Abstract
Developmental research is enhanced by use of multiple methodologies for examining psychological processes. The electroencephalogram (EEG) is an efficient and relatively inexpensive method for the study of developmental changes in brain-behavior relations. In this review, we highlight some of the challenges for using EEG in cognitive development research. We also list best practices for incorporating this methodology into the study of early cognitive processes. Consideration of these issues is critical for making an informed decision regarding implementation of EEG methodology.
Developmental scientists have various research methodologies available for the study of age-related changes in brain-behavior relations. The electroencephalogram (EEG) is considered by many to be one of the most efficient and relatively inexpensive methods for examining these developmental changes. We prefer the EEG because it also allows an examination of developmental changes without dramatic interference with normal ongoing behaviors. We have used EEG methodology to examine correlations between brain electrical activity and working memory performance during infancy (Bell, 2012, 2001) and early childhood (Wolfe & Bell, 2004, 2007) and recall memory performance during toddlerhood (Cuevas, Raj, & Bell, 2012a). We have also used EEG to describe month-to-month changes in brain development during infancy (Bell & Fox, 1992, 1994; Cuevas & Bell, 2011).
EEG measures the electrical potential between two electrodes on the scalp, with evidence that the origin of this electrical signal is in the brain (Pizzagalli, 2007). The EEG signal is spontaneous but context-related; EEG generated during quiet rest is quantitatively different from that generated during cognitive processing. The EEG signal has temporal resolution on the order of milliseconds. Thus, postsynaptic changes are immediately reflected in the EEG, making this methodology outstanding for tracking rapid shifts in brain functioning. Of value to research with younger participants, the electrical signals recorded at the scalp are robust and the techniques by which they are obtained are relatively simple and non-invasive. However, obtaining high-quality signals typically requires a significant amount of training.
This manuscript is a brief review on the use of EEG in cognitive research with infants and young children. The review is intended for the non-EEG researcher; we focus on issues to consider when incorporating EEG into an existing research program. Two of the most popular techniques involve cap/gel or net/saline systems. It is our experience that researchers typically have strong opinions as to which is the better technique, with “better” being defined various ways by different researchers. It is clear from the photograph in Figure 1 that we use the cap/gel system; thus, our comments are focused on that methodology. Advantages and disadvantages of the cap/gel and net/saline systems for developmental populations have been discussed by DeBoer, Scott, and Nelson (2006) and Johnson and colleagues (2001).
Figure 1.
The experimental arrangement for EEG data collection during the infant looking A-not-B task.
EEG Measures
The EEG discussed here is sometimes called “quantitative EEG” and is used for basic research on brain electrical activity during cognitive or affective processing. The EEG signal recorded from the scalp is composed of multiple sine waves cycling at different frequencies. Fourier analysis decomposes the EEG into these different sine waves and estimates the spectral power (in mean square microvolts) at each frequency. This results in information regarding the contribution of each individual frequency to the entire EEG spectrum at a particular electrode site. Power is thought to reflect the excitability of groups of neurons. Power values are usually totaled across frequency bins to form measures of power in a specific frequency band (see frequency band section). The power data should be normalized (e.g., natural log transformation) because EEG power data are usually positively skewed (Davidson, Jackson, & Larson, 2000).
Coherence is the frequency dependent squared cross correlation between two scalp electrode sites which reflects the degree of phase synchrony between them (Gevins, 1989; Nunez, 1981; Thatcher, Walker, & Giudice, 1987). Coherence values (range: 0 to 1) may be related to the strength and number of synaptic connections (Thatcher, 1994), and thus may reflect the level of connectivity between two EEG recording sites. Power and coherence measures both show changes with development and both are associated with cognitive processing (e.g., Bell & Fox, 1992; Cuevas & Bell, 2011).
EEG is typically recorded during specific events of cognitive processing and comparisons are made to the EEG recorded during a resting or baseline period. An advantage of EEG is that the cognitive events can be relatively long, on the order of seconds or even minutes. For example, EEG power and coherence values can be obtained during multiple trials of a working memory task and during the encoding of stimuli for a later recall or recognition memory task. If there are several trials, EEG power and coherence can be averaged across trials. Alternatively, a single prolonged period of information processing, such as during the encoding of a complex stimulus, can be appropriate.
