Abstract
Interleukin (IL)-6, a cytokine featuring redundancy and pleiotropic activity, contributes to host defense against acute environmental stress, while dysregulated persistent IL-6 production has been demonstrated to play a pathological role in various autoimmune and chronic inflammatory diseases. Targeting IL-6 is thus a rational approach to the treatment of these diseases. Indeed, clinical trials of tocilizumab, a humanized anti-IL-6 receptor antibody have verified its efficacy and tolerable safety for patients with rheumatoid arthritis, Castleman's disease and systemic juvenile idiopathic arthritis, resulting in approval of this innovative biologic for treatment of these diseases. Moreover, a considerable number of case reports and pilot studies of off-label use of tocilizumab point to the beneficial effects of tocilizumab for a variety of other phenotypically different autoimmune and chronic inflammatory diseases. Elucidation of the source of IL-6 and of mechanisms through which IL-6 production is dysregulated can thus be expected to lead to clarification of the pathogenesis of various diseases.
Keywords: interleukin-6, a humanized anti-interleukin-6 receptor antibody, tocilizumab, autoimmune, inflammation.
Introduction
Interleukin-6 (IL-6), initially designated as a B cell differentiation factor 1, is a representative cytokine featuring redundancy and pleiotropic activity 2-4. In the early phase of infectious inflammation, IL-6 is produced by monocytes and macrophages immediately after the stimulation of Toll-like receptors (TLRs) with distinct pathogen-associated molecular patterns (PAMPs) 5. In noninfectious inflammations, such as burn or traumatic injury, damage-associated molecular patterns (DAMPs) from damaged or dying cells stimulate TLRs to produce IL-6 6. This acute IL-6 expression plays a central role in host defense by stimulating various cell populations. When acting on hapatocytes, IL-6 strongly induces a broad spectrum of acute-phase proteins such as C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, hepcidin, haptoglobin, and antichymotrypsin, whereas it reduces albumin, cytochrome P 450, fibronectin, and transferrin 7, 8 (Figure 1).
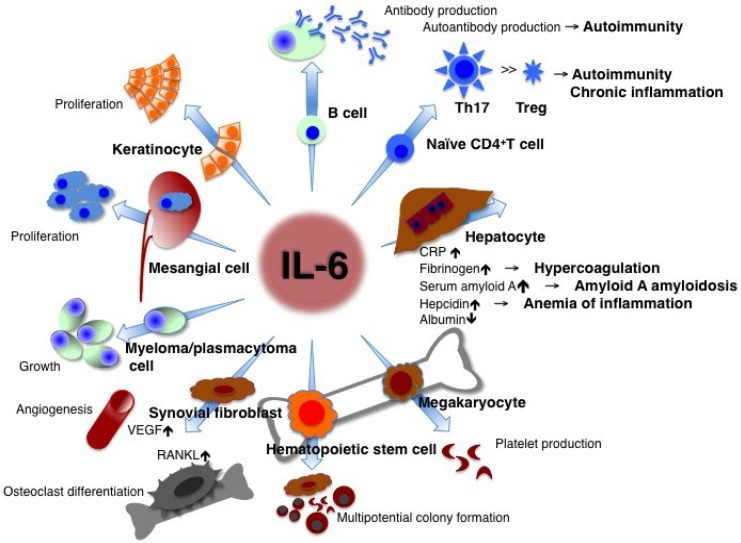
Figure 1.
IL-6 has a pleiotropic effect but its dysregulated persistent production causes the onset and development of various autoimmune and chronic inflammatory diseases. IL-6 is originally found as a B cell stimulatory factor-2, which induces activated B cells into antibody production. IL-6, combined with TGF-β, preferentially induces the differentiation of naïve CD4 positive T cells into Th17 cells whereas IL-6 inhibits TGF-β induced regulatory T cell (Treg) development. As a consequence, Th17/Treg imbalance may cause the onset and progression of autoimmune and chronic inflammatory diseases. IL-6 induces production of acute-phase proteins such as CRP, fibrinogen, serum amyloid A, and hepcidin, whereas it reduces synthesis of albumin in hepatocytes. High persistent levels of serum amyloid A and hepcidin lead to amyloid A amyloidosis and anemia of inflammation, respectively. In bone marrow, IL-6 induces maturation of megakaryocytes into platelets and activation of hematopoietic stem cells. In addition, IL-6 promotes the differentiation of osteoclasts and angiogenesis, the proliferation of keratinocytes and mesangial cells, and the growth of myeloma and plasmacytoma cells. Treg: regulatory T cells; CRP: C-reactive protein; VEGF: vascular endothelial growth factor; RANKL: receptor activator of NF-kappaB ligand.
CRP is a good biomarker of inflammation and is used as such in clinical laboratory tests. Its expression mainly depends on IL-6 9. If the free concentration of the anti-interleukin 6 receptor antibody, tocilizumab is maintained in serum at more than 1 μg/ml, CRP remains negative 10, so that the serum CRP level is a hallmark for checking whether IL-6 activity is completely blocked in vivo. Continuously high levels of hepcidin induced by IL-6 block iron transporter ferroportin 1 in macrophages, hepatocytes, and gut epithelial cells and lead to hypoferremia and anemia of chronic inflammation 11, whereas long-term high levels of SAA result in amyloid A amyloidosis 12. In lymphocytes, IL-6 induces B cell differentiation into immunoglobulin-producing cells 1. When CD4-positive naïve T cells are primed, a specific cytokine prompts their differentiation into an effector T cell subset. IL-6 together with TGF-β preferentially promotes differentiation of IL-17-producing T helper cells (Th17) that play a crucial role in the induction of autoimmune tissue injury, whereas IL-6 inhibits TGF-β-induced regulatory T cell (Treg) differentiation 13, 14. The resultant Th17/Treg imbalance leads to breakage of immunological tolerance and is of pathological importance for the development of various autoimmune and chronic inflammatory diseases 15. IL-6 also induces CD8-positive T cells to generate cytotoxic T cells 16. The function of IL-6 in hematopoiesis is to induce maturation of megakaryocytes into platelets as well as activation of hematopoietic stem cells 17. IL-6 production in bone marrow stromal cells generates the receptor activator of NF-kappaB ligand (RANKL), which is an essential factor for the differentiation and activation of osteoclasts and bone resorption, thus leading to osteoporosis 18. Enhanced angiogenesis and increased vascular permeability are pathological features of inflammation, and these characteristics are due to the excess production of vascular endothelial growth factor (VEGF), which is induced by IL-6 in inflamed lesions such as seen in synovium tissue of rheumatoid arthritis 19. The promotional activities of IL-6, such as the proliferation of keratinocytes or collagen production in dermal fibroblasts, may contribute to autoimmune skin diseases including psoriasis and systemic sclerosis 20, 21. Furthermore, IL-6 stimulates the growth of cells such as myeloma/plasmacytoma cells and mesangial cells 22-24.
