Abstract
Due to its antiapoptotic action, derivatives of the lipid mediator lysophosphatidic acid (LPA) provide potential therapeutic utility in diseases associated with programmed cell death. Apoptosis is one of the major pathophysiological processes elicited by radiation injury to the organism. Consequently, therapeutic explorations applying compounds that mimic the antiapoptotic action of LPA have begun. Here we present a brief account of our decade-long drug discovery effort aimed at developing LPA mimics with a special focus on specific agonists of the LPA2 receptor subtype, which was found to be highly effective in protecting cells from apoptosis. We describe new evidence that 2-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propyl)thio)benzoic acid (GRI977143), a prototypic nonlipid agonist specific to the LPA2 receptor subtype, rescues apoptotically condemned cells in vitro and in vivo from injury caused by high-dose γ-irradiation. GRI977143 shows the features of a radiomitigator because it is effective in rescuing the lives of mice from deadly levels of radiation when administered 24 h after radiation exposure. Our findings suggest that by specifically activating LPA2 receptors GRI977143 activates the ERK1/2 prosurvival pathway, effectively reduces Bax translocation to the mitochondrion, attenuates the activation of initiator and effector caspases, reduces DNA fragmentation, and inhibits PARP-1 cleavage associated with γ-irradiation-induced apoptosis. GRI977143 also inhibits bystander apoptosis elicited by soluble proapoptotic mediators produced by irradiated cells. Thus, GRI977143 can serve as a prototype scaffold for lead optimization paving the way to more potent analogs amenable for therapeutic exploration.
Keywords: LPA, radiomitigator, radioprotection, apoptosis, bystander-apoptosis, distant effects of radiation exposure, countermeasure
INTRODUCTION
Direct and indirect effects of ionizing radiation can result in programmed cell death or malignant transformation due to the accompanying DNA damage, chromosomal aberrations and generation of reactive oxygen species [1, 2]. A decade ago, based on earlier evidence in the literature establishing the prosurvival and antiapopotic actions of lysophosphatidic acid (LPA), our group embarked on a study aimed at the evaluation of LPA and its analogs for the attenuation of programmed cell death elicited by radiation and genotoxic chemotherapeutic agents [3]. Initiating this research project appeared to be timely and justified in the post September 11, 2001 period due to the possible threat of radiation terrorism and because of the ever present possibility of nuclear accidents, such as the one at the Fukushima nuclear plant on March 11, 2011. During this period, we have focused on developing metabolically stabilized analogs of LPA that could be used as long-acting stimulators of the prosurvival signaling mediated by LPA receptors. Research aimed at developing small molecular mimics of LPA was based on our increasing understanding of the ligand recognition of LPA and sphingosine-1-phosphate (S1P) receptors guided by the development and validation of theoretical models of the individual LPA and S1P receptors [4–8]. This project has ultimately steered our drug discovery program to a group of fatty alcohol phosphates, some of which mimicked the actions of LPA in activating all LPA G protein-coupled receptors (GPCR) known at the time [9, 10]. In parallel with the development of LPA mimics, we have begun to study the antiapoptotic mechanisms elicited by LPA using the IEC-6 crypt cell line, which is highly sensitive to genotoxic stress and radiation. These studies provided insight into the essential role of the LPA GPCR-mediated activation of MEKK-ERK1/2 and PI3K-Akt-NFkB prosurvival pathways underlying the antiapoptotic effect of LPA [11, 12]. We have shown that pretreating LPA GPCR with pertussis toxin, or blocking either of these prosurvival kinase pathways inhibited the antiapoptotic effect of LPA [9, 10]. Furthermore, we were able to demonstrate that LPA-induced transphosphorylation of the EGFR, ErbB2, or PDGF receptors was not required for its antiapoptotic effect [11].
A pivotal observation recognized from our early experiments was that LPA not only prevented apoptosis induced by radiation injury when applied prior to the irradiation of the cells but also rescued apoptotically condemned cells when applied two hours postirradiation [3]. With the availability of a long-acting LPA mimic octadecenyl thiophosphate (OTP, [9]), we began to examine the utility of LPA GPCR activation in mouse models of the acute hematopoietic and gastrointestinal radiation syndromes. The hematopoietic and gastrointestinal stem cells are among the most radiation sensitive cells in the body, thus these murine models appeared to be a logical extension of our in vitro studies. We reported in 2007 [13] that LPA or OTP administration to mice exposed to lethal levels of radiation was effective in reducing lethality from the hematopoietic radiation syndrome and reduced radiation-induced injury to the gastrointestinal stem cells by attenuating their apoptosis and enhancing crypt regeneration. In this study, we showed that OTP and LPA were completely ineffective in protecting mice lacking the LPA2 receptor subtype. We also obtained in vitro evidence that LPA or OTP failed to protect RH7777 cells, which do not express LPA1/2/3 GPCR, unless LPA2 was expressed by heterologous transfection of this receptor subtype.
Our research focused on further characterization of OTP and the preclinical development of this compound as a radiomitigator in murine and nonhuman primate models of the acute gastrointestinal radiation syndrome. Radiomitigators are agents that attenuate radiation injury when applied after radiation exposure. We have been studying the unique signaling properties of the LPA2 GPCR responsible for the radiomitigative action of OTP. These studies led to the previously unrealized role of the LPA2 GPCR as a center of a macromolecular signaling complex mediated through unique sequence motifs present in its C-terminal domain [14, 15]. We discovered that LPA2 via a C311xxC half zinc-finger-like motif interacts with the proapoptotic protein Siva-1 from the LIM family of proteins [15]. LIM domain proteins are named after the Lin-11, Isl-1, and Mec-3 proteins, which contain Zn-finger-like domains in their sequences that are improtant for oligomerization and interaction with other proteins. Siva-1 is an immediate-early response gene product whose expression is triggered by the DNA damage-mediated activation of the p53 and E2F1 transcription factors. Siva-1 mediates the progression of apoptosis by making a complex with the antiapoptotic Bcl-XL. Binding of Siva-1 to Bcl-XL reduces the availability of this protein to protect the mitochondrial outer membrane, thus promoting the progression of the mitochondrial apoptosis cascade. However, upon activation of LPA2, the C-terminal domain of LPA2 binds Siva-1 and this complex is withdrawn from GPCR recycling, undergoes polyubiquitination and is degraded in the proteasome [15].
In a subsequent study, we have determined that the LPA2 GPCR makes a ternary complex with two other proteins, the thyroid receptor interacting protein 6 (TRIP6) and the Na+-H+ exchange regulatory factor 2 (NHERF2). LPA2 and TRIP6 contain motifs in their last three C-terminal amino acids that interact with PSD-95, DlgA, and ZO-1 (PDZ) binding domains of proteins. NHERF2 contains tandem PDZ-binding domains near its N-terminus. We showed that TRIP6 with its LIM domain physically binds to the C311xxC motif of LPA2 and at the same time the PDZ motif of TRIP6 binds to the PDZ-binding domain of NHERF2. NHERF2 homodimerizes, leaving an additional PDZ-binding domain available to bind to the S351TL PDZ motif of LPA2. The ternary complex consisting of LPA2 – TRIP6 – 2×(NHERF2) is formed upon LPA or OTP stimulation of the GPCR leading to enhanced, long-lasting activation of the MEKK-ERK1/2 and PI3KAkt-NFkB prosurvival pathways required for the LPA2-mediated antiapoptotic effect [14]. The role of the ternary complex recruitment in the LPA2-mediated antiapoptotic response is supported by the lack of LPA protection against apoptosis when cysteines 311/314 and leucine 351 in the C-terminus of LPA2 are simultaneously mutated to alanine [14].