Another advantage of EEG is that experimental conditions are designed such that attrition is relatively low. Our recent infant EEG work has attrition rates of 0–12% due to excessive movement artifact or crying (Bell, 2012; Cuevas & Bell, 2011; Cuevas, Raj, & Bell, 2012b). Attrition rates for two- and three-year-old children are higher (30–45%), typically due to refusal to wear the EEG cap, cap removal during the session, or excessive movement artifact (Cuevas, Raj, & Bell, 2012a; Morasch & Bell, 2011; Wolfe & Bell, 2007). Attrition rates drop to 20% by age four as children become more tolerant of the EEG cap and exhibit less movement during recording (Wolfe & Bell, 2004, 2007).
The EEG differs from event-related potentials (ERPs), which are waveform analyses of the brain electrical responses within the EEG signal, time-locked to a specific set of stimuli. ERPs are used to capture snapshots of brain responses to discrete stimuli associated with the recognition of familiar and novel objects or sounds. ERP events are brief, typically 500 milliseconds. Many trials are required because the ERP waveform is extracted by averaging across all trials of the same stimulus so that the background EEG signal drops out and the specific response to a particular stimulus is observable. An advantage of ERPs is that the various positive and negative components of the waveform at different latencies from stimulus onset have been associated with specific cognitive processes. A disadvantage is the relatively large number of trials required, such that the attrition rate in ERP studies with infants can range from 35–75% (de Haan, 2006). A discussion of ERPs in studies of cognitive development can be found elsewhere (e.g., DeBoer et al., 2006; Fox, Schmidt, Henderson, & Marshall, 2007).
Challenges for Developmental EEG Research
Spatial and Temporal Resolution
Although EEG is one of the more favorable brain imaging methods for use with infants and children, there is one major caveat of this methodology. The EEG signal has excellent temporal resolution, but it has poor spatial resolution. The skull behaves like a low-pass filter and distorts the underlying brain electrical activity over a large area of the scalp. Furthermore, potentials recorded at the scalp are likely generated by multiple groupings of cortical and subcortical generators spread across a relatively wide area (Pizzagalli, 2007). Thus, a scalp electrode is likely detecting electrical activity generated from non-local groups of neurons, which is why it is better to discuss EEG activity at a specific electrode location rather than resulting from a particular brain area. Use of dense electrode arrays (typically considered to be a minimum of 64 electrodes) may alleviate some of the concerns with spatial resolution. Indeed, dense arrays allow calculation of the source of the electrical signal (Reynolds & Richards, 2009). Of course, the cost of an EEG system is correlated with the number of electrodes.
Application of EEG Electrodes
EEG electrode arrays are available in many different sizes from newborn to adult. After measuring the head and determining appropriate size, affixing the EEG cap to the infant’s or child’s head requires a tremendous amount of practice, planning, and patience, as well as at least two researchers. Application time varies with the type of EEG technique (cap/gel or net/saline) and the number of electrodes. While an infant sits on mother’s lap it is relatively easy for one research assistant to apply the EEG array as another research assistant continuously entertains and distracts the infant with a wide variety of toys, games, or songs. The “entertainer” is critical to the success of the EEG research study with young participants and should be a research assistant savvy to individual differences in participants’ development and temperament.
Toddlers are very challenging in the EEG lab. Depending on the child, EEG application occurs while the toddler either sits on mother’s lap or in a high chair near mother. Snacking on crackers and watching an animated video with a research assistant facilitates this process. Some toddlers may need additional distraction with musical or electronic toys. Playing a computer game with the research assistant sufficiently distracts most preschoolers to allow electrode placement and preparation, although preschoolers are definite in their opinions about whether they wish to contribute EEG data. By middle childhood, participants enjoy chatting with the research assistant during cap placement, especially while sitting in front of a mirror.
It is critical that care be taken during electrode application to insure a high quality EEG signal that is free of non-EEG electrical signals or “noise.” With the cap/gel technique, we use one gel to prepare the scalp and another gel to conduct the electrical signal. These techniques reduce the likelihood of recording non-EEG electrical signals. In the past, researchers had to be mindful of ambient electrical interference from lighting and other equipment in the EEG room. With contemporary EEG bioamplifiers, this is less of a concern.
