi: 10.1002/art.1780340716. [DOI] [PubMed] [Google Scholar]
- 11.Schroder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immuno L. 1987;139:3474–83. [PubMed] [Google Scholar]
- 12.Jorens PG, van Damme J, Dc Backer W, Bossaert L, De Jongh RF, Herman AG, et al. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokinc. 1992;4:592–7. doi: 10.1016/1043-4666(92)90025-m. [DOI] [PubMed] [Google Scholar]
- 13.Gainet J, Dang PM, Chollet MS. Neutrophil Dysfunctions, IL-8, and Soluble L-Selectin Plasma Levels in Rapidly Progressive Versus Adult and Localized Juvenile Periodontitis: Variations According to Disease Severity and Microbial Flora. J Immunol. 1999;163:5013–9. [PubMed] [Google Scholar]
- 14.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Poleski HF. Simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole KL, Gibbs PJ, Evans PR, Sadek SA, Howell WM. Influence of patient and donor cytokine genotypes on renal allograft rejection: evidence from a single center study. Transpl Immunol. 2001;8:259–65. doi: 10.1016/s0966-3274(01)00030-2. [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, et al. Endothelial cell gene expression of a neutrophil chemotactic factor by TNFalpha, LPS, and IL-1 beta. Science. 1989;243:1467–9. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 18.Houshmand B, Rafiei A, Hajilooi M, Mani-Kashani K, Gholami L. E-selectin and L-selectin polymorphisms in patients with periodontitis. J Periodont Res. 2009;44:88–93. doi: 10.1111/j.1600-0765.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 19.Thornton AJ, Strieter RM, Lindley I, Baggiolini M, Kunkel SL. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990;144:2609–13. [PubMed] [Google Scholar]
- 20.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-xB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–11. [PubMed] [Google Scholar]
- 21.Martich GD, Danner RL, Ceska M. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: The effect of anti-inflammatory agents. J Exp Med. 1991;173:1021–4. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of the periodontitis: Summary of developments, clinical implications and future directions. Periodontology 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 23.Michalowicz BS. Genetic and heritable risk factors in periodontal disease. J Periodontol. 1994;65:479–88. doi: 10.1902/jop.1994.65.5s.479. [DOI] [PubMed] [Google Scholar]
- 24.Domiaty S. Gingival crevicular fluid il-8 level in adult periodontitis patients: correlation with clinical attachment level and radiographic bone loss. Eda. 2000;4:801. [Google Scholar]
- 25.Andia DC, Oliveira NF, Letra AM, Nociti FH, Line SR, Souza AP. IL8 Gene Promoter Polymorphism (rs4073) may Contributes to Chronic Periodontitis. J Periodontol. 2011;82:893–9. doi: 10.1902/jop.2010.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YJ, Viana AC, Curtis KM, Orrico SR, Cirelli JA, Scarel-Caminaga RM. Association of haplotypes in the IL8 gene with susceptibility to chronic periodontitis in a Brazilian population. Clin Chim Acta. 2010;411:1264–8. doi: 10.1016/j.cca.2010.05.014. [DOI] [PubMed] [Google Scholar]