IL-6 triggers signal transduction after binding to the IL-6 receptor (IL-6R) 25, 26. There are two forms of IL-6R, a transmembrane 80-kDa form with a short cytoplasmic domain and a soluble form (sIL-6R). After binding of IL-6 to transmembrane IL-6R, the resultant IL-6/IL-6R complex associates with gp130 27-29, and the activated IL-6 receptor complex is formed as a hexameric structure consisting of two molecules each of IL-6, IL-6R and gp130 (so-called a classical signaling) 30, 31. The expression of transmembrane IL-6R is limited to a few cell types but the IL-6/sIL-6R complex can also transduce the IL-6 signal to various cells which do not express transmembrane IL-6R but express gp130 (known as a trans-signaling mechanism) 32, so that IL-6 affects a wide variety of cells.
Pathological Role of IL-6 in Development of Diseases
When IL-6 is synthesized transiently, it promptly participates in the host defense against environmental stress such as infection and injury and at the same time provides an SOS (warning) signal by triggering a broad spectrum of biological events. Once the source of stress is removed from the host, IL-6-mediated activation of the signal transduction cascade is terminated by negatively regulatory systems in conjunction with the normalization of serum IL-6 and CRP levels. However, dysregulated persistent IL-6 production has been implicated in the development of various autoimmune, chronic inflammatory diseases and even cancers 2-4, 33. The reason(s) why such dysregulated continuous IL-6 production is induced remains to be clarified and elucidation of the mechanism(s) underlying persistent IL-6 synthesis in diseases is of particular importance to make their pathogenesis clear. It was found that in human immunodeficiency virus (HIV)-positive cases of multicentric Castleman's diseases all patients were infected with the Kaposi sarcoma-associated herpes virus (KSHV) and that sustained synthesis of both virus-derived IL-6, which directly binds to and stimulates human gp130, and of host-derived human IL-6 contribute to the development of the disease 34.
Moreover, numerous animal models of diseases have also disclosed the pathologic role of IL-6 in disease development and that IL-6 blockade by means of gene-knockout or administration of anti-IL-6 or anti-IL-6R antibody can suppress such disease development either preventively or therapeutically. For example, IL-6 blockade strategy demonstrably limited susceptibility to Castleman's disease-like symptoms in IL-6 transgenic mice 35, as well as in various mouse models of rheumatoid arthritis 36-48, systemic lupus erythematosus 49-51, scleroderma 52, 53, C-peptide-induced myositis 54, experimental autoimmune uveoretinitis 55, 56, experimental autoimmune encephalomyelitis 57, and many other diseases.
Targeting IL-6: All the Way to Treat Autoimmune and Inflammatory Diseases
Because of the pathological role of IL-6 in various diseases, blockade of IL-6 was expected to constitute a novel treatment strategy for these diseases 3, 58, 59. Consequently, a humanized anti-human IL-6R monoclonal antibody (chemical name: tocilizumab, generic name: Actemra outside of the EU or RoActemra inside the EU) was developed, by grafting the complementarity-determining regions of a mouse anti-human IL-6R antibody onto human IgG1. The resultant tocilizumab then blocks IL-6-mediated signal transduction by inhibiting IL-6 binding to transmembrane and soluble IL-6 receptors.
Clinical trials of tocilizumab have demonstrated its outstanding efficacy for rheumatoid arthritis 60-66, systemic juvenile idiopathic arthritis 67-71 and Castleman's disease 72, 73. For patients with moderately to severely active rheumatoid arthritis, tocilizumab is now being used as an innovative drug in more than 90 countries worldwide. As a monotherapy or in combination with disease-modifying antirheumatic drugs, it has significantly suppressed the disease activity and radiographically detected progression of joint deformity, thus improving daily functional activity. Tocilizumab was also approved as the first line biologic for the treatment of systemic juvenile idiopathic arthritis in Japan, India, USA and EU and for Castleman's disease in Japan and India.
Furthermore, favorable results of recent pilot studies, case series or case studies have suggested that tocilizumab may have broad application for other diseases. These diseases include systemic autoimmune diseases such as systemic lupus erythematosus 74-76, systemic sclerosis 77, polymyositis 78, vasculitis syndrome including giant cell arteritis 79-84, Takayasu arteritis 79, 82, 85-87, cryoglobulinemia 88, myeloperoxidase-antineutrophil cytoplasmic antibody-associated crescentic glomerulonephritis 89 and rheumatoid vasculitis 90. The application of tocilizumab may also extend to organ-specific autoimmune diseases including Crohn's disease 91, relapsing polychondritis 92, 93, acquired hemophilia A 94, autoimmune hemolytic anemia 95, 96, as well as to chronic inflammatory diseases such as adult-onset Still's disease 97-113, amyloid A amyloidosis 114-120, polymyalgia rheumatica 79, 84, 121, remitting seronegative symmetrical synovitis with pitting edema 122, Behcet's disease 123, 124, uveitis 125, graft-versus-host diseases 126, 127, and tumor necrosis factor receptor-associated periodic syndrome 128 (Table 1). Some studies have reported that tocilizumab is efficacious for spondyloarthritis 129-135, although others observed only minor effects 136-138. In addition, tocilizumab is reportedly effective for pulmonary arterial hypertension 139-141, atopic dermatitis 142, and sciatica 143. Finally, it was observed that during tocilizumab treatment of patients with rheumatoid arthritis, HbA1c levels and insulin resistance indices such as the homeostasis model assessment of insulin resistance (HOMA-IR) and the leptin-to-adiponectin ratio improved 144, 145, while serum levels of reactive oxygen metabolites decreased 146. It can thus be expected that long-term tocilizumab treatment may offer protection against the progression of atherosclerosis leading to cardiovascular events 147. Indeed, large-scale genetic analyses demonstrated a causal association between IL-6R-related pathways and coronary heart disease 148, 149, and a randomized, open-label, parallel-group, multicenter study to evaluate the rate of cardiovascular events of tocilizumab in comparison to a TNF inhibitor, etanercept in patients with rheumatoid arthritis (ClinicalTrials.gov, Identifier: NCT01331837) is now in progress. To establish broad clinical indications for tocilizumab for various diseases, however, further clinical studies will be needed to verify its efficacy and safety. The current clinical trials are listed in Table 2.
