Abstract
Background
Epidemiologic data on the combined influence of several lifestyle factors on diabetes risk are rare, particularly among older adults.
Objective
Examine how combinations of lifestyle risk factors relate to the 11-year risk of incident diabetes.
Design
Population-based prospective cohort study.
Setting
National Institutes of Health (NIH)-AARP Diet and Health Study.
Participants
114996 men and 92483 women aged 50–71 in 1995–1996 without evidence of heart disease, cancer, or diabetes.
Measurements
A comprehensive survey of demographic characteristics and lifestyle factors, including dietary intake, body weight and height, physical activity, smoking, and alcohol at baseline (1995–1996). Low-risk groups were formed by dichotomizing each lifestyle factor. Incident self-reported physician-diagnosed diabetes was identified with a follow-up survey in 2004–2006.
Results
There were 11031 (9.6%) men and 6969 (7.5%) women who developed new-onset diabetes. The odds for diabetes were 31% (odds ratio (OR): 0.69; 95% confidence interval (CI): 0.68, 0.71) lower for each 1 additional lifestyle factor in the low-risk group among men and 39% (OR: 0.61; 95% CI: 0.60, 0.63) lower among women. Men and women with a diet score, physical activity level, smoking status, and alcohol use all in the low-risk group had ORs for diabetes of 0.61 (95% CI: 0.56, 0.66) and 0.43 (95% CI: 0.34, 0.55), respectively. When absence of overweight or obesity was added, ORs for diabetes were 0.28 (95% CI: 0.23, 0.34) and 0.16 (95% CI: 0.10, 0.24) for men and women, respectively. Results did not differ by family history of diabetes and level of adiposity.
Limitation
The study was observational with potential for residual confounding.
Conclusions
Lifestyle factors, when considered in combination, are associated with a substantial reduction in risk for diabetes.
Keywords: physical activity, diet, obesity, smoking, alcohol, older adults
Diabetes is a prevalent, costly condition associated with substantial morbidity and mortality. In 2010, approximately 25.6 million adults (prevalence of 11.3%) in the United States had diagnosed diabetes, including 10.9 million adults aged ≥ 65 years (prevalence 26.9%) (1). Pharmacological management of diabetes has proven benefits, but these efforts are often costly, include side effects, and may not be as effective as lifestyle interventions (2). Primary prevention of diabetes, therefore, would have major positive public health consequences.
Regular physical activity (3, 4), maintenance of an optimal body weight (5, 6), a healthful diet (7, 8), avoidance of smoking (9, 10), and alcohol use in moderation (11, 12) have each been associated with a lower risk of diabetes. An overall healthy lifestyle that incorporates more than one of these factors may be more effective in lowering risk for diabetes than any single factor; however, epidemiologic data on the combined influence of these lifestyle factors on diabetes risk are rare (13, 14).
The objective of the current study was to examine how combinations of several lifestyle risk factors relate to the 11-year risk of incident diabetes in a large prospective cohort study of adults aged 50 to 71 years. We also sought to determine whether these lifestyle factors differentially influence risk for diabetes between men and women, as well as across other potentially important modifying factors such as a family history of diabetes and overall level of adiposity.
METHODS
Study Participants
The National Institutes of Health (NIH)-AARP Diet and Health cohort was established in 1995–1996 by the US National Cancer Institute (15). Cohort participants included 566401 AARP members ages 50 to 71 in 1995–1996 from six states (California, Florida, Louisiana, New Jersey, North Carolina, Pennsylvania) and two metropolitan areas of the US (Atlanta, Georgia and Detroit, Michigan). All study participants completed a comprehensive dietary survey, including a 124-item food frequency questionnaire and a short survey on demographics, lifestyle, and medical conditions (15). Six months later in 1996–1997, a second questionnaire (termed the risk factor survey) was mailed to the original cohort to provide additional information, including whether a family member (father, mother, brother, or sister) had been diagnosed with diabetes. A follow-up questionnaire was mailed out to surviving participants of the original cohort in 2004–2006 to update exposures and ascertain the occurrence of major chronic conditions, including diabetes. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute. All participants provided written informed consent.
Of the 566401 cohort participants, we excluded 15760 whose baseline questionnaires were completed by proxy, 84584 with previously diagnosed heart disease or cancer, and 38221 with diabetes at baseline (Appendix Figure). We further excluded 3990 with extreme energy intake (i.e., greater than twice the interquartile range of sex-specific log-transformed intake above the 75th or below the 25th percentile), 16705 with missing data on smoking, 9295 with missing data on weight or height, 3234 with missing data on physical activity, 4474 with a body mass index (BMI) < 18.5 kg/m2, and 158465 who did not return the follow-up questionnaire. We further excluded 24194 with an unknown diabetes status at follow-up. We analyzed the data from the remaining 207479 participants, including 114996 men and 92483 women. Information on family history of diabetes was available from approximately 71% of study participants (80848 men and 67180 women) who returned the risk factor survey in 1996–1997.
