Abstract
Adaptive features of innate immunity, recently described as “trained immunity,” have been documented in plants, invertebrate animals, and mice, but not yet in humans. Here we show that bacille Calmette-Guérin (BCG) vaccination in healthy volunteers led not only to a four- to sevenfold increase in the production of IFN-γ, but also to a twofold enhanced release of monocyte-derived cytokines, such as TNF and IL-1β, in response to unrelated bacterial and fungal pathogens. The enhanced function of circulating monocytes persisted for at least 3 mo after vaccination and was accompanied by increased expression of activation markers such as CD11b and Toll-like receptor 4. These training effects were induced through the NOD2 receptor and mediated by increased histone 3 lysine 4 trimethylation. In experimental studies, BCG vaccination induced T- and B-lymphocyte–independent protection of severe combined immunodeficiency SCID mice from disseminated candidiasis (100% survival in BCG-vaccinated mice vs. 30% in control mice). In conclusion, BCG induces trained immunity and nonspecific protection from infections through epigenetic reprogramming of innate immune cells.
Keywords: mycobacterium tuberculosis vaccine, innate immune memory
The general perception in immunology is that innate immunity, as opposed to adaptive immunity, is static and does not adapt to an enhanced functional state. However, there is an increasing body of scientific literature indicating enhanced nonspecific protection against infections after previous exposure to certain microbial components in plants (1), invertebrates (2, 3), and mice (4). In our recent review of the relevant literature, we proposed the term trained immunity for this effect (5). Prominent microbial components that can induce this enhanced effector function are mycobacterial components such as bacille Calmette-Guérin (BCG), complete Freund adjuvant, and muramyl dipeptide (MDP) (6–9).
BCG, the live attenuated vaccine against tuberculosis (TB), is one of the world’s most widely used vaccines (10). It usually is given to newborns, protecting them especially against severe forms of TB (e.g., TB meningitis, disseminated TB) (11). Soon after its introduction in the 1930s, epidemiological studies surprisingly demonstrated that BCG also protects against childhood mortality independent of its effect on TB (12–15). Recent studies corroborated these findings and suggested a reduction in the burden of infections other than TB (16–20). For example, in a case-control study in Brazil, BCG reduced the risk of death from pneumonia by 50% (17). Although little is known about the mechanisms responsible for these protective effects of BCG, macrophages from BCG-vaccinated mice displayed a greater release of oxygen radicals and intracellular fungal killing (8), suggesting an important role of innate immune mechanisms.
In this paper, we explore the mechanisms of the enhanced immune function induced by BCG both in vitro and in vivo.
Results
Monocyte Phenotype Is Modified on BCG Vaccination.
In the first series of experiments, blood was collected from 20 naïve (nonexposed) volunteers, before and after (2 wk and 3 mo) vaccination with BCG (Fig. 1A).
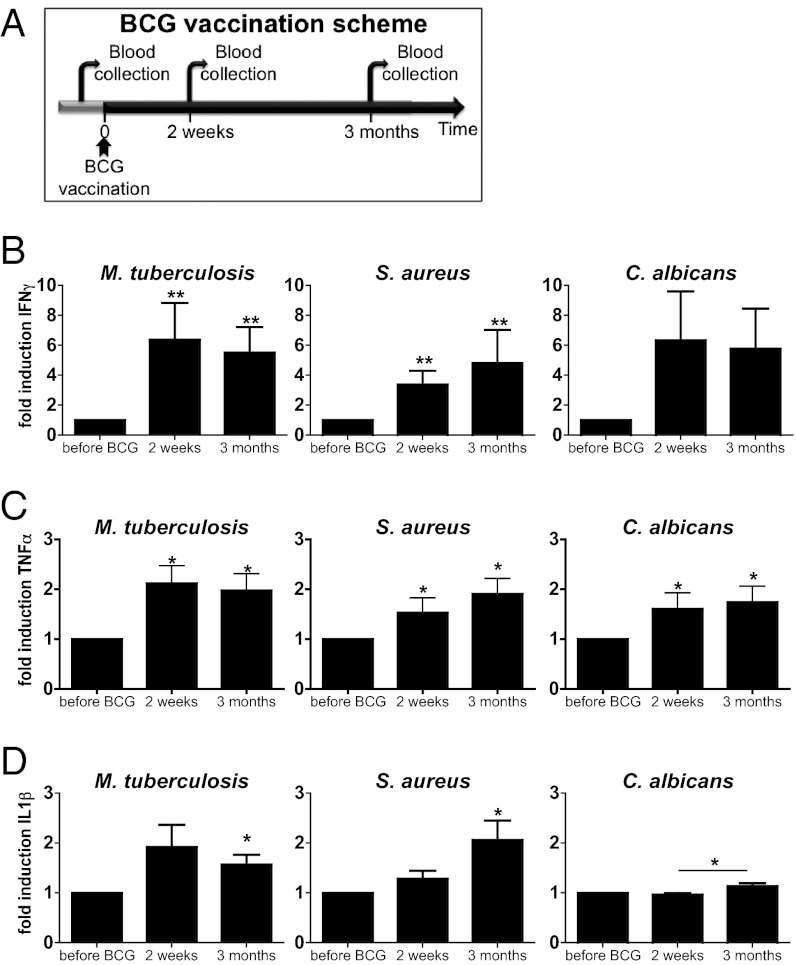
Fig. 1.