Table 1.
Application of IL-6 blockade strategy for various autoimmune and chronic inflammatory diseases.
| Approved or candidate diseases | References | |
|---|---|---|
| RA | > 90 countries worldwide* | 60-66 |
| Systemic JIA | Japan, India, USA and EU* | 67-71 |
| Castleman's disease | Japan and India* | 72,73 |
| SLE | Phase I, case reports | 74-76 |
| Systemic sclerosis | Case series | 77 |
| Polymyositis | Case series | 78 |
| Vasculitis syndrome | Case report & series | 79-90 |
| Crohn's disease | Pilot randomized trial | 91 |
| Relapsing polychondritis | Case reports | 92,93 |
| Acquired hemophilia A | Case report | 94 |
| Autoimmune hemolytic anemia | Case reports | 95,96 |
| Adult-onset Still's disease | Case reports & series | 97-113 |
| Amyloid A amyloidosis | Case reports | 114-120 |
| Polymyalgia rheumatica | Case reports & series | 79,84,121 |
| RS3PE | Case report | 122 |
| Behcet's disease | Case reports | 123,124 |
| Uveitis | Case report | 125 |
| GVHD | Case report & series | 126,127 |
| TRAPS | Case report | 128 |
| Spondyloarthritides | Case reports | 129-135 |
| Pulmonary arterial hypertension | Case reports | 139-141 |
| Atopic dermatitis | Case series | 142 |
| Sciatica | Case series | 143 |
Tocilizumab, a humanized anti-IL-6 receptor antibody, has been approved* as a biological drug for the treatment of RA, Castleman's disease and systemic JIA, and is expected to be applicable to various other autoimmune and chronic inflammatory diseases. RA: rheumatoid arthritis; JIA: juvenile idiopathic arthritis; SLE: systemic lupus erythematosus; RS3PE: remitting seronegative, symmetrical synovitis with pitting edema; GVHD: graft-versus-host disease; TRAPS: tumor necrosis factor-associated periodic syndrome.
Table 2.
Clinical trials of tocilizumab for diseases other than RA.
| Targeted diseases | Identifier |
|---|---|
| ClinicalTrials.gov (USA) | |
| Adult-onset Still's disease | NCT01002781 |
| Relapsing polychondritis | NCT01041248 |
| Type II diabetes, obesity | NCT01073826 |
| Ankylosing spondylitis | NCT01209702 |
| Graves' ophthalmopathy | NCT01297609 |
| Cardiovascular disease in rheumatoid arthritis | NCT01331837 |
| Polymyalgia rheumatica | NCT01396317 |
| Giant cell arteritis | NCT01450137 |
| Acute GVHD | NCT01475162 |
| Non-ST elevation myocardial infarction | NCT01491074 |
| Systemic sclerosis | NCT01532869 |
| Transplant rates in highly sensitized patients awaiting kidney transplantation | NCT01594424 |
| EU Clinical Trials Registry | |
| Ankylosing spondylitis | 2009-017488-40 |
| 2009-017443-34 | |
| Cardiovascular disease in rheumatoid arthritis | 2010-020065-24 |
| Graves' ophthalmopathy | 2010-023841-31 |
| Systemic sclerosis | 2011-001460-22 |
| UMIN-CTR clinical trials (Japan) | |
| ANCA-associated vasculitis | UMIN000002892 |
| Systemic sclerosis | UMIN000005550 |
| Neuromyelitis optica | UMIN000005889 |
| UMIN000007866 | |
| Chronic glomerulonephritis | UMIN000006080 |
| Colorectal cancer | UMIN000007493 |
| Takayasu arteritis | UMIN000007845 |
Current clinical trials of tocilizumab in the USA, EU and Japan are listed. GVHD: graft-versus-host disease; ANCA: anti-neutrophil cytoplasmic antibody.
On the basis of the pathologic role of IL-6 and the outstanding beneficial effect of tocilizumab, targeting IL-6 is a rational strategy for the treatment of various diseases and other biologics of IL-6 inhibitors are also being developed 32. These include fully human anti-IL-6R, anti-IL-6R nanobody, anti-IL-6 antibody, and anti-IL-6/anti-IgG avimer protein consisting of the IgG-binding domain fused to the N-terminus of a 3-domain IL-6 binding region, which results in a 19-kDa heterotetrameric avimer. These novel biologics block IL-6-mediated both classical and trans-signaling pathway by inhibiting IL-6 binding to both transmembrane and soluble IL-6R. By contrast, the fusion protein soluble gp130-Fc selectively targets IL-6/sIL-6R trans-signaling pathway. It is hypothesized that IL-6 trans-signaling is a local and temporal danger signal with fewer and less important physiological functions under non-stressed conditions than classical signaling on the basis of several animal models 32, 150.
Future Directions
IL-6 participates in the host defense against environmental pathogens, whereas dysregulation of IL-6 production has been implicated in the development of various autoimmune and chronic inflammatory diseases 2-4, 33. The pleiotropic activity of IL-6 also indicates that IL-6 blockade represents a rational treatment strategy for various diseases. A good example of the efficacy of such treatment is the dramatic improvement engendered by tocilizumab in amyloid A amyloidosis and anemia of inflammation through inhibition of their respective responsible proteins, SAA and hepcidin synthesis 151, 152. However, the mechanisms through which tocilizumab exerts its therapeutic effects on various phenotypically different autoimmune and inflammatory diseases are not yet well understood. In recent years, it has been shown that Th17 and/or Th1 >> Treg causes the onset of various autoimmune and chronic inflammatory diseases 14, 15. IL-6, in combination with TGF-β, promotes the differentiation of naïve T cells into Th17, but inhibits TGF-β-induced Treg differentiation, indicating that IL-6 is a very important factor for determining the Th17/Treg balance 14. Dysregulated IL-6 production leads to predominance of Th17 over Treg but anti-IL-6R antibody can repair this imbalance. It has been demonstrated in several animal disease models that IL-6 blocking suppresses antigen-specific Th17 and/or Th1 differentiation but induces antigen-specific Treg 47, 48, 55-57. Furthermore, it has been shown that tocilizumab in fact corrects Th17/Treg imbalance in rheumatoid arthritis patients 153. In another study, it was found that tocilizumab induced a significant reduction in the peripheral pre-switch and post-switch memory B cells of rheumatoid arthritis patients 154 and that tocilizumab but not the TNF inhibitor significantly reduced somatic hypermutation in immunoglobulin gene rearrangements in pre-switch memory B cells 155, thus suggesting that modulation of memory B cells may be one possible target for tocilizumab. Moreover, tocilizumab treatment led to a reduction in the pathologic CD38highCD19lowIgDnegative plasma cells of SLE patients 74 and could lessen the survival of plasmablasts, which produce the anti-aquaporin 4 antibody in neuromyelitis optica 156. These findings suggest that the clinical effect of tocilizumab is also mediated through its inhibition of pathological autoantibody production.