Ascertainment of Lifestyle Factors
The lifestyle factors of interest were assessed at baseline in 1995–1996 and included diet, alcohol use, BMI, smoking, and physical activity. The food frequency questionnaire was a grid-based version of the National Cancer Institute Diet History Questionnaire that assessed the frequency of consumption and usual portion size of 124 food items and alcohol use during the previous year (16). The Diet History Questionnaire was validated using two non-consecutive 24-hour dietary recalls that were administered to 2053 randomly chosen NIH-AARP participants (17). Correlation coefficients comparing the Diet History Questionnaire to recall values for fruits and vegetables, polyunsaturated fat, saturated fat, and dietary fiber ranged from 0.5–0.8 (17). The food items, portion sizes, and nutrient database for this food frequency questionnaire was constructed by using the US Department of Agriculture 1994–1996 Continuing Survey of Food Intakes by Individuals (18).
BMI was calculated from self-reported weight and height (kg/m2). Self-reported weight and height are generally known to be accurate (correlation coefficient > 0.9) with agreement somewhat less at extreme values of BMI (19, 20). Participants were asked whether they had ever smoked 100 cigarettes or more in their lifetime to define ever smokers and never smokers. Ever smokers were asked if they currently smoke or whether they had stopped smoking in the last year or 1 to 4, 5 to 9, or 10 years or more previously. Current (and former) smokers were asked to report how many cigarettes/day they usually smoke. Participants were asked to report how often (never, rarely, 1–3 times/month, 1–2 times/week, 3–4 times/week, or >5 times/week) they engaged in physical activity at home or at work for at least 20 minutes that resulted in increased breathing or heart rate or produced perspiration.
Classification of a Low-Risk Lifestyle
In order to determine how combined influences of lifestyle patterns impact the risk for new-onset diabetes, we formed dichotomous scores for each lifestyle factor. To maintain consistency with previous studies examining the relation between diet and diabetes (13, 14), we classified a low-risk dietary pattern based upon a dietary score consisting of glycemic index, the ratio of polyunsaturated to saturated fat, dietary fiber, and trans-fat. Each person received a score of 1 to 5 based upon their quintile of consumption of lower glycemic index foods, a higher polyunsaturated to saturated fat ratio, higher fiber, and lower intake of trans-fat. All scores were then summed to form the dietary score; the top two quintiles were used to classify a low-risk pattern. We defined moderate alcohol consumption as at least 5 g/day with an upper limit of 29.9 g/day for men and 14.9 g/day for women, consistent with current US guidelines for alcohol intake (21). Optimal weight was defined as a BMI 18.5–24.9 kg/m2 (normal weight). For smoking, we defined low-risk as never smoking. Because our interest was in modifiable factors, we also included adults who had quit smoking for 10 years or more in the low-risk smoking category. Finally, for physical activity, we defined low risk as participation in at least 20 minutes of physical activity ≥3–4 times/week, which is approximately equal to current public health recommendations for physical activity (22).
Ascertainment of New-Onset Diabetes
As part of the 1995–1996 baseline survey, participants were asked whether they had ever been told by a doctor that they had diabetes. A similar item was also included on the follow-up questionnaire in 2004–2006 with categorical choices of the year of first diagnosis: before 1985, 1985–1994, 1995–1999, and 2000 to present. Participants who did not report a diagnosis of diabetes at baseline, but reported a diagnosis of diabetes prior to 1995 at follow-up were excluded from analyses. These questions did not differentiate type 2 from type 1 diabetes; however, it has been estimated that nearly 95% of cases identified during adulthood are type 2 (23). In addition, since the current study included adults aged 50 to 71 years at baseline and incident cases diagnosed after 1996, we believe almost all cases should be type 2 diabetes.
Statistical Analysis
Logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) for new-onset diabetes. Sex-specific multivariable models were adjusted for age, race/ethnicity (white; black; Hispanic; Asian, Pacific Islander, Native American combined; missing), educational attainment (high school or less, some college, college graduate), marital status (married or living as married, other), and for women, use of hormone replacement therapy (yes/no). In analyses with the individual lifestyle factors, each of the other lifestyle factors were included in the model. Tests for a linear trend in the OR across categories of increasing low-risk lifestyle risk factors were determined by entering the combined number of low-risk lifestyle factors variable into the model as an ordinal term. Potential effect modification by sex, family history of diabetes, and BMI categories (for all other lifestyle factors combined) was explored in stratified analyses and evaluated by testing the statistical significance of multiplicative interaction terms in models that also included lower order terms. Tests of statistical significance were 2-tailed, with an alpha level of 0.05. SAS version 9.1 (Cary, NC) was used to perform all analyses.
Role of the Funding Source
The funding organization played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript.