BCG vaccination increased the nonspecific production of proinflammatory cytokines. (A) Diagram showing the course of the BCG vaccination trial. Blood was collected from 20 naïve (nonexposed) volunteers before and after (2 wk and 3 mo) vaccination with BCG. (B–D) PBMCs isolated from the 20 volunteers before and after (2 wk and 3 mo) vaccination were stimulated in vitro with sonicated M. tuberculosis, heat-killed S. aureus, and C. albicans blastoconidia. Proinflammatory cytokine production (INF-γ [B], TNF-α [C]), and IL-1β [D]) was assessed by ELISA in the supernatants. *P < 0.05, **P < 0.01.
As expected, 2 wk after BCG vaccination, IFN-γ production induced by Mycobacterium tuberculosis (MTB) was sevenfold higher than it was before vaccination, and this effect remained for at least 3 mo (Fig. 1B). Surprisingly, however, IFN-γ production also increased significantly when cells were exposed to unrelated pathogens, such as yeasts (7.7-fold increase, Fig.1B) or hyphae (5.2-fold increase, Fig. S1A) of Candida albicans and Staphylococcus aureus (3.5-fold induction, Fig. 1B). Exposure of peripheral blood mononuclear cells (PBMCs) to LPS induced production of IFN-γ in very low amounts that did not differ before and after BCG vaccination (Fig. S1B).
Interestingly, in the same series of experiments, production of the proinflammatory cytokines TNF-α and IL-1β also was found to be enhanced when cells isolated from the volunteers after BCG vaccination were exposed to mycobacterial and nonmycobacterial stimuli (Fig. 1 C and D). Of note, the increase in cytokine production persisted 3 mo after BCG vaccination (Fig. 1 B–D).
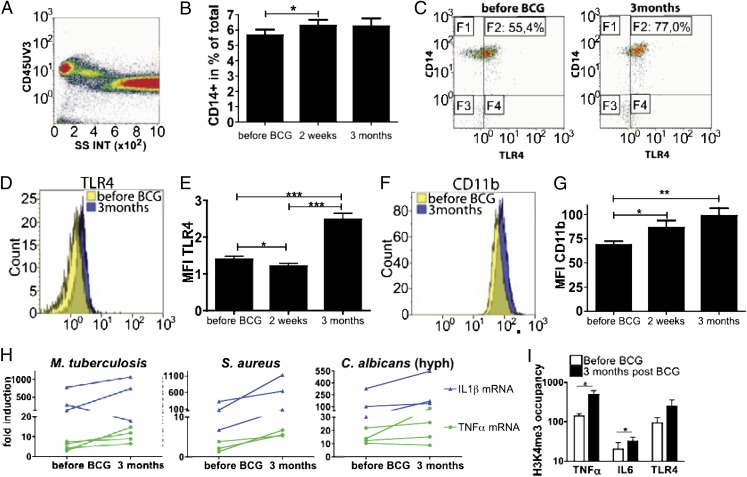
These data demonstrate that BCG alters the functional state of circulating mononuclear cells. To further explore this finding, we investigated the expression of known cell surface activation markers of monocytes. We observed a slight increase in the population of CD14+ monocytes after BCG vaccination (Fig. 2A), all of which expressed the differentiation markers CX3CR1 and CD11b (Fig. S2 C–E). Interestingly, the proportion of CD14+ monocytes expressing Toll-like receptor 4 (TLR4) changed significantly after vaccination (Fig. S2B). In addition, the expression of CD14 (Fig. S2A), TLR4 (Fig. 2 B–D), and CD11b (Fig. 2 E and F) was enhanced on monocytes isolated 2 wk and 3 mo after BCG vaccination compared with their expression before vaccination. The mean fluorescence intensity of the CX3CR1 marker did not change (Fig. S2F). No changes could be observed in the proportion or expression of pattern recognition receptors, such as dectin-1, TLR2, or mannose receptor (Fig. S2 G–K). These observations show that BCG vaccination can induce long-term changes in the phenotype of circulating monocytes, in line with the greater proinflammatory cytokine production after BCG. To examine the level at which the enhanced monocyte function is exerted, we assessed whether the increased cytokine production was the result of enhanced transcription. Indeed, mRNA expression for tnfα and il-1β was increased after BCG vaccination (Fig. 2G).