Because of the therapeutic efficacy of tocilizumab, IL-6 plays a major role in the onset or development of various phenotypically different diseases. IL-6 is produced by a panoply of cells including monocytes, macrophages, dendritic cells, T and B cells, neutrophils, mast cells, fibroblasts, synovial cells, keratinocytes, endothelial cells, stromal cells, mesangial cells, glial cells, neurons, chondrocytes, osteoblasts, smooth muscle cells, and others in response to various stimuli 2-4, 33. Such phenotypic difference of diseases is conceivably due to differences in cells which generate IL-6 through abnormal transcriptional activation of the IL-6 gene 157, 158 and/or inhibition of IL-6 mRNA degradation 159, 160, or dose-dependent effects of IL-6 produced by cells recruited into the organs. Some virus products from KSHV, human immunodeficiency virus (HIV), human lymphotropic virus-1 (HTLV-1) and hepatitis B virus have been reported to affect IL-6 gene activation and/or mRNA degradation 161-168. Therefore clarification of the cell source of IL-6 production and of the mechanism(s) through which dysregulated continuous IL-6 synthesis is induced constitutes an important issue for future studies into the pathogenesis of diseases.
References
- 1.Hirano T, Yasukawa K, Harada H. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 3.Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Katada Y, Suemura M, et al. Interleukin-6. In: Snapper CM, editor. Cytokine regulation of humoral immunity: basic and clinical aspects. New Jersey: John Wiley & Sons Ltd; 1996. pp. 251–72. [Google Scholar]
- 5.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 6.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siewert E, Bort R, Kluge R. et al. Hepatic cytochrome p450 down-regulation during aseptic inflammation in the mouse is interleukin 6 dependent. Hepatology. 2000;32:49–55. doi: 10.1053/jhep.2000.8532. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa T, Hagihara K, Serada S. et al. Transcriptional complex formation of c-Fos, STAT3, and Hepatocyte NF-1alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol. 2008;180:3492–501. doi: 10.4049/jimmunol.180.5.3492. [DOI] [PubMed] [Google Scholar]
- 10.Nishimoto N, Terao K, Mima T. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V. et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obici L, Perfetti V, Palladini G. et al. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005;1753:11–22. doi: 10.1016/j.bbapap.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, Bettelli E, Oukka M. et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 14.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–35. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 15.Miyara M, Gorochov G, Ehrenstein M. et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–55. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Kitahara M, Kishimoto S. et al. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141:1543–9. [PubMed] [Google Scholar]
- 17.Ishibashi T, Kimura H, Shikama Y. et al. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74:1241–4. [PubMed] [Google Scholar]
- 18.Kotake S, Sato K, Kim KJ. et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara H, Song J, Sugimoto M. et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–9. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 20.Grossman RM, Krueger J, Yourish D. et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan MR, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol. 1991;97:686–92. doi: 10.1111/1523-1747.ep12483971. [DOI] [PubMed] [Google Scholar]
- 22.Kawano M, Hirano T, Matsuda T. et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–5. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 23.Suematsu S, Matsuda T, Aozasa K. et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–51. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horii Y, Muraguchi A, Iwano M. et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949–55. [PubMed] [Google Scholar]
- 25.Yamasaki K, Taga T, Hirata Y. et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–8. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–7. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 27.Hibi M, Murakami M, Saito M. et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–57. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto T, Akira S, Narazaki M. et al. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–54. [PubMed] [Google Scholar]
- 29.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Hibi M, Nakagawa N. et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–10. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 31.Boulanger MJ, Chow DC, Brevnova EE. et al. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–4. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 32.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 34.Aoki Y, Narazaki M, Kishimoto T. et al. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpes virus. Blood. 2001;98:3042–9. doi: 10.1182/blood.v98.10.3042. [DOI] [PubMed] [Google Scholar]
- 35.Brandt SJ, Bodine DM, Dunbar CE. et al. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86:592–9. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka T, Kishimoto T. Immunotherapy of tocilizumab for rheumatoid arthritis. J Clin Cell Immunol. 2011:S6–001. [Google Scholar]
- 37.Alonzi T, Fattori E, Lazzaro D. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takagi N, Mihara M, Moriya Y. et al. Blockade of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–21. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Ohshima S, Saeki Y, Mima T. et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasai M, Saeki Y, Ohshima S. et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Ohshima S, Nishioka K. et al. Antigen induced arthritis (AIA) can be transferred by bone marrow transplantation: evidence that interleukin 6 is essential for induction of AIA. J Rheumatol. 2002;29:1176–82. [PubMed] [Google Scholar]
- 42.Wong PK, Quinn JM, Sims NA. et al. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158–68. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 43.Hata H, Sakaguchi N, Yoshitomi H. et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–8. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirota K, Hashimoto M, Yoshitomi H. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–7. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwakura Y, Saijo S, Kioka Y. et al. Autoimmunity induction by human T cell leukemia virus type 1 in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. J Immunol. 1995;155:1588–98. [PubMed] [Google Scholar]