Although highly effective in protecting animals from radiation injury, OTP activates multiple LPA GPCRs including LPA1, which has been linked to apoptosis through anoikis [16, 17] and might attenuate the protective effect of LPA2 stimulation in cells that coexpress both GPCR subtypes. We have recently identified novel nonlipid compounds that are specific agonists of LPA2 and do not activate other LPA GPCRs including LPA1/3/4/5, GPR87, or P2Y10 [18]. One of these nonlipid hits from the Genome Research Institute chemical library, GRI977143 (GRI), has been found to have LPA-like antiapoptotic efficacy in cell based models of apoptosis elicited by Adriamycin, TNFα, or serum withdrawal. The protective effect of GRI was only observed in cells, which express the LPA2 GPCR subtype. In the present article we give an account of our recent in vitro and in vivo studies aimed at testing the radiomitigative efficacy of GRI. Our results show that although GRI is approximately 3-fold less potent than LPA, it remains highly efficacious against radiation-induced apoptosis in vitro and protects mice from the acute hematopoietic radiation syndrome when administered 24 hours after the radiation exposure.
MATERIAL AND METHODS
Materials
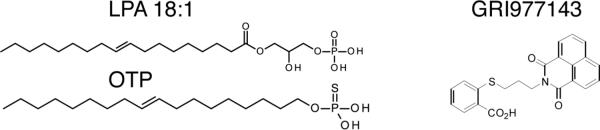
Lysophosphatidic acid (LPA) 18:1 was purchased from Avanti Polar Lipids (Alabaster, AL). Octadecenyl thiophosphate (OTP) was synthesized and provided by RxBio Inc. (Johnson City, TN) as described previously [9]. The LPA2-specific agonist GRI977143 (2-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propyl)thio)benzoic acid, [18]) was obtained from the University of Cincinnati Drug Discovery Center and Life Chemicals (UC-DDC, Cincinnati, OH). The structures of these compounds are shown in figure 1. Stock solutions of LPA and OTP were prepared in phosphate-buffered saline (PBS) with an equimolar complex of charcoal-stripped, fatty acid-free bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO). For the in vitro experiments, a 10 mM stock solution of GRI was prepared in dimethyl sulfoxide (DMSO). For the animal experiments 1 mg/kg GRI was dissolved in a vehicle consisting of PBS, 1% ethanol and 2% propanediol.
Figure 1.
Chemical structures and potencies at the LPA2 receptor of LPA 18:1 (EC50LPA2~ 0.03 μM), OTP (EC50LPA2~ 0.34 μM) and GRI (EC50LPA2~ 3.3 μM).
Kiss et a.
Cell culture
Mouse embryonic fibroblast (MEF) cells used in this study were isolated from LPA1/2 double knock out (DKO) mice. The human LPA2 receptor was reintroduced into these MEF cells by lentiviral transduction. Empty vector-transduced MEF cells were used as a control [14, 15]. MEF growth medium consisted of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (V/V) fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. During serum deprivation, the growth medium was replaced with DMEM containing 0.1% (W/V) BSA.
The rat Intestinal Epithelial Cell line 6 (IEC-6) was purchased from the American Type Culture Collection (Rockville, MD) at passage 13 and was maintained in a humidified 37 °C incubator in an atmosphere of 90% air and 10% CO2. IEC-6 cell growth medium was composed of DMEM, 5% (V/V) heat inactivated FBS, 10 μg/mL insulin and 50 μg/mL gentamicin. The serum starvation medium contained insulin and gentamicin in DMEM without FBS. Cells at passages 16 to 21 were used in all experiments.
The U937 human monocyte lymphoma cell line was a kind gift of Dr. Ryan Yates (Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, TN). Growth medium consisted of RPMl-1640 supplemented with 10% (V/V) FBS and 50 μg/mL gentamicin while serum starvation medium contained 0% FBS.
Induction of apoptosis by direct γ-irradiation
Cell cultures were irradiated on a rotating platform, using a Cs137 γ-source (J.L. Shepherd & Assoc. Mark I, Model 25, San Fernando, CA). MEF cells were plated the day before the irradiation in 48 well plates (for caspase activation or DNA fragmentation assays) or in 10 cm dishes (for PARP-1 cleavage or Bax translocation assays) at a density of 2 × 104 cell/well or 1.5 × 106 cell/dish, respectively. One hour before the irradiation full growth medium was changed to serum starvation medium and the cell cultures were exposed to a dose of 15 Gy γ-irradiation, at a dose rate of 3.2 Gy/min. One hour post irradiation cells were treated with either vehicle, LPA (1–10 μM), OTP (1–10 μM), or GRI (1–10 μM). Caspase activation, DNA fragmentation, PARP-1 cleavage, and Bax translocation were measured 5 h after the irradiation. U937 cells were plated on the day of the irradiation at a density of 2.5 × 105 cell/ml in serum starvation medium and exposed to increasing doses of γ-irradiation (7–55 Gy), at a dose rate of 7.74 Gy/min. Caspase 3 and 7 activation was determined in the irradiated U937 cells 24 h later.
Induction of apoptosis in unirradiated cells using conditioned medium (CM) from γ-irradiated U937 cell cultures
For the generation of irradiated cell CM, U937 cell cultures (5 × 105 cell/ml) were irradiated with a dose of 35 Gy γ-irradiation, using a Cs137 γ-source and a dose rate of 7.74 Gy/min. Twenty four hours later the cells were pelleted by centrifugation at 1000 × g for 5 min and the CM was collected. This CM was supplemented with either vehicle, 10 μM LPA, or 10 μM GRI and applied to 24-h serum-starved IEC-6 cultures (1.7 × 105 cell/well in 48-well plates). Activation of caspase 3 and 7 in the IEC-6 cells treated with the different CM-drug mixtures was measured 24 h later.
Caspase activation assay
To measure caspase activation the cells were lysed in 50 μl Caspase-Glow® reagent (Promega, Madison, WI). After incubation of the cells with shaking for 30 min at room temperature (RT), 200 μl of the lysate was transferred to a 96-well white wall plate and luminescence was measured on a BioTek (Winooski, VT) plate reader. The mean caspase activity in 3 triplicates / experimental group ± SEM was calculated.
DNA fragmentation ELISA
DNA fragmentation was quantified by using the Cell Death Detection® ELISA assay kit (Roche Diagnostics, Penzberg, Germany). Briefly, 20 μl cell lysate was incubated with the anti-histone-biotin plus anti-DNA-peroxidase-conjugated antibody in a 96-well streptavidin-coated plate with shaking at RT for 2 h. After washing the wells three times with the incubation buffer, 100 μl/well 2,2'-azino-di[3-ethylbenzthiazolin-sulfonate substrate was added and absorbance was measured at 405 nm every 3 min for 12 min. Protein concentration was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL). DNA fragmentation was expressed as absorbance units/mg protein/min as previously described [19].