Fig. 2.
BCG alters the phenotype of circulating monocytes in healthy volunteers. (A) Flow cytometry analysis of CD45+ cells. (B) Histogram showing the expression level of CD14+ (% of total cells) in the cells isolated from 20 volunteers before and after BCG vaccination. (C) Flow cytometry analysis of TLR4 and CD14 in cells isolated from one volunteer before and 3 mo after BCG vaccination. (D and F) Overlays of the surface expression level of TLR4 (D) and CD11b (F) within CD14+ cells isolated from one volunteer before and 3 mo after BCG vaccination. (E and G) Average surface expression level of TLR4 (E) and CD11b (G) within the CD14+ monocyte population isolated from 20 volunteers before and after BCG vaccination. *P < 0.05, **P < 0.01, ***P < 0.005. (H) il-1β and tnfα mRNA expression after sonicated M. tuberculosis, heat-killed S. aureus and C. albicans hyphae in vitro stimulation of the PBMCs isolated from volunteers before and 3 mo after BCG vaccination (n ≥ 3). (I) ChIP analysis of the enrichment of H3K4me3 at the promoter of tnfα, il6 and tlr4 in human monocytes isolated from three subjects before and 3 mo after BCG vaccination. *P < 0.05, paired t test.
Increased H3K4 Trimethylation in Monocytes After BCG Vaccination.
As there is accumulating evidence that histone modifications (both acetylation and methylation) are crucial for long-term transcriptional regulation during inflammation (21–23), the involvement of epigenetic mechanisms was assessed. Increased trimethylation of histone H3 at lysine 4 (H3K4), which has been associated with an increased transcription of proinflammatory cytokine genes (22), might represent the mechanism responsible for the long-term modulation of monocyte-derived cytokines. In line with this hypothesis, H3K4 trimethylation was found to be significantly increased at the level of cytokine and TLR4 promoters in the circulating cells collected 3 mo after vaccination with BCG compared with the values before BCG vaccination (Fig. 2H).
BCG-Induced Protection Is T- and B-Cell Independent.
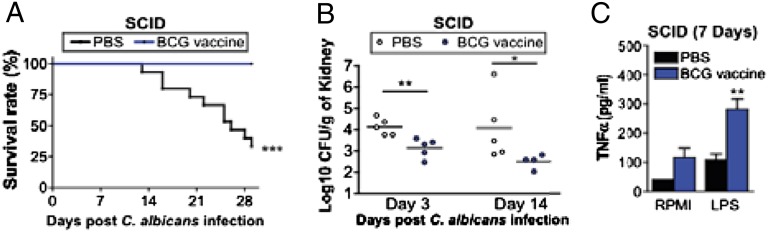
To establish whether BCG-induced trained immunity is indeed mediated by innate immune cells (e.g., monocytes) and independent of T and B lymphocytes, we injected either BCG or saline in two groups of severe combined immunodeficiency (SCID) mice that lack both T and B cells. Two weeks later, both groups of mice were injected with a lethal inoculum of C. albicans. The BCG-vaccinated mice had a significantly better survival rate than the saline-injected animals (Fig. 3A), and this was accompanied by a decreased fungal burden in the kidneys, the target organ of disseminated candidiasis in mice (Fig. 3B). These data strongly support the notion that BCG can induce nonspecific protection against nonmycobacterial infections through functional reprogramming of innate immune cells, such as monocytes. To demonstrate that the trained immunity function of monocytes also may be found after BCG vaccination of SCID mice in vivo, we performed an additional experiment in vaccinated mice. As previously, SCID mice lacking functional T and B cells and vaccinated with BCG (or with control PBS) were infected with C. albicans, and 1 wk later spleen monocytes were restimulated in vitro with LPS, a classic inducer of innate immunity responses that is not related to mycobacteria. Innate proinflammatory cytokine production induced by LPS was significantly higher in the BCG-vaccinated mice (Fig. 3C).