- 46.Atsumi T, Ishihara K, Kamimura D. et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–90. doi: 10.1084/jem.20020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujimoto M, Serada S, Mihara M. et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–9. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 48.Iwanami K, Matsumoto I, Tanaka-Watanabe Y. et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–63. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 49.Mihara M, Takagi N, Takeda Y. et al. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang B, Gardner DB, Griswold DE. et al. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119:296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pflegerl P, Vesely P, Hantusch B. et al. Epidermal loss of JunB leads to a SLE phenotype due to hyper IL-6 signaling. Proc Natl Acad Sci USA. 2009;106:20423–8. doi: 10.1073/pnas.0910371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitaba S, Murota H, Terao M. et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol. 2012;80:165–76. doi: 10.1016/j.ajpath.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Yoshizaki A, Yanaba K, Ogawa A. et al. Immunization with DNA topoisomerase I and Freund's complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011;63:3575–85. doi: 10.1002/art.30539. [DOI] [PubMed] [Google Scholar]
- 54.Okiyama N, Sugihara T, Iwakura Y. et al. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum. 2009;60:2505–12. doi: 10.1002/art.24689. [DOI] [PubMed] [Google Scholar]
- 55.Hohki S, Ohguro N, Haruta H. et al. Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp Eye Res. 2010;91:162–70. doi: 10.1016/j.exer.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Haruta H, Ohguro N, Fujimoto M. et al. Blockade of interleukin-6 signaling suppresses not only Th17 but also interphotoreceptor retinoid binding protein-specific Th1 by promoting regulatory T cells in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52:3264–71. doi: 10.1167/iovs.10-6272. [DOI] [PubMed] [Google Scholar]
- 57.Serada S, Fujimoto M, Mihara M. et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041–6. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS lett. 2011;585:3699–709. doi: 10.1016/j.febslet.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 60.Nishimoto N, Yoshizaki K, Miyasaka N. et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–9. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 61.Maini RN, Taylor PC, Szechinski J, et al; CHARISMA Study Group. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 62.Nishimoto N, Miyasaka N, Yamamoto K. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–4. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones G, Sebba A, Gu J. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smolen JS, Beaulieu A, Rubbert-Roth A, et al; OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 65.Genovese MC, McKay JD, Nasonov EL. et al. Interleukin-6 receptor inhibition with TCZ reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the TCZ in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 66.Emery P, Keystone E, Tony HP. et al. IL-6 receptor inhibition with TCZ improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre randomized placebo controlled trial. Ann Rheum Dis. 2008;67:1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woo P, Wilkinson N, Prieur AM. et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–8. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokota S, Miyamae T, Imagawa T. et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–25. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 69.Yokota S, Imagawa T, Mori M. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 70.De Benedetti F, Brunner H, Ruperto N. et al. Tocilizumab in patients with systemic juvenile idiopathic arthritis: efficacy data from the placebo-controlled 12-week part of the phase 3 TENDER trial. Arthritis Rheum. 2010;62(Suppl 10):1434. [Google Scholar]
- 71.De Benedetti F, Brunner H, Ruperto N. et al. Efficacy and safety of tocilizumab (TCZ) in patients with systemic juvenile idiopathic arthritis (SJIA): tender 52-week data. Pediat Rheumatol. 2011;9(Suppl 1):164. [Google Scholar]
- 72.Nishimoto N, Sasai M, Shima Y. et al. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 73.Nishimoto N, Kanakura Y, Aozasa K. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 74.Illei GG, Shirota Y, Yarboro CH. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–52. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maeshima K, Ishii K, Torigoe M. et al. Successful tocilizumab and tacrolimus treatment in a patient with rheumatoid arthritis complicated by systemic lupus erythematosus. Lupus. 2012 doi: 10.1177/0961203312441046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Makol A, Gibson LE, Michet CJ. Successful use of interleukin 6 antagonist tocilizumab in a patient with refractory cutaneous lupus and urticarial vasculitis. J Clin Rheumatol. 2012;18:92–5. doi: 10.1097/RHU.0b013e31823ecd73. [DOI] [PubMed] [Google Scholar]
- 77.Shima Y, Kuwahara Y, Murota H. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 2010;49:2408–12. doi: 10.1093/rheumatology/keq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narazaki M, Hagihara K, Shima Y. et al. Therapeutic effect of tocilizumab on two patients with polymyositis. Rheumatology (Oxford) 2011;50:1344–6. doi: 10.1093/rheumatology/ker152. [DOI] [PubMed] [Google Scholar]
- 79.Seitz M, Reichenbach S, Bonel HM. et al. Rapid induction of remission in large vessel vasculitis by IL-6 blockade. A case series. Swiss Med Wkly. 2011;141:w13156. doi: 10.4414/smw.2011.13156. [DOI] [PubMed] [Google Scholar]
- 80.Beyer C, Axmann R, Sahinbegovic E. et al. Anti-interleukin 6 receptor therapy as rescue treatment for giant cell arteritis. Ann Rheum Dis. 2011;70:1874–5. doi: 10.1136/ard.2010.149351. [DOI] [PubMed] [Google Scholar]
- 81.Sciascia S, Rossi D, Roccatello D. Interleukin 6 blockade as steroid-sparing treatment for 2 patients with giant cell arteritis. J Rheumatol. 2011;38:2080–1. doi: 10.3899/jrheum.110496. [DOI] [PubMed] [Google Scholar]