Immunoblot analysis
Bax translocation was detected in cytosolic fractions of samples prepared following the protocol of the Cell Fractionation Kit-Standard (MitoSciences, Eugene, OR). Cytosolic fractions were precipitated by 75% (W/V) trichloroacetic acid, dissolved in 50 mM non-neutralized Tris buffer pH 10 and 6× Laemmli buffer and boiled for 5 min. To measure PARP-1 cleavage samples were prepared as described previously [20]. Proteins were separated on 12% SDS-PAGE followed by wet transfer to PVDF membranes. After 2 h blocking in 5% non-fat milk the following primary antibodies were used: pERK1/2, PARP-1, BAX (1:1000, Cell Signaling Technology, Beverly, MA), actin (1:10000, Sigma-Aldrich). Protein bands were visualized on X-ray films using anti-rabbit-HRPO secondary antibody (1:20000, Promega) and the SuperSignal detection system (Thermo Fisher Scientific Inc.).
ELISA determination of ERK1/2 activation
Activation of ERK1/2 was detected using the MAPmate® Phospho ERK/MAP Kinase 1/2 (Thr185/Tyr187) and the Milliplex® Map Total ERK/MAP Kinase 1/2 Detection kits (Millipore, Billerica, MA). Cells were plated in 24 well plates (1.2 × 105 cell/well) the day before the experiment, serum starved 2 hours and treated with vehicle, LPA (3μM), or GRI (1–10μM) for 5 min. After treatment cells were lysed in 140 μl/well lysis buffer-assay buffer mixture (1:1) supplemented with 2% protease inhibitor cocktail (Sigma-Aldrich). Twenty five μl of the samples were transferred to separate wells of a 96-well filter plate containing either the phosphorylated or total ERK1/2 beads and incubated at 4 °C overnight. The following day the lysates were removed by vacuum filtration, the plate content was washed twice with assay buffer and 25 μl of biotin-labeled detection antibody was added. After a 2-h incubation at RT the detection antibody was removed by vacuum filtration. The individual samples were incubated with a mixture of 25 μl streptavidin-phycoerythrin and 25 μl cell signaling detection buffer at RT for 15 min. At the end of the incubation the reagents were removed by filtration, 150 μl assay buffer was added to each well and the immunoreactivity was detected using a Luminex (Austin, TX) plate reader.
Radiomitigation of the acute hematopoietic radiation syndrome in mice
Experimental procedures were reviewed and approved by the IACUC of the University of Tennessee Health Science Center. Ten-week old C57BL6 female mice (Charles River Laboratories International Inc., Wilmington, MA) were acclimated for one week in the vivarium. The mice were divided into two groups (15 animals/group) and exposed to 6.6 Gy γ-irradiation from the Cs137 γ-source at a dose-rate of ~320 cGy/min. Twenty four hours after the irradiation the animals were treated with either vehicle or 1 mg/kg GRI via intraperitoneal injection. The mice received no additional supportive care in this study. Animal survival was recorded twice daily up to 30 days, which was the endpoint of this experiment.
Determination of colony growth of irradiated MEF cells in soft agar cultures
Poly-HEMA-coated (Sigma-Aldrich) 6 well plates containing a 0.6% bottom agar layer and a 0.35% top agar layer were used. For these layers, Noble agar (BD, Franklin Lake, NJ) and full growth MEF medium without penicillin-streptomycin was used. Unirradiated and 15 Gy-γ-irradiated LPA2 MEF cells were mixed with the top agar layer (105 cell/ml) 2 h after the irradiation and 1 h after receiving vehicle or 10 μM GRI. The cells were fed every 5 days with fresh growth medium and colonies were counted 2 weeks later under a light microscope at 100 × magnification. A positive score was given to colonies with a diameter ≥ 500 μm. Results were expressed as mean number of colonies per well ± SD. MEFs overexpressing c-Myc and Tbx2 are immortal but not transformed [21]. Such MEF cells, a kind gift from Prof. Martejn van Lohuizen (Netherlands Cancer Institute, Amsterdam, NL), transduced with the Tbx2 transcriptional repressor with or without the oncogenic RasV12 mutant served as immortalized non-transformed controls and transformed controls, respectively in these experiments.
Gene expression profiling with real-time quantitative PCR (QPCR)
Relative transcript level of the following gene products was quantified using QPCR: LPA1/2/3/4/5/6, GPR87, P2Y10, GPR35 and autotaxin (ENPP2). A list of receptor-specific primers used in the QPCR experiments is included in supplemental Table 1. RNA isolation was performed using the TRIzol® reagent (Life Technologies, Grand Island, NY). cDNA synthesis was performed using the ThermoScript™ RT-PCR System (Life Technologies), and quantitative PCR was done with the RT2 RealTime™ SYBR Green/ROX PCR Master Mix kit (Qiagen Inc., Valencia, CA). Amplification was performed for 40 cycles consisting of 15 sec at 95 °C and 60 sec at 60 °C using an ABI Model 7300 Real-Time PCR machine (Life Technologies Corporation, Carlsbad, CA). To compare relative expression levels each sample was normalized on the basis of its GAPDH gene content, which was used as an internal control. Quantitative values were obtained from the threshold cycle value (Ct) using the Comparative Ct Method (ΔΔCt) as described previously [22].
Statistical analysis
Data are expressed as means ± SD or SEM. Experiments were performed at least three times unless otherwise indicated and statistical significance was determined using Student's t-test. P < 0.05 was regarded as statistically significant.
RESULTS
The LPA2 specific agonist GRI protects against γ-irradiation induced apoptosis
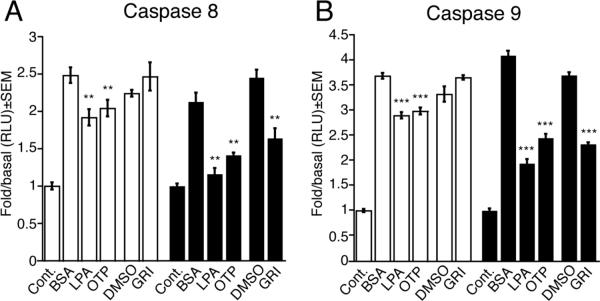
We have previously shown that GRI is a specific agonist of LPA2 (EC50 ~3.3 μM) and when applied at low micromolar concentrations (1–10 μM) protects cells from various forms of apoptosis induced by Adriamycin, serum–withdrawal, or TNFα treatments [18]. Here we have extended these studies to examine the antiapoptotic properties of GRI in different models of γ-irradiation induced apoptosis in vitro and in vivo. We first examined the effect of GRI on activation of the initiator caspase 9 induced by γ-irradiation using LPA2-transduced and control vector-transduced MEF cells, derived from LPA1 and LPA2 DKO mice. Serum-starved vector-transduced and LPA2 MEF cells irradiated with 15 Gy γ-irradiation were treated with 10 μM GRI, 3 μM LPA or 3 μM OTP one h after the irradiation. We chose the one h delay for the drug treatment to model radiomitigation as opposed to radioprotection. In this latter case the drug is applied as pretreatment to the cells. Caspase 9 activation was determined 5 h after drug treatment (Figure 2 A). The concentrations chosen were the same we previously found effective in other models of apoptosis [18]. GRI selectively protected LPA2 MEF cells and reduced caspase 9 activation by 37% ± 1% but was without any effect in the vector-transduced MEF cells. In contrast, LPA and OTP mitigated the effects of radiation-induced caspase 9 activation in both LPA2 MEF cells and the vector-transduced cells. LPA decreased caspase 9 activation in LPA2 MEF cells by 53% ± 2% compared to 22% ± 2% in vector-transduced cells. Administration of OTP resulted in a 40% ± 2% decrease in caspase 9 activation in LPA2 MEF cells and a 19% ± 2% decrease in vector-transduced cells (Fig. 2 A). These observations suggest that GRI mitigates γ-irradiation induced apoptosis via an LPA2 receptor-dependent inhibition of caspase 9, whereas LPA and OTP which are nonselective ligands of multiple LPA receptors have additional molecular targets expressed in the MEF cells derived from LPA1 and LPA2 double KO mice (see below).