Fig. 3.
BCG vaccination protects mice against lethal C. albicans infection through a T-/B-lymphocyte–independent mechanism. (A) Survival rate of SCID mice infected with live C. albicans (2 × 106 cfu/mouse) injected i.v. The mice were vaccinated i.v. either with PBS (control) or BCG 14 d before inoculation with a lethal C. albicans dose (n ≥ 15 per group, two independent experiments). (B) Fungal burden of kidneys from control- and BCG-vaccinated SCID mice 3 and 14 d after the lethal C. albicans infection (n = 5). (C) TNF-α production of spleen monocytes after restimulation in vitro with LPS from control- and BCG-vaccinated SCID mice 7 d after the lethal C. albicans infection (n = 5). *P < 0.05, **P < 0.01, and ***P < 0.005 vs. control (PBS) animals.
Training Effects of BCG Vaccination Are NOD2 and Rip2 Dependent.
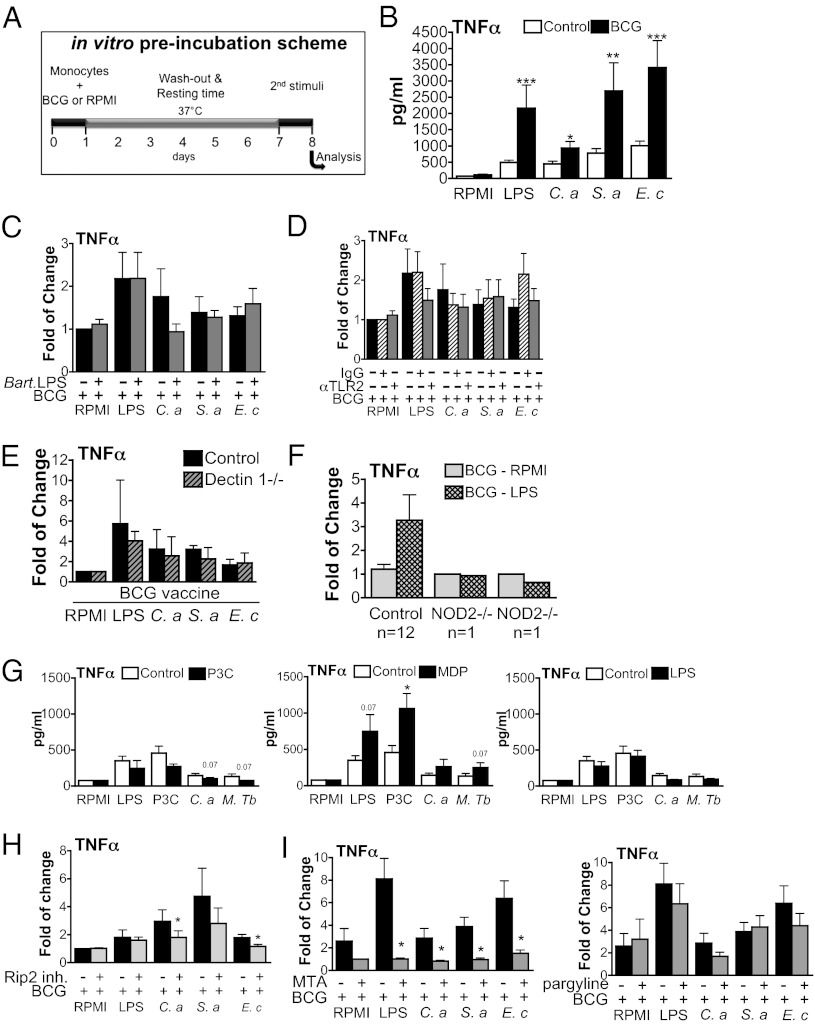
The mechanisms responsible for training by BCG were explored further in vitro using human cells. Freshly isolated human monocytes were preincubated with either culture medium or BCG for 24 h, followed by washing of the cells. After a washout period of 7 d, during which the cells were incubated solely with culture medium, various secondary stimuli were added to the cell culture (Fig. 4A). During the first week, the monocytes had a viability rate >95% when trained with either culture medium or BCG; however, the experiments could not be extended beyond 10 d because of the death of primary monocytes in longer-term cultures (based on morphology and trypan blue exclusion). Preincubation of cells with BCG markedly increased production of the proinflammatory cytokine TNF-α after the secondary stimulus (Fig. 4B), reproducing the effects observed during BCG vaccination. Remarkably, BCG increased production of cytokines induced by a secondary nonmycobacterial challenge, such as purified TLR ligands or whole heat-killed bacteria.