RESULTS
In terms of age and sex, the 207479 participants who were included in the analysis did not differ substantially from those of the 220357 participants who were excluded. However, participants who were included in the analysis were somewhat more likely to be white (93.5% vs. 89.7%) and report that their current health status at baseline was excellent or very good (64.2% vs. 55.0%) as well as less likely to report their health status was fair or poor (5.5% vs. 10.4%) (Appendix Table 1).
Table 1 displays the characteristics of men and women included in the current study overall and according to number of lifestyle factors in the low-risk category. Overall, nearly 55% of men and about 37% of women were college graduates. About 87% of men and 47% of women reported being married or living as married. Approximately 30% met criteria for two low-risk lifestyle factors. Men and women with a greater number of low-risk lifestyle factors were slightly older and more likely to be white or Hispanic, college educated, married, have a lower total energy intake, and consume more fruits and vegetables.
Table 1.
Characteristics of Participants According to Number of Lifestyle Factors in the Low-Risk Category Stratified by Sex, NIH-AARP Diet and Health Studya
| Men |
Women |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 0 | 1 | 2 | 3 | 4 | 5 | All | 0 | 1 | 2 | 3 | 4 | 5 | |
| Participants, n | 114996 | 4722 | 21891 | 34502 | 31537 | 17716 | 4628 | 92483 | 4949 | 21688 | 27749 | 23229 | 12700 | 2168 |
| % of men/women | -- | 4.1 | 19.0 | 30.0 | 27.4 | 15.4 | 4.0 | -- | 5.4 | 23.5 | 30.0 | 25.1 | 13.7 | 2.3 |
| Age at baseline, y | 61.6 | 60.0 | 60.9 | 61.5 | 62.0 | 62.2 | 62.3 | 61.4 | 60.1 | 61.0 | 61.4 | 61.7 | 61.8 | 61.6 |
| Race/ethnicity, %b | ||||||||||||||
| White | 94.3 | 94.4 | 94.3 | 94.2 | 94.3 | 94.4 | 95.2 | 93.4 | 90.9 | 91.6 | 92.1 | 92.6 | 93.7 | 96.5 |
| Black | 1.9 | 2.9 | 2.5 | 2.3 | 1.7 | 1.2 | 0.5 | 4.0 | 6.5 | 5.3 | 4.3 | 3.3 | 1.9 | 1.1 |
| Hispanic | 3.1 | 2.1 | 2.5 | 2.8 | 3.4 | 3.7 | 3.7 | 2.9 | 1.6 | 2.3 | 2.7 | 3.3 | 3.8 | 1.9 |
| BMI, kg/m2 | 26.9 | 29.3 | 28.7 | 27.6 | 26.4 | 24.7 | 23.2 | 26.5 | 30.4 | 29.4 | 26.9 | 24.8 | 22.8 | 22.2 |
| Never smoker, % | 35.3 | 0.0 | 27.9 | 35.4 | 39.8 | 43.4 | 44.2 | 47.6 | 0.0 | 42.1 | 51.1 | 54.0 | 55.7 | 48.5 |
| College, %c | 54.7 | 36.0 | 43.4 | 50.9 | 58.7 | 68.0 | 75.5 | 37.1 | 23.4 | 28.6 | 34.8 | 42.3 | 49.1 | 55.7 |
| Married, %d | 87.1 | 84.1 | 86.9 | 87.6 | 87.8 | 87.0 | 87.4 | 47.1 | 38.7 | 44.4 | 46.8 | 49.3 | 51.7 | 55.5 |
| Family history of diabetes, %e | 23.8 | 26.0 | 25.3 | 24.2 | 24.0 | 21.7 | 19.2 | 27.2 | 30.3 | 30.1 | 27.5 | 26.2 | 23.6 | 18.9 |
| Alcohol, g/day, median | 4.8 | 1.8 | 1.8 | 3.3 | 6.5 | 10.1 | 13.9 | 1.1 | 0.9 | 0.8 | 1.0 | 1.3 | 2.5 | 9.0 |
| Hormone replacement therapy,%f | 47.9 | 39.2 | 42.5 | 46.5 | 51.3 | 55.6 | 59.5 | |||||||
| Dietary intake per day | ||||||||||||||
| Total energy, kcal | 2016.7 | 2200.5 | 2096.0 | 2055.4 | 1978.3 | 1901.1 | 1870.1 | 1552.5 | 1646.4 | 1621.2 | 1553.6 | 1513.4 | 1482.3 | 1465.7 |
| Total fruits, cup/1000 kcal, median | 1.3 | 0.7 | 1.0 | 1.2 | 1.5 | 1.8 | 1.9 | 1.7 | 1.0 | 1.3 | 1.6 | 2.0 | 2.3 | 2.3 |
| Total vegetables, cup/1000 kcal, median | 1.9 | 1.5 | 1.6 | 1.8 | 2.0 | 2.2 | 2.3 | 2.3 | 1.8 | 1.9 | 2.2 | 2.5 | 2.8 | 3.0 |
| Total fat, g/1000 kcal | 33.7 | 37.8 | 36.8 | 34.9 | 32.5 | 30.0 | 28.4 | 32.9 | 37.6 | 36.4 | 33.7 | 30.8 | 28.1 | 27.3 |