Figure 2.
Inhibition of the initiator caspase 9 (Panel A) and caspase 8 (Panel B) in LPA1/2 double KO MEF cells transduced with empty vector (open bars) or the human LPA2 GPCR (filled bars). GRI (10 μM), LPA (3 μM) and OTP (3 μM) were added to the cells 1 h after irradiation with 15 Gy and caspase activation was measured 5 h later. Bars represent the mean of at least three independent experiments. (*p < 0.05, **p < 0.01, ***p < 0.001).
Kiss et al.
One example of the crosstalk between the intrinsic and extrinsic pathways of apoptosis is the activation of caspase 8, which serves as the initiator caspase of the extrinsic apoptotic pathway, during intrinsic apoptosis [23, 24]. We tested whether GRI-mediated inhibition of caspase 8 could play a role in the radiomitigation against γ-irradiation-induced apoptosis. Vector- or LPA2-transduced MEF cells were irradiated with 15 Gy γ-irradiation and treated with 10 μM GRI, 3 μM LPA, or 3 μM OTP one hour postirradiation and caspase 8 activation was detected 5 h after drug treatment. In LPA2 MEF cells irradiation-induced caspase 8 activation was mitigated by 33% ± 5% with GRI, by 34% ± 1% with OTP, and by 45% ± 4% in the presence of LPA. GRI did not alter caspase 8 activation in the vector-transduced cells. However, administration of LPA and OTP resulted in 23% ± 4% and 18% ± 4% mitigation of caspase 8 in vector-transduced MEF cells, respectively (Fig. 2 B). These data indicate that the radiomitigative antiapoptotic effect of GRI extends to γ-irradiation-induced caspase 8 and 9 inhibition via LPA2 receptor-mediated signaling.
GRI mitigates γ-irradiation-induced executioner caspase 3 and 7 activation
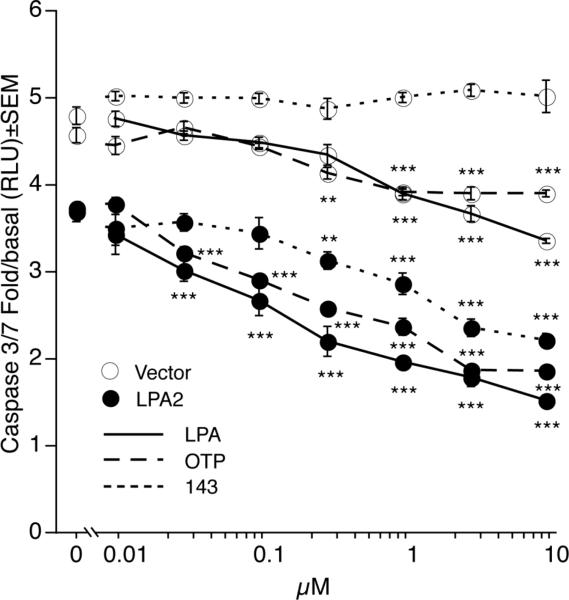
Activation of caspase 3 and 7 represent common steps in the final execution stage of the intrinsic and extrinsic apoptotic pathways by committing the cell to apoptosis [25]. Vector- or LPA2-transduced MEF cells were serum-starved, irradiated with 15 Gy and treated with increasing doses of GRI, LPA, and OTP in the 0.01 μM-10 μM concentration range 1 h postirradiation (Fig. 3). Caspase 3 and 7 activation were measured 5 h later. Administration of GRI dose-dependently protected LPA2 MEF cells by mitigating caspase 3 and 7 activation, which was significant at concentrations at and above 300 nM. The radiomitigative effects of LPA and OTP were detectable already at as low a concentration as 30 nM resulting in 19% ± 3 % and 14% ± 2% decrease in caspase 3 and 7 activation, respectively. Thus, based on this assay GRI was 3-fold less potent than LPA or OTP. The GRI compound did not alter caspase 3 and 7 activation in the vector-transduced cells whereas, LPA and OTP showed a dose dependent mitigation, although to a lesser degree than was observed in the LPA2-transduced MEFs (Fig. 3).
Figure 3.
Dose-response relationship of the inhibition of executioner caspases 3 and 7 by LPA, OTP and GRI in LPA1/2 double KO MEF cells transduced with empty vector (open symbols) or the human LPA2 GPCR (filled symbols). GRI significantly reduced caspase 3 and 7 activation significantly above 300 nM only in the LPA2 receptor-transduced cells, while the effect of LPA and OTP manifested in the vector-transduced cells as well. Data points represent the mean of at least three independent experiments. (* p < 0.05, ** p < 0.01, *** p < 0.001 compared to irradiated vehicle treated cells).
Kiss et al.
Effect of GRI on DNA fragmentation, Bax translocation and PARP-1 cleavage induced by γ-irradiation
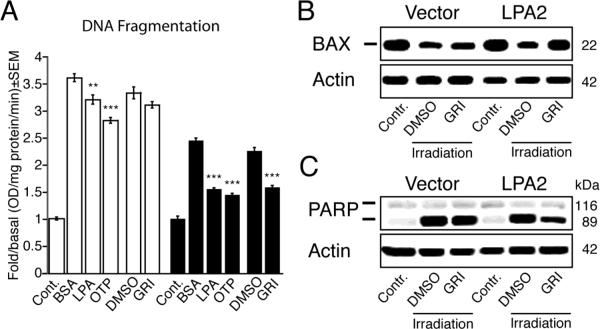
DNA fragmentation is recognized as one of the most characteristic biochemical features of apoptosis and occurs downstream of caspase 3 and 7 activation [26]. Serum-starved vector- and LPA2-transduced MEF cells irradiated with 15 Gy γ-irradiation were treated with 10 μM GRI, 3 μM LPA, or 3 μM OTP 1 h after irradiation. DNA fragmentation was determined 5 h later. GRI selectively protected LPA2 MEF cells reducing DNA fragmentation by 25% ± 2% relative to vehicle control. LPA decreased DNA fragmentation in LPA2 MEF cells by 37% ± 1%, whereas OTP decreased DNA fragmentation by 41% ± 1%. Both LPA and OTP had protective action in the vector-transduced cells reducing DNA fragmentation by 11% ± 2% and 22% ± 1%, respectively (Fig. 4 A). These data indicate that the LPA2 receptor-mediated antiapoptotic effect of GRI in the γ-irradiation-induced apoptosis model extends to the inhibition of DNA fragmentation in addition to inhibition of caspases 3, 7, 8 and 9.
Figure 4.