Fig. 4.
BCG primes the production of proinflammatory cytokines. (A) Diagram showing the course of the in vitro preincubation experiment. Cells were preexposed to culture medium or BCG vaccine for 24 h (first stimulation—training). After the first stimulus was washed, the cells were incubated for 7 d in culture medium supplemented with serum. Afterward, a second in vitro stimulation (second stimulation) of cytokine production with various pattern recognition receptor ligands was performed for an additional 24 h. (B) BCG training in vitro using freshly isolated adherent monocytes. (C and D) Inhibition of TLR4 or TLR2 does not affect the training effects induced by BCG. (E and F) BCG training of monocytes is severely affected in cells obtained from NOD2-deficient volunteers (F) but not from dectin-1–deficient volunteers (E). (G) The training effects induced by BCG could be reproduced with MDP but not with Pam3Cys or LPS. (H and I) TNF-α production in BCG-primed monocytes in the absence or presence of Rip2/p38 inhibitor (I), the histone demethylase inhibitor pargyline, or the histone methyltransferase inhibitor MTA (H). (C–I) The ratios of cytokine production in BCG-primed vs. nonprimed monocytes are presented. Data presented in (E) are the mean of two independent experiments (n = 1 + 1). Data presented in (F) were obtained from 12 healthy volunteers (controls) and two different NOD2-deficient individuals (NOD2−/−). (B–D and G–I) *P < 0.05, **P < 0.01, ***P < 0.005. Data are presented as mean ± SD (n ≥ 6). The Wilcoxon signed rank test was used to detect significant differences.
In a following set of experiments, we assessed the receptors and signaling pathways responsible for these effects. Blockade of TLR2 or TLR4 receptors by specific inhibitors, or the use of monocytes from a dectin-1–deficient individual, did not abolish the training ability induced by BCG (Fig. 4 C–E). In contrast, monocytes isolated from patients with a complete NOD2 deficiency due to the homozygous presence of the 1022insC frameshift mutation could not mount an increased cytokine response upon training with BCG (Fig. 4F). This is a strong argument that NOD2, but not TLRs or the C-type lectin receptor dectin-1, is required for the training of monocytes by BCG. This conclusion is supported further by experiments showing that only the NOD2-specific ligand MDP, but not the TLR4 ligand LPS or the TLR2 ligand Pam3Cys, could mimic the effects of BCG preincubation on cytokine production by monocytes (Fig. 4G). In line with this effect of MDP, an inhibitor of the Rip2 kinase also impaired BCG-induced training (Fig. 4H). These data are supported further by a recent study demonstrating priming effects of NOD2 engagement for stimulation of human macrophages with mycobacteria (24).
Finally, as H3K4me3 was increased after BCG vaccination in vivo, we investigated whether blocking histone methylation might reverse monocyte training in vitro. Indeed, inhibition of histone methylation by the methyltransferase inhibitor MTA (5′-deoxy-5′-methylthio-adenosine) almost completely reversed the training induced by BCG (Fig. 4I). In contrast, the histone demethylase inhibitor pargyline did not influence the BCG-induced training effects.
Discussion
Until now, the nonspecific adaptive features of innate immunity (25) that have been demonstrated in plants, invertebrates, and mice and that we termed trained immunity (5) have not been demonstrated to be active in vivo in humans, and the molecular substrate of trained immunity has not yet been identified. In the present study, we demonstrate that monocytes can be functionally reprogrammed, or “trained,” to exhibit an enhanced and lasting phenotype after vaccination with BCG. These data point to the mononuclear phagocyte as the cell that mediates the nonspecific protection against reinfection after BCG vaccination. Our data also imply that vaccination with BCG induces two types of immune response: on one hand it induces a classic specific immune response involving antigen-specific T cells and memory leading to protection against TB, whereas on the other hand it induces adaptive trained immunity based on functional reprogramming of mononuclear phagocytes that induces protective effects not only against tuberculosis, but also against other infections. In a combination of in vivo and in vitro experiments, we demonstrate that a NOD2-mediated epigenetic change at the level of histone methylation (H3K4me3) is the mechanism through which BCG enhances innate immune responses.