EEG-Appropriate Tasks
A challenge for both behavioral and psychophysiological investigations with developmental populations is identifying tasks that are engaging (e.g., maintain visual attention) and require responses that are within the participants’ behavioral repertoire (e.g., looking, reaching). Most of the cognitive tasks used in adult EEG research are inappropriate for younger populations because they require receptive and productive language skills (i.e., instructions, stimuli, responses), include numerous trials that lengthen the experimental protocol, and use methods that are not engaging for infants and children. When designing age-appropriate EEG tasks, researchers must also consider the influence of gross motor movements, eye blinks or lateral eye movement, and unrelated changes in behavioral state (e.g., crying or drowsiness during a cognitive task), which contaminate the EEG recording (i.e., artifacts; see next section). In order for EEG collection to be worthwhile, researchers must have sufficient artifact-free EEG available for data analysis. Since only portions of the EEG that are associated with the cognitive process of interest are included in data analysis, researchers should identify tasks that require minimal gross motor movement. Likewise, state changes can be reduced by assessing participants at optimal points in their sleep-wake and eating cycles.
The development of the looking A-not-B task (Bell & Adams, 1999) provides an example of global and local changes in task design that can be implemented to reduce motor artifacts. The reaching A-not-B task creates too many motor artifacts for data analysis. Clearly, eye movements (looking A-not-B) produce fewer artifacts than reaching movements (reaching A-not-B). Smaller changes in task design can also increase the amount of artifact-free data. During the looking task, infants were less likely to lean and/or reach if they were not within reaching distance of the table (i.e., location of the desirable object; see Figure 1). Importantly, within-subjects comparisons of performance of looking and reaching versions of the A-not-B task suggest that the same cognitive processes are required for both versions (Bell & Adams, 1999; Cuevas & Bell, 2010).
Identifying Artifacts
After data collection is complete, a significant amount of time is required to process the EEG data. It is essential to eliminate portions of the EEG record that are contaminated by gross motor movements or eye blinks (i.e., artifact scoring) prior to data analysis. The EEG signal is of such small amplitude that motor movements and eye blinks tend to overpower the EEG signal, in effect wiping it out. Simultaneous recording of electrooculogram (EOG; i.e., recording of eye movement: blinks and lateral eye movements) and electromyogram (EMG; i.e., recording of muscle movement) can facilitate artifact detection. Eye blink and lateral eye movement artifacts, however, are also readily identified via visual inspection of the EEG at frontal electrodes. Although eye blink correction algorithms are often used on adult EEG recordings, there has been some concern that the algorithms may filter out maturational change in frontal EEG power in young research participants (Somsen & van Beek, 1998). Thus, selecting artifact-free data may yield a more accurate portrayal of the EEG developmental record (Somsen & van Beek, 1998).
Best Practices for Developmental EEG Research
Frequency Band Selection
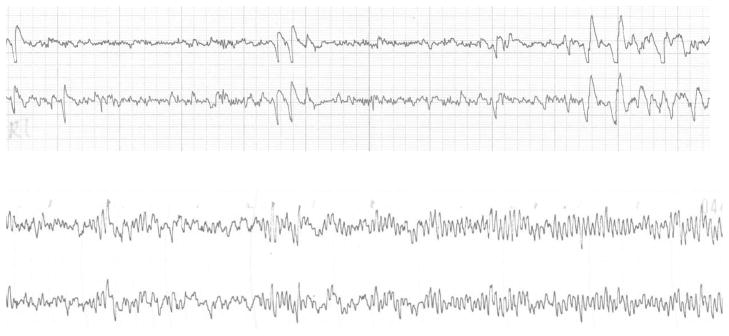
The EEG is comprised of rhythmic activity oscillating at different frequencies. This rhythmic activity is theorized to emerge from interactions between the thalamus and cortex (see Pizzagalli, 2007, for review). For adults, specific frequency bands (e.g., 4–8 Hz; 8–13 Hz) are associated with different behaviors or mental processes. One challenge for developmental EEG researchers is understanding how infant/child frequency bands are related to adult frequency bands. As can be seen in Figure 2, there are differences in both the frequency and amplitude of infant and adult EEG. Early longitudinal research demonstrated that (a) infant EEG is at a much lower frequency than adult EEG and (b) there are substantial changes in the peak frequency from infancy through early childhood (see Bell, 1998; Bell & Fox, 1994, for reviews). Accordingly, developmental EEG researchers have questioned the appropriateness of using traditional adult frequency bands (Pivik et al., 1993).