- 82.Salvarani C, Magnani L, Catanoso M. et al. Tocilizumab: a novel therapy for patients with large-vessel vasculitis. Rheumatology (0xford) 2012;51:151–6. doi: 10.1093/rheumatology/ker296. [DOI] [PubMed] [Google Scholar]
- 83.Vinit J, Bielefeld P, Muller G. et al. Efficacy of tocilizumab in refractory giant cell arteritis. Joint Bone Spine. 2012;79:317–8. doi: 10.1016/j.jbspin.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Christidis D, Jain S, Das Gupta B. Successful use of tocilizumab in polymyalgic onset biopsy positive GCA with large vessel involvement. BMJ Case Reports. 2011 doi: 10.1136/bcr.04.2011.4135. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishimoto N, Nakahara H, Yoshio-Hoshino N. et al. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197–200. doi: 10.1002/art.23373. [DOI] [PubMed] [Google Scholar]
- 86.Salvarani C, Magnani L, Catanoso MG. et al. Rescue treatment with tocilizumab for Takayasu arteritis resistant to TNF-α blockers. Clin Exp Rheumatol. 2012 [Epub ahead of print] [PubMed] [Google Scholar]
- 87.Bredemeier M, Rocha CM, Barbosa MV. et al. One-year clinical and radiological evolution of a patient with refractory Takayasu's arteritis under treatment with tocilizumab. Clin Exp Rheumatol. 2012 [Epub ahead of print] [PubMed] [Google Scholar]
- 88.Cohen C, Mekinian A, Saidenberg-Kermanach N. et al. Efficacy of tocilizumab in rituximab-refractory cryoglobulinemia vasculitis. Ann Rheum Dis. 2012;71:628–9. doi: 10.1136/annrheumdis-2011-200501. [DOI] [PubMed] [Google Scholar]
- 89.Sumida K, Ubara Y, Suwabe T. et al. Complete remission of myeloperoxidase-antineutrophil cytoplasmic antibody-associated crescentic glomerulonephritis complicated with rheumatoid arthritis using a humanized anti-interleukin 6 receptor antibody. Rheumatology (0xford) 2011;50:1928–30. doi: 10.1093/rheumatology/ker222. [DOI] [PubMed] [Google Scholar]
- 90.Sumida K, Ubara Y, Takemoto F. et al. Successful treatment with humanized anti-interleukin 6 receptor antibody for multidrug-refractory and anti-tumour necrosis factor-resistant systemic rheumatoid vasculitis. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S133. [PubMed] [Google Scholar]
- 91.Ito H, Takazoe M, Fukuda Y. et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Kawai M, Hagihara K, Hirano T. et al. Sustained response to tocilizumab, anti-interleukin-6 receptor antibody, in two patients with refractory relapsing polychondritis. Rheumatology (Oxford) 2009;48:318–9. doi: 10.1093/rheumatology/ken468. [DOI] [PubMed] [Google Scholar]
- 93.Narshi CB, Allard SA. Sustained response to tocilizumab, anti-IL-6 antibody, following anti-TNF-α failure in a patient with relapsing polychondritis complicated by aortitis. Rheumatology (Oxford) 2012;51:952–3. doi: 10.1093/rheumatology/ker451. [DOI] [PubMed] [Google Scholar]
- 94.Nishida S, Kawasaki T, Kashiwagi H. et al. Successful treatment of acquired hemophilia A, complicated by chronic GVHD, with tocilizumab. Mod Rheumatol. 2011;21:420–2. doi: 10.1007/s10165-010-0411-6. [DOI] [PubMed] [Google Scholar]
- 95.Yuzuriha A, Saitoh T, Koiso H. et al. Successful treatment of autoimmune hemolytic anemia associated with multicentric Castleman disease by anti-interleukin-6 receptor antibody (tocilizumab) therapy. Acta Haematol. 2011;126:147–50. doi: 10.1159/000328426. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Hernandez FJ, Gonzalez-Leon R, Castillo-Palma MJ. et al. Tocilizumab for treating refractory haemolytic anemia in a patient with systemic lupus erythematosus. Rheumatology (Oxford) 2012 doi: 10.1093/rheumatology/kes072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 97.Iwamoto M, Nara H, Hirata D. et al. Humanized monoclonal anti-interleukin-6 receptor antibody for treatment of intractable adult-onset Still's disease. Arthritis Rheum. 2002;46:3388–9. doi: 10.1002/art.10620. [DOI] [PubMed] [Google Scholar]
- 98.Nakahara H, Mima T, Yoshino-Hoshino N. et al. A case report of a patient with refractory adult-onset Still's disease who was successfully treated with tocilizumab over 6 years. Mod Rheumatol. 2009;19:69–72. doi: 10.1007/s10165-008-0116-2. [DOI] [PubMed] [Google Scholar]
- 99.De Bandt M. Saint-Marcoux B. Tocilizumab for multirefractory adult-onset Still's disease. Ann Rheum Dis. 2009;68:153–4. doi: 10.1136/ard.2008.088179. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto K, Nagashima T, Takatori S. et al. Glucocorticoid and cyclosporine refractory adult onset Still's disease successfully treated with tocilizumab. Clin Rheumatol. 2009;28:485–7. doi: 10.1007/s10067-009-1097-z. [DOI] [PubMed] [Google Scholar]
- 101.Cunha ML, Wagner J, Osawa A. et al. The effect of tocilizumab on the uptake of 18FDG-PET imaging in patients with adult-onset Still's disease. Rheumatology (Oxford) 2010;49:1014–6. doi: 10.1093/rheumatology/kep441. [DOI] [PubMed] [Google Scholar]
- 102.Sumida K, Ubara Y, Hoshino J. et al. Etanercept-refractory adult-onset Still's disease with thrombotic thrombocytopenic purpura successfully treated with tocilizumab. Clin Rheumatol. 2010;29:1191–4. doi: 10.1007/s10067-010-1418-2. [DOI] [PubMed] [Google Scholar]
- 103.Yoshimura M, Makiyama J, Koga T. et al. Successful treatment with tocilizumab in a patient with refractory adult-onset Still's disease (AOSD) Clin Exp Rheumatol. 2010;28:141–2. [PubMed] [Google Scholar]
- 104.Perdan-Pirkmajer K, Praprotnik S, Tomsic M. A case of refractory adult-onset Still's disease successfully controlled with tocilizumab and a review of the literature. Clin Rheumatol. 2010;29:1465–7. doi: 10.1007/s10067-010-1553-9. [DOI] [PubMed] [Google Scholar]
- 105.Naniwa T, Ito R, Watanabe M. et al. Case report: successful use of short-term add-on tocilizumab for multirefractory systemic flare of adult-onset Still's disease. Clin Rheumatol. 2010 doi: 10.1007/s10067-010-1562-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 106.Kishida D, Okuda Y, Ohnishi M. et al. Successful tocilizumab treatment in a patient with adult-onset Still's disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol. 2011;21:215–8. doi: 10.1007/s10165-010-0365-8. [DOI] [PubMed] [Google Scholar]
- 107.Thonhofer R, Hiller M, Just H. et al. Treatment of refractory adult-onset Still's disease with tocilizumab: report of two cases and review of the literature. Rheumatol Int. 2011;31:1653–6. doi: 10.1007/s00296-010-1631-y. [DOI] [PubMed] [Google Scholar]