Inhibition of radiation-induced DNA fragmentation (panel A), Bax translocation (panel B) and PARP-1 cleavage (panel C) by LPA, OTP, and GRI in LPA1/2 double KO MEF cells transduced with empty vector (open bars on panel A) or the human LPA2 GPCR (filled bars on panel A). The concentrations of the ligands in the DNA fragmentation experiments was: GRI (10 μM), LPA (3 μM), and OTP (3 μM). Note that GRI selectively protected LPA2-transduced MEF cells compared with LPA or OTP (Panel A, * p < 0.05, ** p < 0.01, *** p < 0.001). γ-irradiation induced cytosolic Bax depletion (Panel B) and PARP-1 cleavage (Panel C) were effectively reduced by 10 μM GRI. Bars and western blots shown are representative of three experiments.
Kiss et al.
Bax translocation to the mitochondrion during intrinsic apoptosis occurs upstream of caspase 3 and 7 activation and DNA fragmentation [27, 28]. Because GRI effectively inhibited γ-irradiation induced initiator and effector caspase activation and DNA fragmentation, we next investigated its effect on Bax translocation to the mitochondria. Serum-starved vector- and LPA2-transduced MEF cells irradiated with 15 Gy γ-irradiation were exposed to vehicle or 10 μM GRI 1 h after the irradiation and Bax translocation was determined 5 h later. As shown in figure 4 B, GRI prevented γ-irradiation-induced Bax translocation to the mitochondria. This effect was absent in vector-transduced MEF cells and only seen in LPA2-transduced MEF cells. These findings indicate that in addition to inhibition of initiator and effector caspases, GRI inhibits γ-irradiation induced Bax translocation to the mitochondria, which is an early step of the intrinsic apoptosis cascade.
One of the several targets of caspase 3 and 7 during apoptosis is the DNA repair enzyme PARP-1, which is inactivated following cleavage by the executor caspases [29, 30]. Because GRI inhibited caspase 3 and 7 activation in γ-irradiated LPA2 MEF cells, we examined its effect on PARP-1 cleavage. Serum-starved vector- and LPA2-transduced MEF cells were irradiated with 15 Gy γ-irradiation and treated with 10 μM GRI 5 h after the irradiation. Cleavage of PARP-1 enzyme was determined 5 h later. GRI inhibited γ-irradiation-induced PARP-1 cleavage only in LPA2 MEF cells whereas in the vector-transduced cells GRI treatment had no effect (Fig. 4).
Radiomitigative effect of LPA and OTP is mediated mainly by LPA2 receptor
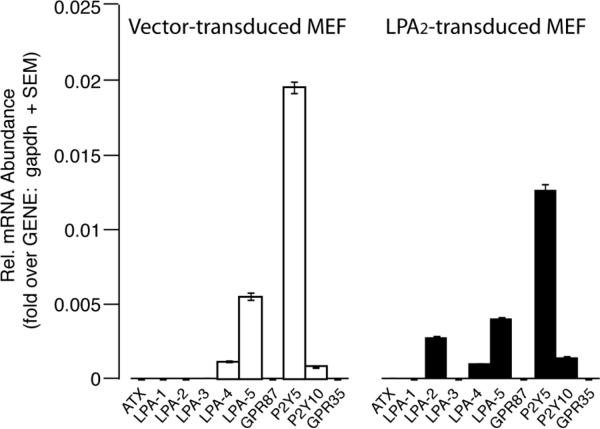
By using GRI, an LPA2 selective agonist, and MEF cells derived from LPA1&2 DKO mice and reconstituted with LPA2 receptor or empty vector, we demonstrated the unique role of LPA2 receptor in the protection against γ-irradiation. However, the small but significant radiomitigation detected in the vector-transduced cells in the presence of LPA and its non receptor selective mimic OTP suggested the existence of additional LPA receptors on the MEF cells. To test this hypothesis we performed quantitative real-time PCR using primers specific to LPA1/2/3/4/5/6 receptors, GPR87, P2Y10, GPR35 and autotaxin. As shown in figure 5, both vector- and LPA2-transduced MEF cells express appreciable amounts of LPA4/5/6 and P2Y10, which can explain the radiomitigating effect of LPA and OTP detected in the vector-transduced MEF cells. However the efficacy of radiomitigation mediated by one or more of these LPA receptors is much less than the protection mediated by the LPA2 receptor.
Figure 5.
Expression profiling of LPA receptor transcripts and autotaxin (ATX) by quantitative RT-PCR in MEF cells transduced with empty vector (open bars) or the human LPA2 receptor (filled bars). Note that the vector-transduced MEF cells express appreciable amounts of LPA4/5/6 and P2Y10 receptor transcripts. LPA GPCR expression was normalized to GAPDH gene content of the cells.
Kiss et al.
GRI protects against radiation-induced bystander apoptosis
It has been shown that irradiated cells either via soluble factors or by communication through gap junctions can induce changes in the neighboring intact cells, known as the “bystander radiation effect” or distant effects of radiation exposure, which could in turn lead to the apoptosis of these cells [31, 32]. Because GRI protected γ-irradiated LPA2 MEF cells against direct radiation-induced apoptosis, we extended our experiments to a model of γ-irradiation-induced bystander apoptosis. In this model [33] we used CM from irradiated U937 cells to elicit apoptosis in unirradiated IEC-6 cells that endogenously express multiple LPA GPCRs including LPA2 [13].
First, we determined the radiosensitivity of U937 cells by measuring γ-radiation-induced apoptosis through caspase 3 and 7 activation 24 h after irradiation with doses in the 7–55 Gy range (Fig. 6 A). Based on the radiation-dose response of U937 cells we chose the 35 Gy dose that caused a near maximal level of caspase 3 and 7 activation. Second, we tested the effect of CM collected from U937 cells irradiated with 7–35 Gy in IEC-6 cells by measuring caspase 3 and 7 activation 24 h after the exposure to CM (Fig. 6 B). CM was highly effective in eliciting apoptosis in nonirradiated IEC-6 cell cultures indicated by the activation of the main executional caspases 3 and 7. Third, we determined the effect of GRI on the bystander apoptosis using CM harvested from 35 Gy-irradiated U937 cells. The CM was supplemented with either 10 μM GRI, 10 μM LPA, or vehicle and transferred to nonirradiated IEC-6 cell cultures that were already serum-starved for 24 h. Bystander apoptosis of IEC-6 cells was determined 24 h later by measuring caspase 3 and 7 activation. The CM induced robust caspase 3 and 7 activation in the IEC-6 cells (Fig. 6 C) GRI decreased caspase 3 and 7 activation in the IEC-6 cells by 24% ± 3%, whereas LPA caused a 42% ± 5% inhibition (Fig. 6 C). These data highlight that stimulation of LPA2 receptor by GRI protects not only against direct radiation damage but also against the bystander apoptosis induced by proapoptotic mediators present in the CM.
Figure 6.
Effect of LPA and GRI on the bystander apoptosis elicited by conditioned medium of irradiated U937 cells. Radiation dose-dependence of caspase 3 and 7 activation in U937 cells (Panel A). Dose-response relationship of bystander apoptosis measured by caspase 3 and 7 activation in IEC-6 cells elicited by CM from γ-irradiated (7–35 Gy) U937 cells (Panel B). Effect of GRI (10 μM) and LPA (10 μM) on the bystander apoptosis of IEC-6 cells induced by 24-h CM from U937 cells irradiated with 35 Gy (Panel C). Bars are representative of three experiments. (* p < 0.05, ** p < 0.01, *** p < 0.001).