Figure 2.
Adult (top) and 8-month-old infant (bottom) EEG tracings of F3 and F4 (left and right medial frontal) scalp locations. There are differences in the amplitude and frequency of infant and adult EEG. The large deflections in the adult tracing are eye blinks. Eyeblinks in the infant record are difficult to see because of the high amplitude of the infant tracing.
Pivik and colleagues (1993) recommend two approaches for determining appropriate frequency bands for developmental population: small band and wide band. For the wide band method, EEG analyses are computed for a wide frequency band that includes all frequencies in which there is evidence of power (e.g., Jones, Field, Fox, Davalos, Lundy, & Hart, 1998). This method, however, is not used as often as the small band method. Researchers using the small band method examine individual spectra and define frequency bands that center around the peaks in the spectrum. This technique has been applied to longitudinal data to reveal that 6–9 Hz is the dominant frequency band during quiet wakefulness from infancy (i.e., 5 or 7 months) through early childhood (i.e., 48 months; Bell & Fox, 1992; Marshall, Bar-Haim, & Fox, 2002). Stroganova and colleagues (e.g., Stroganova, Orekhova, & Posikera, 1999) have identified an overlapping infant frequency band using a narrow frequency bin method in which narrow frequency bins (e.g., 0.4 Hz) are analyzed and frequency bands are determined by significant findings at adjacent bins. Specifically, the 5.2–9.6 Hz band in 8- to 11-month-old infants exhibits changes in power values at occipital sites during the absence of visual input (i.e., a “lights off” period) that is analogous to changes exhibited by adult 8–13 Hz alpha band during an eyes closed period (Stroganova et al., 1999). Thus, the 6–9 Hz band has been identified by independent investigations, using different approaches, as the dominant frequency band during early development.
The 6–9 Hz band has emerged as a standard in developmental EEG research, and some researchers refer to it as “infant alpha” (Stroganova & Orekhova, 2007). This frequency band, however, also exhibits some characteristics of adult theta, and may be a hybrid of the adult theta and alpha bands. Much like the adult 8–13 Hz alpha and 4–8 Hz theta bands, the infant/child 6–9 Hz band has been used to examine a variety of different constructs, (e.g., visual attention, emotion expression and regulation, working memory and inhibitory control; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Morasch & Bell, 2011; Orekhova, Stroganova, & Posikera, 2001). Relatively little work, however, has examined the functional significance of other infant/child frequency bands (e.g., Bell, 2002; Cuevas, Raj, & Bell, 2012a, 2012b; Jing, Gilchrist, Badger, & Pivik, 2010). Clearly, age-appropriate frequency bands are critical for developmental EEG research, and future research will enhance our understanding of infant-adult frequency band relations.
Number of Electrodes
With technological advances, it is now possible to quickly apply an EEG array with 128 or more electrodes to the head of an infant or young child. Researchers who use these dense array techniques typically average across clusters of electrodes to calculate EEG power values (e.g., Grieve, Myers, Stark, Housman, & Fifer, 2005). Researchers who use 32 or fewer electrodes tend to report power values for individual scalp electrodes. The number of scalp electrodes is typically determined by the research question. If information about electrical activity at specific scalp locations is of interest, then the standard 19 electrode montage (i.e., the 10/20 system; Jasper, 1957) may suffice. If information about the source of the electrical signal is of interest, then dense arrays are required.
Reference Electrode
As noted earlier, the EEG signal reflects the electrical potential between two electrodes, one of which is considered the “active” electrode site (i.e., the site of interest) and the other the reference. Choice of appropriate reference electrode location has been the source of much disagreement in the psychophysiology literature in recent years. Researchers have demonstrated that various reference electrode locations (Cz and linked ears, for example) produce different EEG results. The pros and cons of various reference electrode locations are discussed by others (Davidson et al., 2000; Hagemann, Naumann, & Thayer, 2001; Stroganova & Orekhova, 2007). In our work, we record EEG using Cz as the reference electrode and then re-reference the data via software to an average reference configuration (Lehmann, 1987). This re-referencing eliminates concerns that power values at each active site reflect interelectrode distance as much as they reflect electrical potential. At this point, there is debate about how many electrodes are adequate for average referencing.