- 108.Sabnis GR, Gokhale YA, Kulkarni UP. Tocilizumab in refractory adult-onset Still's disease with aseptic meningitis-efficacy of interleukin-6 blockade and review of the literature. Semin Arthritis Rheum. 2011;40:365–8. doi: 10.1016/j.semarthrit.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Rech J, Ronneberger M, Englbrecht M. et al. Successful treatment of adult-onset Still's disease refractory to TNF and IL-1 blockade by IL-6 blockade. Ann Rheum Dis. 2011;70:390–2. doi: 10.1136/ard.2010.129403. [DOI] [PubMed] [Google Scholar]
- 110.Kobayashi M, Takahashi Y, Yamashita H. et al. Benefit and a possible risk of tocilizumab therapy for adult-onset Still's disease accompanied by macrophage-activation syndrome. Mod Rheumatol. 2011;21:92–6. doi: 10.1007/s10165-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 111.Puechal X, DeBandt M, Berthelot JM. et al. Tocilizumab in refractory adult Still's disease. Arthritis Care Res. 2011;63:155–9. doi: 10.1002/acr.20319. [DOI] [PubMed] [Google Scholar]
- 112.Sekkach Y, Elqatni M, Khattabi AE. et al. Antagonists of interleukin-6 (tocilizumab), in adult refractory still disease. Presse Med. 2011;40:e333–7. doi: 10.1016/j.lpm.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 113.Suematsu R, Ohta A, Matsuura E. et al. Therapeutic response of patients with adult Still's disease to biologic agents: multicenter results in Japan. Mod Rheumatol. 2011 doi: 10.1007/s10165-011-0569-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 114.Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:2997–3000. doi: 10.1002/art.22118. [DOI] [PubMed] [Google Scholar]
- 115.Nishida S, Hagihara K, Shima Y. et al. Rapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis. 2009;68:1235–6. doi: 10.1136/ard.2008.099267. [DOI] [PubMed] [Google Scholar]
- 116.Sato H, Sakai T, Sugaya T. et al. Tocilizumab dramatically ameliorated life-threatening diarrhea due to secondary amyloidosis associated with rheumatoid arthritis. Clin Rheumatol. 2009;28:1113–6. doi: 10.1007/s10067-009-1185-0. [DOI] [PubMed] [Google Scholar]
- 117.Inoue D, Arima H, Kawanami C. et al. Excellent therapeutic effect of tocilizumab on intestinal amyloid a deposition secondary to active rheumatoid arthritis. Clin Rheumatol. 2010;29:1195–7. doi: 10.1007/s10067-010-1422-6. [DOI] [PubMed] [Google Scholar]
- 118.De La Torre M, Arboleya L, Pozo S. et al. Rapid and sustained response to tocilizumab, anti-interleukin-6 receptor antibody, in a patient with nephritic syndrome secondary to systemic juvenile idiopathic arthritis-related amyloidosis. NDT Plus. 2011;4:178–80. doi: 10.1093/ndtplus/sfr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Magro-Checa C, Navas-Parejo Casado A, Borrego-Garcia E. et al. Successful use of tocilizumab in a patient with nephritic syndrome due to a rapidly progressing AA amyloidosis to latent tuberculosis. Amyloid. 2011;18:235–9. doi: 10.3109/13506129.2011.613962. [DOI] [PubMed] [Google Scholar]
- 120.Hattori Y, Ubara Y, Sumida K. et al. Tocilizumab improves cardiac disease in a hemodialysis patient with AA amyloidosis secondary to rheumatoid arthritis. Amyloid. 2012;19:37–40. doi: 10.3109/13506129.2011.636460. [DOI] [PubMed] [Google Scholar]
- 121.Hagihara K, Kawase I, Tanaka T. et al. Tocilizumab ameliorates clinical symptoms in polymyalgia rheumatica. J Rheumatol. 2010;37:1075–6. doi: 10.3899/jrheum.091185. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka T, Hagihara K, Shima Y. et al. Treatment of a patient with remitting seronegative, symmetrical synovitis with pitting oedema with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Rheumatology (Oxford) 2010;49:824–6. doi: 10.1093/rheumatology/kep412. [DOI] [PubMed] [Google Scholar]
- 123.Hirano T, Ohguro N, Hohki S. et al. A case of Behcet's disease treated with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Mod Rheumatol. 2012;22:298–302. doi: 10.1007/s10165-011-0497-5. [DOI] [PubMed] [Google Scholar]
- 124.Shapiro LS, Farrell J, Haghighi AB. Tocilizumab treatment for neuro-Behcet's disease, the first report. Clin Neurol Neurosurgery. 2011;114:297–8. doi: 10.1016/j.clineuro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 125.Muselier A, Bielefeld P, Bidot S. et al. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocular Immunol Inflamm. 2011;19:382–3. doi: 10.3109/09273948.2011.606593. [DOI] [PubMed] [Google Scholar]
- 126.Gergis U, Arnason J, Yantiss R. et al. Effectiveness and safety of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient with refractory GI graft-versus-host disease. J Clin Onocol. 2010;28:e602–4. doi: 10.1200/JCO.2010.29.1682. [DOI] [PubMed] [Google Scholar]
- 127.Drobyski WR, Pasquini M, Kovatovic K. et al. Tocilizumab for the treatment of steroid refractory graft versus host disease. Biol Blood Marrow Transplant. 2011;17:1862–8. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vaitla PM, Radford PM, Tighe PJ. et al. Role of interleukin-6 in a patient with tumor necrosis factor receptor-associated periodic syndrome: Assessment of outcomes following treatment with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum. 2011;63:1151–5. doi: 10.1002/art.30215. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka T, Kuwahara Y, Shima Y. et al. Successful treatment of reactive arthritis with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Arthritis Rheum. 2009;61:1762–4. doi: 10.1002/art.24899. [DOI] [PubMed] [Google Scholar]
- 130.Henes JC, Horger M, Guenaydin I. et al. Mixed response to tocilizumab for ankylosing spondylitis. Ann Rheum Dis. 2010;69:2217–8. doi: 10.1136/ard.2009.126706. [DOI] [PubMed] [Google Scholar]
- 131.Wendling D, Bossert M, Prati C. Short-term effect of IL-6 inhibition in spondylarthritis. Joint Bone Spine. 2010;77:624–5. doi: 10.1016/j.jbspin.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 132.Brulhart L, Nissen MJ, Chevallier P. et al. Tocilizumab in a patient with ankylosing spondylitis and Crohn's disease refractory to TNF antagonists. Joint Bone Spine. 2010;77:625–6. doi: 10.1016/j.jbspin.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Shima Y, Tomita T, Ishii T. et al. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorated clinical symptoms and MRI findings of a patient with ankylosing spondylitis. Mod Rheumatol. 2011;21:436–9. doi: 10.1007/s10165-011-0416-9. [DOI] [PubMed] [Google Scholar]