Kiss et al.
GRI activates prosurvival responses mediated by ERK1/2 kinases
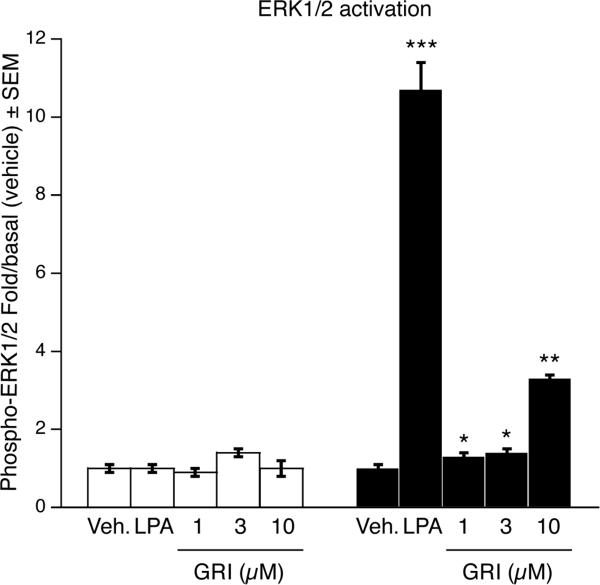
Activation of ERK1/2 has been shown to be a required step in the antiapoptotic action downstream of LPA2 receptor activation [13, 14]. We examined the activation of ERK1/2 kinases in vector- and LPA2-transduced MEF cells upon stimulation with GRI. The cells were serum starved for 2 h followed by 5-min treatment with 1 μM, 3 μM or 10 μM GRI, or 3 μM LPA as a positive control. Five min after treatment cells were harvested and a bead-based ELISA assay was performed to detect activation of ERK1/2 kinases. Administration of 10 μM GRI resulted in a significant 3.3-fold increase in the activation of ERK1/2 kinases in the LPA2-transduced MEF cells whereas no ERK1/2 activation could be detected in the vector-transduced cells (Fig. 7).
Figure 7.
Effect of GRI (1-3-10 μM) and LPA (3 μM) on ERK1/2 phosphorylation. GRI dose-dependently activated the ERK1/2 kinases in LPA2-transduced MEF cells (filled bars) and had no effect in the vector-transduced cells (open bars). Activation of ERK1/2 kinases was detected using ELISA beads against phospho- and total-ERK1/2 kinases and a Luminex® plate reader (* p < 0.05, ** p < 0.01 compared to vehicle).
Kiss et al.
GRI does not promote malignant transformation in γ-irradiated MEF cells
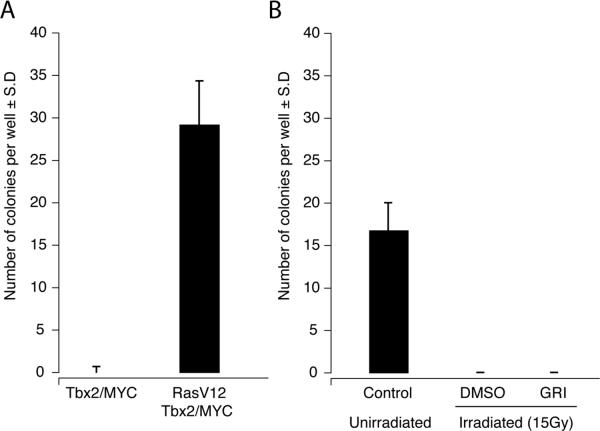
Ionizing radiation can lead to malignant transformation and tumorigenesis due to mutagenesis, mitotic stimulation, and alterations in the tissue microenvironment [34]. To examine whether the radiomitigative activity of GRI promotes malignant transformation in MEF cells rescued from γ-irradiation induced apoptosis, soft-agar assays were carried out. Unirradiated or 15 Gy γ-irradiated LPA2 MEF cells were treated with 10 μM GRI or vehicle and plated in soft-agar plates. Cells received fresh growth medium every 5 days and colonies were counted 2 weeks after plating. As shown in Fig. 8, GRI did not promote colony formation in irradiated LPA2-transduced MEF cells. In contrast, oncogenic V12Ras-transformed MEF cells showed a robust colony growth under these assay conditions.
Figure 8.
Effect of GRI (10 μM) on γ-irradiation-induced colony formation in soft-agar cultures of LPA2-transduced MEF cells. Panel A shows colony growth of Tbx2 and Tx2+V12 Ras transformed MEF in soft agar using conditions identical to that used in the experiment with LPA2-trasduced MEF in panel B. Note the robust colony growth of the Ras-transformed cells. In panel B, colony formation of unirradiated control LPA2-transduced MEF cells or 15 Gy γ-irradiated LPA2-transduced MEF cells treated with vehicle or 10 μM GRI was determined under a light microscope, two weeks after plating the cells in the soft agar. Note that colony growth was reduced by irradiation in the vehicle treated cells and GRI treatment did not enhance colony growth.
Kiss et al.
Effect of GRI on the hematopoietic acute radiation syndrome
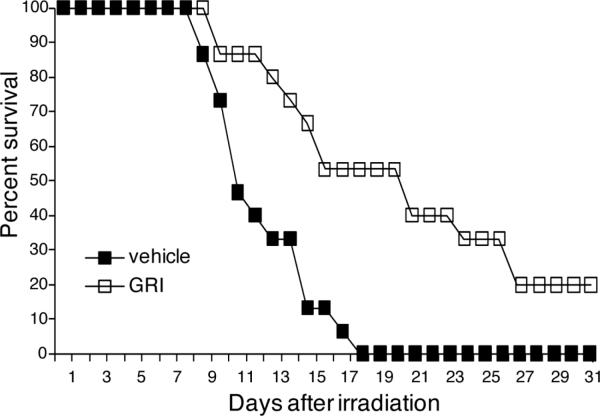
Targeting the LPA2 receptor, OTP has been shown to act as a radiomitigator and rescue irradiated mice against lethal doses of γ-irradiation [13]. Because the actions of GRI mimicked those of OTP in the γ-irradiation apoptosis model in vitro, we extended our study to a murine model of the acute hematopoietic radiation syndrome. Ten-weeks old C57BL6 mice were exposed to 6.6 Gy total body γ-irradiation (~LD100/20) and were treated with vehicle or a single 1 mg/kg subcutaneous dose of GRI 24 h postirradiation. Survival of the animals was recorded for a 30-day period. In the GRI treated group 50% of the animals survived until the 20th day and 20% of the animals survived until the 30th day post irradiation, which was the endpoint of the study. On the other hand, 50% of the untreated animals died by 11 days and 100% died by 19 days after irradiation (Fig. 9).
Figure 9.
Effect of GRI (1 mg/kg administered intraperitoneally at +24 h postirradiation) on mortality from the acute hematopoietic radiation syndrome in C57BL6 mice exposed to ~6.6 Gy γ-radiation at a dose rate of ~320 cGy/min. GRI significantly increased mean survival time (p < 0.01) and decreased mortality in treated mice compared to the vehicle group. Fifty % of the animals in the GRI treated group (open squares) survived up to 20 days and 20% survived up to 31 days after γ-irradiation (~LD80/30) compared to the vehicle-treated group (open squares).
Kiss et al.