The mechanisms activated by BCG vaccination are almost certainly multiple and complex: classical adaptive immune memory, epigenetic reprogramming (“training”) of innate immunity as described here, and possibly also secondary nonspecific effects of adaptive immunity on innate immune responses (e.g., increased cytokines, as shown by Strutt et al. (26)). Conceptually, it is important to discern classical adaptive immunity mediated by T and B lymphocytes from the trained immunity mediated by innate immune cells. In this respect, trained immunity reflects an increase in nonspecific antimicrobial capacity through epigenetic reprogramming, similar to the concept of adaptive characteristics of innate immunity, as described by Mantovani et al. (25, 27). Moreover, whereas adaptive immune cell–mediated responses take some time to become robust, innate (trained) immunity becomes apparent much sooner. This was also demonstrated recently in epidemiological studies in which the beneficial effects of BCG in newborn children already were apparent within days of vaccination (28). The rapidity of the nonspecific adaptive characteristics of innate immunity underlines that heterologous adaptive responses cannot fully explain trained immunity.
The impact of the training effects on innate immune responses has become apparent in our experiments in which SCID mice were vaccinated with BCG: these mice were protected from mortality, and kidney burdens were reduced to 20–40% of the levels in the unvaccinated mice after a secondary lethal infection with C. albicans. Earlier studies with BCG in experimental schistosomiasis demonstrated similar protection, which was at least partially T-cell independent (29). There appears to be an obvious link between these experimental results and the nonspecific protection against nonmycobacterial disease conferred by BCG (8, 9, 12–20). In the present study, we provide unique mechanistic insights into the processes mediating the adaptive features of innate immunity, or trained immunity, in humans. The modified methylation status of cytokine promoters after BCG vaccination in human monocytes, as well as the blockade of the in vitro training effects with methyltransferase inhibitors, suggests that the innate immune response in humans can be reprogrammed epigenetically. Similar mechanisms have been demonstrated before in plants, during so-called systemic acquired resistance (1), and in invertebrates (30); however, both classes of organisms are devoid of adaptive immune responses. The present study teaches us that in the presence of adaptive immunity—as is the case in mammals—training of innate immunity also is operational and serves to enhance resistance to infection.
Another important point for discussion is whether the training effects of BCG vaccination are the result of epigenetic reprogramming of innate immune cells exclusively, or whether long-term BCG infection also may play a role. Little is known about how long BCG stays viable in the human body. Fortunately, however, a very recent study by Minassian et al. (31) reports that 4 wk after vaccination, only 50% of individuals still displayed viable BCG at the vaccination site. Although we cannot fully exclude a low BCG persistence in a minority of the volunteers, it is to be expected that in most of them, all the microorganisms were cleared after 3 mo. Moreover, in our in vitro experimental setting of trained immunity, monocytes were trained with live BCG as well as with inert MDP, highlighting that live BCG persistence is not mandatory for the training.
It should be stressed that trained immunity in mammals is not induced solely by BCG. Indeed, murine CMV infection can induce protection from reinfection in a T/B-cell–independent and natural killer cell–dependent fashion (4, 32), and systemic infection with C. albicans induced T/B-cell–independent nonspecific protection in mice (33). The role of epigenetic reprogramming was not investigated in these studies.
In conclusion, in this study we provide firm evidence that innate immunity in humans has adaptive features and that it has the capacity to display an enhanced response upon reinfection. This process of trained immunity likely represents a paradigm shift in immunity, as it demonstrates the existence of (nonspecific) immunological memory in the absence of adaptive immune responses. Better insights into the relative role of adaptive immune memory and epigenetic reprogramming of innate immunity (trained immunity) may have important consequences for vaccine design, more specifically with regard to the selection of antigens and development of adjuvants. In this respect, answers remain to be provided regarding the strength and duration of epigenetically induced trained immunity. In this context, it is important to note that BCG and the NOD2-ligand MDP have long been known to induce nonspecific protective effects against infections (7, 15) and neoplasms (34), providing the hope that trained immunity can indeed be harnessed for preventive and therapeutic purposes.
Materials and Methods
Subjects.
Subjects (age range, 20–36 y) who were scheduled to receive a BCG vaccination at the public health service, because of travel or work in TB-endemic countries, were asked to participate in this trial. Twenty healthy individuals were included between August and November 2010. Informed consent was obtained from the human subjects included. Blood was drawn before, 2 wk after, and 3 mo after the BCG vaccination. The study was approved by the Arnhem-Nijmegen Ethical Committee.
In vitro cytokine stimulation experiments were performed with PBMCs isolated from buffy coats obtained from healthy volunteers (Sanquin Bloodbank, Nijmegen, The Netherlands).
PBMC Stimulation Assays.