- 134.Cohen JD, Ferreira R, Jorgensen C. Ankylosing spondylitis refractory to tumor necrosis factor blockade responds to tocilizumab. J Rheumatol. 2011;38:1527. doi: 10.3899/jrheum.110265. [DOI] [PubMed] [Google Scholar]
- 135.Koumakis E, Feydy A, Kahan A. et al. Interleukin 6 blockade in spondyloarthritis. J Rheumatol. 2012;39:1097–8. doi: 10.3899/jrheum.110955. [DOI] [PubMed] [Google Scholar]
- 136.Dudler J, Aubry-Rozier B. Tocilizumab in axial spondylarthropathies: about 18 cases. Ann Rheum Dis. 2010;70:128. [Google Scholar]
- 137.Del Castillo Pinol N, Gossec L, Sparsa L. et al. Tocilizumab for treatment of refractory spondyarthritis: report of 5 patients. Ann Rheum Dis. 2011;70:343. [Google Scholar]
- 138.Ogata A, Umegaki N, Katayama I. et al. Psoriatic arthritis in two patients with an inadequate response to treatment with tocilizumab. Joint Bone Spine. 2012;79:85–7. doi: 10.1016/j.jbspin.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 139.Taniguchi K, Shimazaki C, Fujimoto Y. et al. Tocilizumab is effective for pulmonary hypertension associated with multicentric Castleman's disease. Int J Hematol. 2009;90:99–102. doi: 10.1007/s12185-009-0346-x. [DOI] [PubMed] [Google Scholar]
- 140.Arita Y, Sakata Y, Sudo T. et al. The efficacy of tocilizumab in a patient with pulmonary arterial hypertension associated with Castleman's disease. Heart Vessels. 2010;25:444–7. doi: 10.1007/s00380-009-1215-5. [DOI] [PubMed] [Google Scholar]
- 141.Furuya Y, Satoh T, Kuwana M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheumatol. 2010. Article ID 720305. [DOI] [PMC free article] [PubMed]
- 142.Navarini AA, French LE, Hofbauer GFL. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol. 2011;128:1128–30. doi: 10.1016/j.jaci.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 143.Ohtori S, Miyagi M, Eguchi Y. et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J. 2012 doi: 10.1007/s00586-012-2183-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogata A, Morishima A, Hirano T. et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70:1164–5. doi: 10.1136/ard.2010.132845. [DOI] [PubMed] [Google Scholar]
- 145.Schultz O, Oberhauser F, Saech J. et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hirao M, Yamasaki N, Oze H. et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2135-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matthijs Boekholdt S, Stores ESG. The interleukin-6 pathway and atherosclerosis. Lancet. 2012;379:1176–8. doi: 10.1016/S0140-6736(12)60361-4. [DOI] [PubMed] [Google Scholar]
- 148.IL-6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF. et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.The Interleukin-6 Receptor Mendelian Randomization Analysis (IL-6R MR) Consortium, Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Waetzig GH, Rose-John S. Hitting a complex target: an update on interleukin-6 trans-signaling. Expert Opin Ther Targets. 2012;16:225–36. doi: 10.1517/14728222.2012.660307. [DOI] [PubMed] [Google Scholar]
- 151.Tanaka T, Hagihara K, Hishitani Y, et al. Tocilizumab for the treatment of AA amyloidosis. In: Guvenc IA, editor. Amyloidosis - An insight to disease of systems and novel therapies. Croatia: INTECH Open Access Publisher; 2011. pp. 155–70. [Google Scholar]
- 152.Song SN, Tomosugi N, Kawabata H. et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116:3627–34. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- 153.Samson M, Audia S, Janikashvili N. et al. Inhibition of IL-6 function corrects Th17/Treg imbalance in rheumatoid arthritis patients. Arthritis Rheum. 2012 doi: 10.1002/art.34477. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 154.Roll P, Muhammad K, Schumann M. et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255–64. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- 155.Muhammad K, Roll P, Seibold T. et al. Impact of IL-6 receptor inhibition on human memory B cells in vivo: impaired somatic hypermutation in preswitch memory B cells and mutational targeting in memory B cells. Ann Rheum Dis. 2011;70:1507–10. doi: 10.1136/ard.2010.141325. [DOI] [PubMed] [Google Scholar]
- 156.Chihara N, Aranami T, Sato W. et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA. 2011;108:3701–6. doi: 10.1073/pnas.1017385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 158.Matsusaka T, Fujikawa K, Nishio Y. et al. Transcriptional factor NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao W, Liu M, Kirkwood KL. P38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J Biol Chem. 2008;283:1778–85. doi: 10.1074/jbc.M707573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iwasaki H, Takeuchi O, Teraguchi S. et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol. 2011;12:1167–75. doi: 10.1038/ni.2137. [DOI] [PubMed] [Google Scholar]
- 161.Kang JG, Pripuzova N, Majerciak V. et al. Kaposi's sarcoma-associated herpesvirus ORF57 promotes escape of viral and human interleukin-6 from microRNA-mediated suppression. J Virol. 2011;85:2620–30. doi: 10.1128/JVI.02144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin Z, Keamey P, Plaisance K. et al. Pivotal advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2010;87:25–34. doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leung K, Nabel GJ. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–8. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 164.Ballard DW, Bohnlein E, Lowenthal JW. et al. HTLV-1 tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–5. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 165.Scala G, Ruocco MR, Ambrosino C. et al. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994;179:961–71. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ambrosino C, Ruocco MR, Chen X. et al. HIV-1 Tat induces the expression of the interleukin-6 (IL6) gene by binding to the IL6 leader RNA and by interacting with CAAT enhancer-binding protein beta (NF-IL6) transcription factors. J Biol Chem. 1997;272:14883–92. doi: 10.1074/jbc.272.23.14883. [DOI] [PubMed] [Google Scholar]
- 167.Mahe Y, Mukaida N, Kuno K. et al. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT /enhancer-binding protein-like cis-elements. J Biol Chem. 1991;266:13759–63. [PubMed] [Google Scholar]
- 168.Ohno H, Kaneko S, Lin Y. et al. Human hepatitis B virus X protein augments the DNA binding of nuclear factor for IL-6 through its basic-leucine zipper domain. J Med Virol. 1999;58:11–8. doi: 10.1002/(sici)1096-9071(199905)58:1<11::aid-jmv2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]