| Saturated fat, g/1000 kcal | 10.5 | 12.5 | 12.0 | 11.0 | 10.0 | 8.9 | 8.2 | 10.2 | 12.2 | 11.7 | 10.5 | 9.3 | 8.2 | 7.9 |
| Polyunsaturated/saturated fat | 0.76 | 0.65 | 0.67 | 0.73 | 0.80 | 0.86 | 0.91 | 0.82 | 0.72 | 0.74 | 0.80 | 0.86 | 0.92 | 0.94 |
| Trans fat, g/1000 kcal | 2.3 | 2.7 | 2.7 | 2.5 | 2.2 | 1.9 | 1.7 | 2.3 | 2.7 | 2.6 | 2.4 | 2.0 | 1.7 | 1.6 |
| Glycemic index | 54.0 | 55.7 | 55.3 | 54.4 | 53.4 | 52.7 | 52.3 | 53.4 | 55.4 | 54.9 | 53.6 | 52.5 | 51.6 | 51.1 |
| Fiber, g/1000 kcal | 10.4 | 7.7 | 8.5 | 9.7 | 11.2 | 12.6 | 13.3 | 11.7 | 8.7 | 9.6 | 11.2 | 12.9 | 14.4 | 14.4 |
Abbreviations: NIH-AARP, National Institutes of Health-AARP.
All values are means unless otherwise stated.
763 men and 757 women were missing data on race/ethnicity.
1821 men and 2031 women were missing data on educational attainment.
246 men and 376 women were missing data on marital status.
Among 80,848 men and 67,180 women who participated in the risk factor survey in 1996–1997.
278 women were missing data on hormone replacement therapy.
We identified 11031 (9.6%) men and 6969 (7.5%) women with new-onset diabetes. Table 2 shows the adjusted ORs and 95% CIs for diabetes according to each single lifestyle factor in the low-risk group compared to the high risk group among men and women. Each lifestyle factor was associated with diabetes; BMI displayed the strongest association. Compared to men and women who had a BMI ≥25.0 kg/m2 at baseline, those who were normal weight (BMI 18.5–29.9 kg/m2) had odds for incident diabetes 70% (OR: 0.30; 95% CI: 0.29, 0.32) and 78% (OR: 0.22; 95% CI: 0.21, 0.24) lower, respectively. The ORs for the remaining lifestyle factors ranged from 0.76–0.85 for men and 0.63–0.84 for women. The ORs for smoking indicate that men benefit more than women from not smoking (P-interaction by sex 0.003), while the ORs for BMI and alcohol indicate that women benefit more than men from a lower BMI and moderate alcohol consumption (P-interaction by sex < 0.001 for both). The ORs for the remaining lifestyle factors did not differ by sex (P-interaction by sex > 0.050, for all).
Table 2.
Adjusted OR and 95% CI for New-Onset Diabetes According to Single Lifestyle Risk Factors Stratified by Sex
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Cases | ORa | 95% CI | n (%) | Cases | ORa | 95% CI | |
| Body mass index, kg/m2 | ||||||||
| 18.5–24.9 | 36841 (32.0) | 1443 | 0.30 | 0.29, 0.32 | 43541 (47.1) | 1140 | 0.22 | 0.21, 0.24 |
| ≥ 25.0 | 78155 (68.0) | 9598 | 1.00 | referent | 48942 (52.9) | 5829 | 1.00 | referent |
| Diet scoreb | ||||||||
| Top two quintiles | 50518 (43.9) | 4061 | 0.85 | 0.82, 0.89 | 40796 (44.1) | 2439 | 0.82 | 0.78, 0.87 |
| Bottom three quintiles | 64478 (56.1) | 6970 | 1.00 | referent | 51687 (55.9) | 4530 | 1.00 | referent |
| Smoking | ||||||||
| Never or quit ≥ 10 years | 92898 (80.8) | 8281 | 0.76 | 0.72, 0.80 | 70319 (76.0) | 5044 | 0.84 | 0.79, 0.89 |
| Current or quit ≤ 10 years | 22098 (19.2) | 2750 | 1.00 | referent | 22164 (24.0) | 1925 | 1.00 | referent |
| Moderate alcohol consumptionc | ||||||||
| Yes | 39903 (34.7) | 3226 | 0.81 | 0.78, 0.85 | 13237 (14.3) | 565 | 0.63 | 0.57, 0.69 |
| No | 75093 (65.3) | 7805 | 1.00 | referent | 79246 (85.7) | 6404 | 1.00 | referent |
| Regular physical activityd | ||||||||
| Yes | 59350 (51.6) | 4650 | 0.76 | 0.73, 0.79 | 40620 (43.9) | 2272 | 0.77 | 0.73, 0.82 |
| No | 55646 (48.4) | 6381 | 1.00 | referent | 51863 (56.1) | 4697 | 1.00 | referent |
Abbreviations: OR, odds ratio; CI, confidence interval.