The mononuclear cell fraction was isolated from the blood by density centrifugation and diluted 1:1 in pyrogen-free saline over Ficoll-Paque (Pharmacia Biotech). Cells were washed twice in saline and resuspended in culture medium (Roswell Park Memorial Institute [RPMI] medium; Invitrogen) supplemented with 10 μg/mL gentamicin, 10 mM l-glutamine, and 10 mM pyruvate. Cells were counted in a Coulter counter (Coulter Electronics), and the number was adjusted to 5 × 106 cells/mL. A total of 5 × 105 mononuclear cells in a 100-μL volume was added to round-bottom 96-well plates (Greiner) with RPMI or sonicated MTB H37Rv (1 μg/mL end concentration), heat-killed C. albicans, heat-killed C. albicans hyphae (1 × 106 microorganisms/mL, strain UC820), S. aureus (1 × 106 microorganisms/mL), or Escherichia coli LPS (Sigma-Aldrich, 1 ng/mL). After 24 h or 48 h, the supernatants were stored at −20 °C. Cytokine concentrations were assessed in the supernatants using ELISA.
For ChIP analysis, adherent monocytes from the subjects were obtained in a six-well plate by incubating 15 × 106 PBMCs for 1 h. The adherent monocytes then were collected before further treatment for chromatin immunoprecipitation.
Buffy coats of blood from anonymous blood donors were used for the in vitro “training” experiments. To isolate PBMCs, the previously described procedure was used. Monocytes were used after adherence for 1 h in flat-bottom 96-well plates. For monocyte training, RPMI, BCG (1 μg/mL BCG vaccine SSI from the Netherlands Vaccine Institute), MDP (10 μg/mL), Pam3Cys (10 μg/mL), or LPS (1 ng/mL) was added for 24-h incubation at 37 °C. Thereafter, the supernatant was discarded and replaced with fresh RPMI with 10% serum. After 7 d at 37 °C, the supernatant was discarded and the cells were stimulated with E. coli LPS (10 ng/mL), sonicated MTB H37Rv (1 μg/mL), S. aureus (1 × 106 microorganisms/mL), or RPMI as a control for an additional 24 h. Subsequently, the supernatants were stored at −20 °C until ELISA was performed. In some representative experiments, the final cell number after initial training with control medium, BCG, or MDP was assessed using Hoechst 33342 fluorescent stain and showed little variation. The normalized cytokine levels based on the number of cells actually present in the well showed the same trend as the nonnormalized cytokine levels.
In the “inhibition” experiments, before priming with BCG, the adherent monocytes were preincubated for 1 h with Bartonella LPS (1 μg/mL), anti-TLR2 antibody and anti-IgG (10 μg/mL; eBioscience), Rip2/p38 inhibitor (1 μM; Sigma-RBI), pargyline (3 μM; Sigma-Aldrich), or MTA (1 mM; Sigma-Aldrich).
Cytokine Measurements.
Cytokine measurements of TNF-α, IL-1β, and IFN-γ were performed in the supernatants using commercial ELISA kits from R&D Systems (TNF-α and IL-1β) or Sanquin (IL-6 and IFN-γ). In a small proportion of baseline samples in which cytokine concentrations were beyond the detection limit, these outliers were excluded from the analysis.
FACS Analysis.
Cells were phenotypically analyzed by 10- and 5-color flow cytometry using a Coulter Navios and Coulter Cytomics FC 500 cytometer (Beckman Coulter), respectively, and evaluated using Kaluza 1.1 software (Beckman Coulter). The cells were washed with PBS with 1% BSA before being labeled with fluorochrome-conjugated mAbs. After incubation for 30 min at 4 °C in the dark, the cells were washed twice to remove unbound antibodies and analyzed. For cell surface staining, the following mAbs were used: CD11b (IM25814), CD14-ECD (IM2707U), and CD45-PC7 (IM3548) (all from Beckman Coulter); CD284-PE (TLR 4) (312806), CD206-PE (321106), and CX3CR1-FITC (341606) (all from Biolegend); CD282-APC (558319) (from BD Biosciences); and dectin-1-APC (FAB1859A) (from R&D Systems).
Quantitative RT- PCR.