Baseline
A period of resting physiology is essential to EEG research for multiple reasons. First, individual differences in baseline EEG have been associated with cognitive, emotional, and motor processes/skills (e.g., Bell & Fox, 1997; Benasich, Gao, Choudhury, & Harris, 2008; Smith & Bell, 2010). Baseline analyses are particularly useful with developmental populations because they permit the examination of brain-behavior relations when EEG recording during task performance is not permissible (i.e., too much motor movements). Second, researchers interested in task-related changes in EEG measures typically use a baseline period for comparisons (e.g., event-related desynchronization; Marshall, Young, & Meltzoff, 2011; task-related changes; Cuevas et al., 2012a, 2012b). Thus, obtaining an accurate baseline measure is critical to interpretation of EEG findings.
Adults and older children can be instructed to remain still and either close their eyes or fixate on a screen in order to obtain a period of resting physiology (i.e., quiet wakefulness). As noted in the Appropriate Task section, these procedures would be inappropriate for research with infants and young children. Developmental researchers define baseline as a period of quiet wakefulness/attention and use stimuli that are likely to elicit the same affective and cognitive state in the majority of participants. Common procedures for an infant baseline period include watching colorful balls move around a bingo wheel or watching someone blow bubbles. Toddlers and preschoolers, on the other hand, are typically shown abstract visual images or nonarousing video clips during the baseline phase. Thus, as with task design, it is important to use an age-appropriate baseline while also accounting for the influence of state changes and other artifacts.
Data Analyses
The amount of artifact in the EEG record can vary widely by condition and/or by individual participant. Thus, it is important to examine the amount of EEG data available for analyses after the EEG power and coherence values have been computed by the Fourier analysis software. Each research lab should determine the amount of data they consider to be appropriate for their analyses. It is important to keep in mind that including participants with drastically different amounts of data (e.g., seconds versus minutes) can result in questionable data analyses and conclusions. Furthermore, for research designs where participants are assigned to experimental groups, it is important to verify that the amount of EEG data between the groups (baseline, task-related) is similar. It is also critical to examine the EEG power and coherence values for outliers. As with behavioral datasets, careful handling of the EEG data can help the researcher verify the quality of each EEG recording and the integrity of the dataset.
Finally, because EEG measures typically are collected across different conditions at frequent intervals, the measures tend to be highly correlated, especially for adjacent conditions. This violates the sphericity assumption of repeated-measures ANOVA and can lead to invalid statistical results. Thus, researchers should use Huynh-Feldt, Geisser-Greenhouse, or other similar corrections to alter the degrees of freedom and the significance of the F-ratio. The epsilon value should be reported in manuscripts (Jennings, 1987). An appropriate alternative is to analyze data with repeated-measures MANOVA, which does not assume sphericity. No corrections are required when using MANOVA to analyze EEG data.
Implications for ERP Studies
With slight modification, these best practices for EEG research are also applicable to ERP research studies. Rather than focusing on appropriate selection of frequency band, ERP studies focus on appropriate selection of waveform components for infants and young children, which vary in latency and amplitude from adult ERP components. The “baseline” in ERP studies is the part of the waveform prior to stimulus onset and is typically 100 milliseconds. Issues regarding number of electrodes in the montage, selection of reference electrode, and data analysis are similar for EEG and ERP studies.
Conclusions
As can be seen, there are many considerations for developmental scientists interested in adopting EEG methodology for their cognitive development research. We raised four issues and suggested five best practices. Clearly, determining whether EEG is worth the time, effort, and cost is important to consider prior to incorporating this technique into an existing research program. Even for the experienced EEG researcher, the amount of power and coherence data generated by an EEG study can be overwhelming (i.e., the number of conditions, electrodes, and frequency bands/bins). An EEG study is definitely not the context to apply the adage “more is better.”