Adjusted for age, race/ethnicity, educational attainment, marital status, each of the other lifestyle risk factors in the table, and for women, use of hormone replacement
Defined as a dietary score consisting of glycemic index, trans-fat, dietary fiber, and the ratio of polyunsaturated to saturated fat.
Moderate alcohol consumption is defined as 5–29.9 g/day for men and 5–14.9 g/day for women.
Regular physical activity is defined as participation in at least 20 minutes of physical activity ≥3–4 times/week that caused increases in breathing or heart rate or produced perspiration.
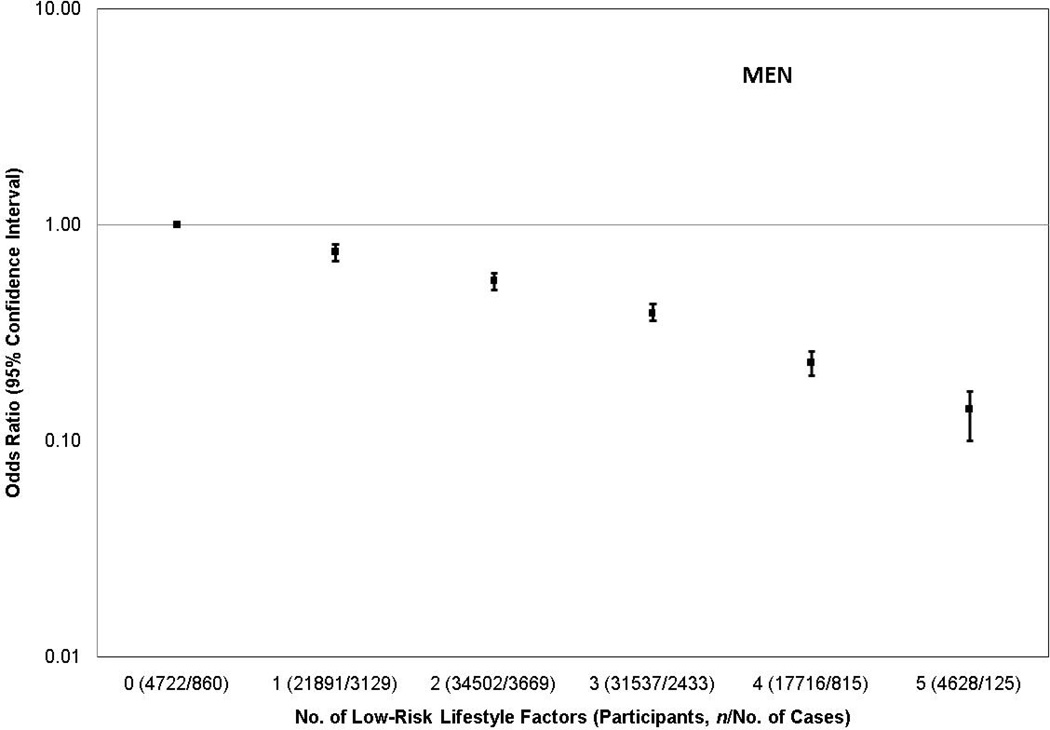
Figure 1 displays the adjusted ORs and 95% CIs for new-onset diabetes according to the combined number of lifestyle factors in the low-risk category for men and women. A strong inverse dose-response relation was observed in both sexes (P-trend < 0.001, for both). When evaluated ordinally, odds for diabetes were 31% lower (OR: 0.69; 95% CI: 0.68, 0.71) for each 1 additional lifestyle factor in the low-risk group among men and 39% lower (OR: 0.61; 95% CI: 0.60, 0.63) among women (P-interaction by sex < 0.001). When BMI was not included, ORs for those with 1, 2, 3, and 4 factors in the low-risk category for men were 0.79 (95% CI: 0.73, 0.86), 0.66 (95% CI: 0.61, 0.72), 0.56 (95% CI: 0.51, 0.61), and 0.45 (95% CI: 0.41, 0.51), respectively, and 0.82 (95% CI: 0.76, 0.89), 0.68 (95% CI: 0.63, 0.75), 0.54 (95% CI: 0.49, 0.60), and 0.32 (95% CI: 0.25, 0.41) for women (P-trend < 0.001, for both sexes), compared to similar adults who had no lifestyle factors in the low-risk category.