DISCUSSION
The first objective of this article was to give an account of the evolution of the concept that ligand activation of the LPA2 receptor can mitigate radiation injury in vitro and in vivo. Over the past decade, starting with the natural ligand LPA we have systematically investigated the effect of LPA2 GPCR stimulation to find that it attenuates various forms of apoptosis including those caused by direct exposure to ionizing radiation or indirect exposure to products released by irradiated cells known as bystander apoptosis. A pivotal discovery we made during these studies was the recognition that LPA stimulation of cells can prevent not only apoptosis but also rescue apoptotically condemned cells if given within hours after radiation exposure in vitro. This observation provided the foundation for exploring the radiomitigative actions of LPA analogs that can now provide radiomitigation when applied even at 24 h after radiation exposure in mice.
A second objective of our paper was to give an insight into ongoing research in our laboratory aimed at developing a second generation of LPA-based radioprotective/radiomitigative pharmacons. This development strategy is based on our successes with OTP, a metabolically stabilized LPA analog with a long biological half life in excess of 10 h [35].
Through an in silico drug discovery study we have been able to identify several nonlipid scaffolds that are specific agonists of LPA2 and show efficacy against apoptosis induced by Adriamycin, serum withdrawal, or TNFα treatments [18]. One of these primary hit compounds, GRI977143, was less potent than but equally efficacious as LPA or OTP in attenuating caspase 3 and 7 activation when applied at a 3-fold higher concentration (Fig. 3). Our experiments showed that by activating the LPA2 receptor GRI effectively reduces cytosolic Bax translocation, activation of initiator and effector caspases, DNA fragmentation and PARP-1 cleavage associated with γ-irradiation induced intrinsic apoptosis. These actions of GRI were only detectable in MEF cells transduced by the human LPA2 receptor and were completely absent in vector-transduced MEFs. In contrast to GRI, we have also noticed that LPA and OTP had a slight attenuating effect in vector-transfected MEF cells. Quantitative RT-PCR analysis showed that the MEF cells derived from LPA1 and LPA2 double KO mouse embryos express appreciable amounts of LPA4/5/6 and P2Y10, which can explain the radiomitigating effect of LPA and OTP (Fig. 5). Even though GRI is less efficacious than LPA in activating Ca2+-mobilization via LPA2 (EC50= 3.3 μM vs. 0.03 μM, respectively, [18]), GRI was highly effective in protecting LPA2-transduced MEF cells compared to the protection exerted by LPA in vector-transduced cells. Both, the LPA2-specific GRI compound and the pan agonist LPA showed the highest level of protection in LPA2 expressing MEFs. This comparison suggests that activation of LPA2 provides effective radiomitigation compared to the activation of the other LPA receptor subtypes expressed in these MEF cells and activated by LPA. Nonetheless, this observation requires further studies with MEF cells expressing each individual LPA GPCR subtype.
We also examined the effect of LPA and GRI in a model of radiation-induced bystander apoptosis in vitro. This model has relevance to the radiomitigative action of LPA analogs because in the animal experiments the LPA analogs were not present during the first 24 h postirradiation when the initial wave of radiation-elicited apoptosis takes place. Nevertheless, administration of OTP or GRI at +24 h postirradiation is effective in protecting the lives of the animals. We hypothesize that GRI-mediated activation of LPA2 receptors in the tissues exerts some of its protective action by attenuating bystander effects of radiation injury that occur 24 – 48 h post injury and are possibly mediated by agents similar to those present in the CM of irradiated U937 cells in our in vitro model [33]. This observation prompts further research toward the identification of these soluble mediators that could passively transfer apoptosis from U937 cells to unirradiated IEC-6 cells. Whether these factors are also present in the biological fluids of irradiated animals becomes a very intriguing question. Taken together, our results obtained with GRI in the different γ-irradiation injury models consistently suggest that this compound exerts a radiomitigative action and is capable of rescuing apoptotically condemned cells in vitro and in vivo. In this context we were surprised to find that malignant transformation of the irradiated and rescued MEF cells did not show enhancement after GRI treatment (Fig. 8). This observation will need to be followed up in vivo but already hints that GRI-treated cells have been able to repair DNA damage that otherwise could have led to a high-rate of transformation revealed by growth in soft agar.
CONCLUSIONS
These observations with the initial characterization of GRI should be taken as a prelude to a more in depth analysis of the action of LPA2-specific pharmacons in radiation-induced or other forms of apoptosis. We remain enthusiastic about these initial results as they provide the first line of evidence that selective pharmacological stimulation of the LPA2 receptor subtype is sufficient to protect against the acute hematopoietic radiation syndrome in vivo. We also recognize that GRI is a weak, although specific LPA2 agonist, which must undergo lead optimization to yield radiomitigative drug candidates suitable for human use. These studies are currently ongoing in our laboratories.
Supplementary Material
HIGHLIGHTS
The nonlipid compound GRI977143 is a specific agonist of the LPA2 receptor subtype
GRI977143 rescues apoptotically condemned cells from radiation-induced programmed cell death
GRI977143 reduces mortality of mice exposed to lethal levels of γ-irradiation
ACKNOWLEDGMENTS
This research was supported by a grant (AI80405) from the National Institutes of Health, National Institute of Allergy Medical Countermeasures Radiological and Nuclear Threats Program and by the Van Vleet Endowment for Basic Oncology Research. The authors thank Dr. Jerold Chun (Scripps Institute) for providing the LPA1 and LPA2 mice, Dr. William Seibel (University of Cincinnati) for his assistance with the similarity search of the UC-DCC library leading to the identification of LPA2-specific hits, Dr. Martejns van Lohuizen (Netherlands Cancer Institue) for the Tbx2 and Ras transformed MEF cells, and Drs. Andrea DiCarlo-Cohen and Bert Maidment at the National Institute of Allergy and Infectious Diseases for their encouragement and support.