Acknowledgments
Preparation of this manuscript was supported by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
References
- Bell MA. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In: Garreau B, editor. Neuroimaging in child neuropsychiatric disorders. Berlin: Springer-Verlag; 1998. pp. 97–111. [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017.S0048577201393174. doi: 10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83:251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Adams SE. Comparable performance on looking and reaching version of the A-not-B task at 8 months of age. Infant Behavior and Development. 1999;22:221–235. doi: 10.1016/S0163-6383(99)00010-7. [DOI] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. doi: 10.1111/1467-8624.ep9301210012. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between electroencephalographic frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 314–345. [Google Scholar]
- Bell MA, Fox NA. Individual differences in object permanence at 8 months: Locomotor experience and brain electrical activity. Developmental Psychobiology. 1997;31:287–297. doi: 10.1002/(SICI)1098-2302(199712)31:4<287::AID-DEV6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Developmental progression of looking and reaching performance on the A-not-B task. Developmental Psychology. 2010;46:1363–1371. doi: 10.1037/a0020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80:119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Raj V, Bell MA. A frequency band analysis of two-year-olds’ memory processes. International Journal of Psychophysiology. 2012-a;83:315–322. doi: 10.1016/j.ijpsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Raj V, Bell MA. Functional connectivity and infant spatial working memory: A frequency band analysis. Psychophysiology. 2012-b;49:271–280. doi: 10.1111/j.1469-8986.2011.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. Cambridge, UK: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- *.DeBoer T, Scott LS, Nelson CA. Methods of acquiring and analyzing infant event-related potentials. In: de Haan M, editor. Infant EEG and event-related potentials. London: Psychology Press; 2006. pp. 5–38. This chapter provides advantages and disadvantages of cap/gel and net/saline EEG techniques. The authors also provide best practice guidelines for ERP research with younger populations. [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and event-related potentials. London: Psychology Press; 2006. pp. 101–143. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- *.Fox NA, Schmidt LA, Henderson HA, Marshall P. Developmental psychophysiology: Conceptual and methodological issues. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 453–481. This chapter provides a comprehensive review of issues important to developmental research using EEG, ERP, and cardiovascular measures. [Google Scholar]
- Gevins AS. Dynamic functional typography of cognitive tasks. Brain Topography. 1989;2:37–56. doi: 10.1007/BF01128842. [DOI] [PubMed] [Google Scholar]
- Grieve PG, Myers MM, Stark RI, Housman S, Fifer WP. Topographic localization of electrocortical activation in newborn and two- to four-month-old infants in response to head-up tilting. Acta Paediatricia. 2005;94:1756–1763. doi: 10.1080/08035250510042933. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jennings JR. Editorial policy on analyses of variance with repeated measures. Psychophysiology. 1987;24:474–475. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jing H, Gilchrist JM, Badger TM, Pivik RT. A longitudinal study of differences in electroencephalographic activity among breastfed, milk formula-fed, and soy formula-fed infants during the first year of life. Early Human Development. 2010;86:119–125. doi: 10.1016/j.earlhumdev.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Johnson MH, de Hann M, Oliver A, Smith W, Hatzakis H, Tucker LA, Csibra G. Recording and analyzing high-density event-related potentials with infants using the Geodesic Sensor Net. Developmental Neuropsychology. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Davalos M, Lundy B, Hart A. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior & Development. 1998;21:537–541. [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of electroencephalography and clinical neuropsychology, rev. ser. Vol. 1: Methods of analysis of brain electrical and magnetic signals. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasch KC, Bell MA. The role of inhibitory control in behavioral and physiological expressions of toddler executive function. Journal of Experimental Child Psychology. 2011;108:593–606. doi: 10.1016/j.jecp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P. Electrical fields of the brain. New York: Oxford; 1981. [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- *.Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. This chapter reviews important issues for all EEG research irrespective of participant age. [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Developmental Neuropsychology. 2009;34:312–329. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Bell MA. Stability in infant frontal asymmetry as a predictor of toddlerhood internalizing and externalizing behaviors. Developmental Psychobiology. 2010;52:158–167. doi: 10.1002/dev.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen R, van Beek B. Ocular artifacts in children’s EEG: selection is better than correction. Biological Psychology. 1998;48:281–300. doi: 10.1016/s0301-0511(98)00041-6. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV. EEG and infant states. In: de Haan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2007. pp. 251–287. [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization: Origins of human cognitive development. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 232–266. [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]