Figure 1.
Adjusted odds ratios and 95% confidence intervals for new-onset diabetes according to number of lifestyle factors in the low-risk category stratified by sex. The P-trend for both men and women was < 0.001. The low-risk lifestyle factors included a body mass index 18.5–24.9 kg/m2, never smoker or quit ≥ 10 years, top two quintiles of a diet score, alcohol use 5–29.9 g/day for men or 5–14.9 g/day for women, or participation in at least 20 minutes of physical activity ≥3–4 times/week. Adjusted for age, race/ethnicity, educational attainment, marital status, and for women, use of hormone replacement therapy.
We examined the association of specific combinations of factors with the odds for diabetes with a particular focus on lifestyle behaviors. As shown in Table 3, when low-risk combinations of only physical activity and diet (which included approximately 25% of men and women) were considered after simultaneously adjusting for the other three lifestyle factors, the OR for diabetes was 0.72 (95% CI: 0.69, 0.76) for men and 0.71 (95% CI: 0.66, 0.77) for women (P-interaction by sex 0.23). When smoking was added to physical activity and diet, which together included about 1 in 5 adults, men and women with all three of these lifestyle factors in the low-risk group had a 32% and 33% lower odds for diabetes, respectively (P-interaction by sex 0.26). Those with physical activity, diet, smoking, and alcohol consumption in the low risk groups (9% and 3% of men and women, respectively) had 39% and 57% lower odds for diabetes among men and women, respectively (P-interaction 0.003). All five factors in the low-risk group (2–4% of adults) was associated with a 72% lower odds for diabetes among men and 84% lower odds for women (P-interaction by sex 0.006).
Table 3.
Adjusted OR and 95% CI for New-Onset Diabetes According to Specific Combinations of Lifestyle Factors in the Low-Risk Category Stratified by Sex
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of factors in low-risk categorya | n (%) | Cases | ORb | 95% CI | n (%) | Cases | ORb | 95% CI |
| 2: diet top two quintiles, regular physical activity | 30724 (26.7) | 2072 | 0.72 | 0.69, 0.76 | 22323 (24.1) | 1056 | 0.71 | 0.66, 0.77 |
| 84272 (73.3) | 8959 | 1.00 | referent | 70160 (75.9) | 5913 | 1.00 | referent | |
| 3: two above plus never smoking or quit ≥ 10 years | 27187 (23.6) | 1743 | 0.68 | 0.64, 0.72 | 18533 (20.0) | 831 | 0.67 | 0.62, 0.73 |
| 87809 (76.4) | 9288 | 1.00 | referent | 73950 (80.0) | 6138 | 1.00 | referent | |
| 4: three above plus moderate alcohol consumption | 10614 (9.2) | 571 | 0.61 | 0.56, 0.66 | 3109 (3.4) | 71 | 0.43 | 0.34, 0.55 |
| 104382 (90.8) | 10460 | 1.00 | referent | 89374 (96.6) | 6898 | 1.00 | referent | |
| 5: four above plus BMI 18.5–24.9 kg/m2 | 4628 (4.0) | 125 | 0.28 | 0.23, 0.34 | 2168 (2.3) | 25 | 0.16 | 0.10, 0.24 |
| 110368 (96.0) | 10906 | 1.00 | referent | 90315 (97.7) | 6944 | 1.00 | referent | |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index.
Referent group is all other categories.
Adjusted for age, race/ethnicity, educational attainment, marital status, each of the other lifestyle risk factors not already included in the model, and for women, use of hormone replacement therapy.
In order to examine potential effect modification of the association between the lifestyle factors and new-onset diabetes by a family history of diabetes, we performed stratified analyses. The ORs according to number of factors in the low-risk category were similar for both men and women with and without a family history of diabetes (P-interaction by family history 0.120 and 0.114 for men and women, respectively) (Appendix Table 2). Similar inverse dose-response associations were observed among men and women whose family history of diabetes was unknown.
To examine whether the association of the lifestyle factors with risk for new-onset diabetes varied according to level of adiposity, we performed analyses stratified by categories of BMI according to the remaining four lifestyle factors in the low-risk category (Appendix Table 3). Strong inverse dose-response associations were observed between the combined number of lifestyle factors in the low-risk group and risk for diabetes within each category of BMI (P-interaction by BMI 0.122 and 0.62 for men and women, respectively). These results were not substantially altered after additional adjustment for BMI as a continuous variable within each stratum.
DISCUSSION
In this large prospective cohort study of men and women age 50 to 71 years at baseline, individuals with a low-risk lifestyle profile, including not smoking, engaging in regular physical activity, consuming a healthful diet, using alcohol in moderation, and having an optimal body weight, had a dramatically lower risk for incident diabetes than individuals who did not have a low-risk profile. Each 1 additional lifestyle factor in the low-risk group was associated with a 31% and 39% lower risk for diabetes among men and women, respectively. This combined influence of lifestyle, while slightly more strongly associated with a lower risk for diabetes among women, was similarly observed among individuals with and without a family history of diabetes and those with higher and lower levels of overall adiposity.