ABBREVIATIONS
- Akt
protein kinase B
- Bax
Bcl-2-associated X protein
- Bcl-XL
B-cell lymphoma XL
- BSA
bovine serum albumin
- CM
conditioned medium
- Cs
Cesium
- Ct
threshold cycle value
- DMEM
Dulbeco's modified Eagle medium
- DMSO
dimethyl sulfoxide
- DlgA
Drosophila disc large tumor suppressor
- E2F
E2F transcription factor
- EGFR
Endothelial growth factor receptor
- ENPP2
autotoxin
- ErbB2
v-erb-b2 erythroblastic leukemia viral oncogene homolog 2
- ERK1/2
extracellular signal regulated kinases 1/2
- FBS
fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GPCR
G protein-coupled receptor
- GRI
Genome Research Institute
- HRPO
Horseradish peroxidase
- IACUC
Institutional Animal Care and Use Committee
- IEC-6
intestinal epithelial cell line 6
- KO
knockout
- LIM
Lin-11, Isl-1 and Mec-3 proteins
- LPA
lysophosphatidic acid
- MEF
mouse embryonic fibroblast
- MEKK
Map-E kinase kinase
- NFκB
nuclear factor κB
- NHERF2
Na+-H+ exchange regulatory factor 2
- OTP
octadecenyl thiophosphate
- PARP-1
poly (ADP-ribose) polymerase 1
- PBS
phosphate-buffered saline
- PDGF
Platelet derived growth factor
- PDZ
PSD95/Dlg/ZO-1 domain
- p53
protein 53
- PI3K
Phosphoinositide-3-kinase
- poly-HEMA
poly(2-hydroxyethyl methacrylate)
- PSD95
postsynaptic density protein 95
- QPCR
real-time quantitative polymerase chain reaction
- RH7777
McArdle rat hepatoma cell line
- RT
room temperature
- S1P
sphingosine-1-phosphate
- TNFα
Tumor necrosis factor α
- TRIP6
thyroid receptor interacting protein 6
- UC-DDC
University of Cincinnati Drug Discovery Center
- U937
human monocyte lymphoma cell line
- Zn
Zinc
- ZO-1
Zonula Occludens 1 protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiation research. 2011;175:405–415. doi: 10.1667/RR2461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nambiar D, Rajamani P, Singh RP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011;728:139–157. doi: 10.1016/j.mrrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- [3].Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- [4].Fujiwara Y, Osborne DA, Walker MD, Wang DA, Bautista DA, Liliom K, Van Brocklyn JR, Parrill AL, Tigyi G. Identification of the hydrophobic ligand binding pocket of the S1P1 receptor. J Biol Chem. 2007;282:2374–2385. doi: 10.1074/jbc.M609648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- [6].Parrill AL, Wang D, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate. J Biol Chem. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- [7].Valentine WJ, Godwin VI, Osborne DA, Liu J, Fujiwara Y, Van Brocklyn J, Bittman R, Parrill AL, Tigyi G. FTY720 (Gilenya) phosphate selectivity of sphingosine 1-phosphate receptor subtype 1 (S1P1) G protein-coupled receptor requires motifs in intracellular loop 1 and transmembrane domain 2. The Journal of biological chemistry. 2011;286:30513–30525. doi: 10.1074/jbc.M111.263442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang DA, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. A single amino acid determines lysophospholipid specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. J Biol Chem. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- [9].Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg Med Chem Lett. 2006;16:633–640. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- [10].Virag T, Elrod DB, Liliom K, Sardar VM, Parrill AL, Yokoyama K, Durgam G, Deng W, Miller DD, Tigyi G. Fatty Alcohol Phosphates are Subtype-Selective Agonists and Antagonists of LPA Receptors. Mol Pharmacol. 2003;63:1032–1042. doi: 10.1124/mol.63.5.1032. [DOI] [PubMed] [Google Scholar]
- [11].Deng W, Poppleton H, Yasuda S, Makarova N, Shinozuka Y, Wang DA, Johnson LR, Patel TB, Tigyi G. Optimal lysophosphatidic acid-induced DNA synthesis and cell migration but not survival require intact autophosphorylation sites of the epidermal growth factor receptor. J Biol Chem. 2004;279:47871–47880. doi: 10.1074/jbc.M405443200. [DOI] [PubMed] [Google Scholar]
- [12].Deng W, Wang DA, Gosmanova E, Johnson LR, Tigyi G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am J Physiol Gastrointest Liver Physiol. 2003;284:G821–829. doi: 10.1152/ajpgi.00406.2002. [DOI] [PubMed] [Google Scholar]
- [13].Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, Johnson LR, Parrill AL, Miller DD, Tigyi G. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, Lin FT. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lin FT, Lai YJ, Makarova N, Tigyi G, Lin WC. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J Biol Chem. 2007;282:37759–37769. doi: 10.1074/jbc.M705025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Furui T, LaPushin R, Mao M, Khan H, Watt SR, Watt MA, Lu Y, Fang X, Tsutsui S, Siddik ZH, Bast RC, Mills GB. Overexpression of edg-2/vzg-1 induces apoptosis and anoikis in ovarian cancer cells in a lysophosphatidic acid-independent manner. Clin Cancer Res. 1999;5:4308–4318. [PubMed] [Google Scholar]
- [18].Kiss GN, J.I. F, Gupte R, Lee S-C, Liu J, Nusser N, Ray RM, Lin F-T, Parrill AL, Sümegi B, Miller DD, Tigyi GJ. Virtual Screening for LPA2-Specific Agonists Reveals Nonlipid Compounds with Antiapoptotic Actions. Mol. Pharm. 2012 doi: 10.1124/mol.112.079699. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ray RM, Bhattacharya S, Johnson LR. Mdm2 inhibition induces apoptosis in p53 deficient human colon cancer cells by activating p73- and E2F1-mediated expression of PUMA and Siva-1. Apoptosis. 2011;16:35–44. doi: 10.1007/s10495-010-0538-0. [DOI] [PubMed] [Google Scholar]
- [20].Valentine WJ, Kiss GN, Liu J, E S, Gotoh M, Murakami-Murofushi K, Pham TC, Baker DL, Parrill AL, Lu X, Sun C, Bittman R, Pyne NJ, Tigyi G. (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. Cell Signal. 2010;22:1543–1553. doi: 10.1016/j.cellsig.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taghavi P, Verhoeven E, Jacobs JJ, Lambooij JP, Stortelers C, Tanger E, Moolenaar WH, van Lohuizen M. In vitro genetic screen identifies a cooperative role for LPA signaling and c-Myc in cell transformation. Oncogene. 2008;27:6806–6816. doi: 10.1038/onc.2008.294. [DOI] [PubMed] [Google Scholar]
- [22].Guo H, Makarova N, Cheng Y, E S, Ji RR, Zhang C, Farrar P, Tigyi G. The early- and late stages in phenotypic modulation of vascular smooth muscle cells: differential roles for lysophosphatidic acid. Biochim Biophys Acta. 2008;1781:571–581. doi: 10.1016/j.bbalip.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramidea nd etoposide-induced apoptosis. J Biol Chem. 2004;279:40755–40761. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- [24].Vit JP, Guillouf C, Rosselli FF. Futile caspase-8 activation during the apoptotic cell death induced by DNA damaging agents in human B-lymphoblasts. Exp Cell Res. 2001;269:2–12. doi: 10.1006/excr.2001.5284. [DOI] [PubMed] [Google Scholar]
- [25].Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Widlak P, Garrard WT. Roles of the major apoptotic nuclease-DNA fragmentation factor-in biology and disease. Cell Mol Life Sci. 2009;66:263–274. doi: 10.1007/s00018-008-8472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ghibelli L, Diederich M. Multistep and multitask Bax activation. Mitochondrion. 2010;10:604–613. doi: 10.1016/j.mito.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [28].Walensky LD, Gavathiotis E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–454. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- [30].Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair. 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- [31].Rzeszowska-Wolny J, Przybyszewski WM, Widel M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur J Pharmacol. 2009;625:156–164. doi: 10.1016/j.ejphar.2009.07.028. [DOI] [PubMed] [Google Scholar]
- [32].Baskar R. Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis. Genome Integr. 2010;1:13. doi: 10.1186/2041-9414-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim EM, Yang HS, Kang SW, Ho JN, Lee SB, Um HD. Amplification of the gamma-irradiation-induced cell death pathway by reactive oxygen species in human U937 cells. Cell Signal. 2008;20:916–924. doi: 10.1016/j.cellsig.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [34].Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- [35].Kosanam H, Ma F, He H, Ramagiri S, Gududuru V, Tigyi GJ, Van Rompay K, Miller DD, Yates CR. Development of an LC-MS/MS assay to determine plasma pharmacokinetics of the radioprotectant octadecyl thiophosphate (OTP) in monkeys. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2379–2383. doi: 10.1016/j.jchromb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.