In general, our results are consistent with the limited published research on the role of multiple lifestyle risk factors in the etiology of diabetes. In a relatively homogeneous cohort of middle-age female health professionals of high socioeconomic status, women with all 5 lifestyle factors in the low-risk group had a relative risk for diabetes of 0.09 (95% CI: 0.05, 0.17) (13). In another smaller scale study of older adults, each 1 additional lifestyle factor in the low-risk group was associated with a 35% lower risk for diabetes (14). However, in the study of older adults, limited power was available to compare risks between the sexes as well as across other potentially important modifiable factors. Large prospective studies such as ours are especially valuable for determining a precise dose response gradient for the connection between lifestyle and diabetes. Our large cohort also enabled us to estimate risks of diabetes according to narrow categories of family history and adiposity separately for men and women with great precision.
Each of the 5 modifiable lifestyle factors was independently associated with risk for incident diabetes; increased adiposity displayed the strongest association. The likely causal effects of adiposity on diabetes risk do not deserve further elaboration here (13). However, even after adjustment for adiposity, regular physical activity, a healthful diet, not smoking, and moderate alcohol use still predicted a lower risk for diabetes. This suggests that these lifestyle factors exert their effects on diabetes risk independent of their effects on adiposity. Since these lifestyle factors also exert at least part of their effects on diabetes risk through adiposity, adjustment for adiposity in these models may reflect overadjustment, underestimating the full impact of these lifestyle factors in the etiology of diabetes.
In the current study, compared to men and women who were not moderate drinkers, we observed a 19% and 37% lower risk of diabetes among moderate drinking men and women, respectively. Insulin resistance is an important factor in the development of diabetes, and light to moderate alcohol consumption has been associated with enhanced insulin sensitivity in several observational studies (24–26). In a controlled trial among nondiabetic postmenopausal women, consumption of 30 g/day of alcohol for 8 weeks resulted in decreases in fasting insulin, as well as increases in triglycerides and insulin sensitivity (27). Moderate alcohol consumption also has noted anti-inflammatory effects (28, 29), representing an additional mechanism by which moderate alcohol consumption may lower the risk of developing diabetes.
Multiple prospective cohort studies have determined the association between active smoking and risk for diabetes (30). Much less is known about the influence of smoking cessation. In a recent study, diabetes risk was elevated in recent quitters, compared to never smokers, but decreased gradually to 0 after 12 years (31). Among never smokers as well as those who successfully quit smoking for a decade or more before baseline, we observed a 24% and 16% reduction in risk of diabetes among men and women, respectively. Smoking negatively affects insulin sensitivity and pancreatic β-cell functioning (32, 33), has pro-inflammatory effects (34), and increases central obesity (35), all of which have been implicated in the development of diabetes.
We tested whether those who were overweight or obese, but otherwise followed a low-risk lifestyle pattern had a lower risk for developing diabetes. Our findings suggested that the remaining lifestyle factors were associated with a lower risk for diabetes among normal weight, overweight, and obese men and women. Similar inverse dose-response associations between a low-risk lifestyle profile and diabetes risk was observed irrespective of a family history of diabetes. The former findings imply, in the context of diabetes risk, that overweight and obese adults may benefit by adopting other low-risk lifestyle behaviors. The latter findings are particularly important given that many individuals may mistakenly believe that their family history of diabetes assures their own eventual development of diabetes; however, these individuals may at least delay the development of diabetes by achieving a healthy lifestyle.
The major strengths of the current study include its large sample size including men and women, its prospective design, and detailed epidemiologic profiles. A large number of diabetes cases permitted stratification by several characteristics simultaneously and provided increased power to detect modest associations with risk. However, this study also had some limitations. First, we had only a single baseline determination of the factors that contributed to the combined number of lifestyle risk factors with no consideration for changes in these factors that may have occurred prior to or after exposure assessment. Second, misclassification of some lifestyle risk factors, particularly diet and physical activity, is likely. Third, in a large study such as this, we had to rely upon a self-reported diagnosis of diabetes. The self-report of diabetes has shown substantial agreement when compared to medical records (kappa=0.76), high specificity (99.7%), but low sensitivity (66.0%) (36). Individuals with a poor lifestyle may have experienced closer medical attention, potentially overestimating the occurrence of diabetes. In a compensating fashion, those with a healthier lifestyle may also have been more likely to have access to or seek medical attention, overestimating the incidence of diabetes among those with a healthy lifestyle. Fourth, the current study was limited to participants of the follow-up survey in 2004–2006. This may have induced a selection bias if the lifestyle risk factors included in the current study were associated with participation in the follow-up survey differentially by diabetes status. Finally, although we adjusted for major sociodemographic characteristics and lifestyle factors simultaneously, residual confounding by unmeasured or inadequately measured factors may exist. However, since many of the risk estimates were of substantial magnitude it is unlikely that all of the risk difference can be explained by residual confounding.
We found that a low-risk profile incorporating 5 lifestyle factors was strongly associated with a lower risk for new-onset diabetes among older adults. Each 1 additional factor in the low-risk group was associated with a substantial reduction in risk for diabetes. While this combined influence of lifestyle was slightly more strongly associated with a lower risk for diabetes among women, it was similarly observed among those with and without a family history of diabetes and those with higher and lower levels of adiposity. These results provide evidence for a tremendous combined impact of lifestyle on diabetes risk reduction in older adults. Public health efforts should continue to support the achievement and maintenance of an optimal body weight, adoption of healthy and attainable physical activity and dietary goals as well as preventing the initiation of smoking and promoting its cessation. Although there are appropriate concerns regarding the wide-spread public health recommendation of moderate alcohol use in the prevention of diabetes, this study supports guidelines that do not exclude alcohol use in moderation among those without contraindications.
Supplementary Material
ACKNOWLEDGEMENTS
Funding: The NIH-AARP Diet and Health study was supported by the Intramural Research Program of the NIH.
Footnotes
Author Contributions: Dr. Reis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Reis, Loria, Sorlie
Acquisition of the data: Reis
Analysis and interpretation of the data: Reis, Loria, Sorlie, Park, Hollenbeck, Schatzkin
Drafting of the manuscript: Reis
Critical revision of the manuscript for important intellectual content: Reis, Loria, Sorlie, Park, Hollenbeck, Schatzkin
Statistical analysis: Reis
Obtained funding: Schatzkin
Administrative, technical, or material support: Park
Study supervision: Reis
Financial Disclosures: None.
REFERENCES
- 1.Centers for Disease Control and Prevention. (US Department of Health and Human Services, Centers for Disease Control and Prevention) National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. 2011
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch J, Helmrich SP, Lakka TA, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156(12):1307–1314. [PubMed] [Google Scholar]
- 4.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 5.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. Jama. 2010;303(24):2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84(2):427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 7.Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27(11):2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 8.Salmeron J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi N, Nakamura K, Matsuo Y, Suzuki K, Tatara K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann Intern Med. 2000;133(3):183–191. doi: 10.7326/0003-4819-133-3-200008010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Manson JE, Stampfer MJ, et al. Cigarette smoking and the risk of diabetes in women. Am J Public Health. 1993;83(2):211–214. doi: 10.2105/ajph.83.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajani UA, Hennekens CH, Spelsberg A, Manson JE. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med. 2000;160(7):1025–1030. doi: 10.1001/archinte.160.7.1025. [DOI] [PubMed] [Google Scholar]
- 12.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Blair SN. Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care. 2000;23(1):18–22. doi: 10.2337/diacare.23.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169(8):798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute Division of Cancer Control and Population Sciences. Diet History Questionnaire. Available from: URL: http://www.riskfactor.cancer.gov/DHQ.
- 17.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 18.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152(3):279–286. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. Monographs in Epidemiology and Biostatistics Volume 30. [Google Scholar]
- 20.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132(6):1156–1163. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Agriculture US Department of Health and Human Services. Dietary Guidelines for Americans. 2005
- 22.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Jama. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. (US Department of Health and Human Services, Centers for Disease Control and Prevention) National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. 2008
- 24.Kiechl S, Willeit J, Poewe W, et al. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study) Bmj. 1996;313(7064):1040–1044. doi: 10.1136/bmj.313.7064.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarus R, Sparrow D, Weiss ST. Alcohol intake and insulin levels. The Normative Aging Study. Am J Epidemiol. 1997;145(10):909–916. doi: 10.1093/oxfordjournals.aje.a009050. [DOI] [PubMed] [Google Scholar]
- 26.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17(2):115–119. doi: 10.2337/diacare.17.2.115. [DOI] [PubMed] [Google Scholar]
- 27.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. Jama. 2002;287(19):2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 28.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357(9258):763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 29.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56(11):1130–1136. doi: 10.1038/sj.ejcn.1601459. [DOI] [PubMed] [Google Scholar]
- 30.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 31.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 152(1):10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance--a potential link with the insulin resistance syndrome. J Intern Med. 1993;233(4):327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 33.Somm E, Schwitzgebel VM, Vauthay DM, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149(12):6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 34.Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 160(3):458–463. doi: 10.1016/j.ahj.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett-Connor E, Khaw KT. Cigarette smoking and increased central adiposity. Ann Intern Med. 1989;111(10):783–787. doi: 10.7326/0003-4819-111-10-783. [DOI] [PubMed] [Google Scholar]
- 36.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.