For quantitative RT-PCR (qRT-PCR), adherent monocytes were primed with either culture medium or β-glucan and subjected after 7 d to a second stimulation with 10 ng/mL LPS, 10 μg/mL Pam3Cys, or 1 × 105/mL heat-killed C. albicans for 4 h. Samples were treated with TRIzol Reagent (Invitrogen), and total RNA purification was performed. Isolated RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative PCR (qPCR) was performed using the SYBR Green method. The following primers were used in the reaction (5′-3′): TNF-α forward: TGGCCCAGGCAGTCAGA, and TNF-α reverse: GGTTTGCTACAACATGGGCTACA; IL-1β forward: GCCCTAAACAGATGAAGTGCTC, and IL-1β reverse: GAACCAGCATCTTCCTCAG; and β2-microglobulin forward: ATGAGTATGCCTGCCGTGTG, and β2-microglobulin reverse: CCAAATGCGGCATCTTCAAAC (Biolegio). Each sample was analyzed following a relative quantification model with efficiency correction, and β2-microglobulin was used as a housekeeping gene. The mRNA expression level of nonprimed and nonstimulated samples was used as a reference. The results are presented as the ratios of mRNA production by β-glucan in primed vs. nonprimed monocytes.
Chromatin Immunoprecipitation.
For ChIP, adherent monocytes were cultured as described earlier (see PBMC Stimulation Assays). ChIP was performed using antibodies against H3K4me3 (Diagenode). ChIPed DNA was processed further for qPCR analysis. The following primers were used in the reaction (5′-3′): TNF-α forward: CAGGCAGGTTCTCTTCCTCT, and TNF-α reverse: GCTTTCAGTGCTCATGGTGT; IL-6 forward: TCGTGCATGACTTCAGCTTT, and IL-6 reverse: GCGCTAAGAAGCAGAACCAC; TLR4 forward: GTCCCTGCTCTGCTACCTTG, and TLR4 reverse: TTGAAAGGAGCAGGGTGACT; and myoglobin forward: AGCATGGTGCCACTGTGCT, and myoglobin reverse: GGCTTAATCTCTGCCTCATGAT.
Mouse Experiments.
PrkdcSCID mice (abbreviated SCID) were obtained from Charles River Wiga. Female mice between 6 and 8 wk of age were used. The mice were fed with sterilized laboratory chow (Hope Farms) and water ad libitum. The mice were housed in a pathogen-free facility. The experiments were approved by the Ethics Committee on Animal Experiments of Radboud University, Nijmegen. The mice first were injected with BCG vaccine SSI (750 μg/mouse) in a 100-μL volume of sterile pyrogen-free PBS (PBS) or with PBS alone. Fourteen days later, the mice were infected i.v. with a lethal dose of C. albicans blastoconidia (1 × 104 cfu/mouse). Survival then was monitored and kidney fungal burden was assessed 3 and 14 d after the C. albicans injection. To assess cytokine production, splenocytes from BCG or vehicle-vaccinated mice were retrieved 7 d after the i.v. infection with C. albicans and stimulated in vitro with LPS (10 ng/mL). Cytokine concentrations were measured using a specific RIA, as described previously (35) in supernatants collected after 48 h of incubation at 37 °C in 5% CO2 in a 48-well plate.
Statistical Analysis.
Differences were analyzed using the Wilcoxon signed rank test or Friedman test for paired samples. P < 0.05 was considered statistically significant. Unless otherwise stated, data are shown as the cumulative results of levels obtained in all volunteers (mean + SEM).
Supplementary Material
Acknowledgments
J.Q. and M.G.N. were supported by a Vici Grant from the Netherlands Organization for Scientific Research (to M.G.N.). R.v.C. was supported by a Vidi Grant from the Netherlands Organization for Scientific Research. S.S. was supported by the Higher Education Commission of Pakistan. D.C.I. was supported by the European Seventh Framework Programme ALLFun Project. R.J.X. was supported by US National Institutes of Health Grants AI 062773, DK 043351, and DK 83756 and the Helmsley Trust.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202870109/-/DCSupplemental.
See Commentary on page 17317.
References
- 1.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 2.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netea MG, Quintin J, van der Meer JW. Trained immunity: A memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Gorhe DS. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund’s complete adjuvant. Nature. 1967;216:1242–1244. doi: 10.1038/2161242a0. [DOI] [PubMed] [Google Scholar]
- 7.Chedid L, et al. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci USA. 1977;74:2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van ’t Wout JW, Poell R, van Furth R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand J Immunol. 1992;36:713–719. doi: 10.1111/j.1365-3083.1992.tb03132.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma T, Suenaga T, Yoshida I, Azuma M. Mechanisms of enhanced resistance of Mycobacterium bovis BCG-treated mice to ectromelia virus infection. Infect Immun. 1983;42:567–573. doi: 10.1128/iai.42.2.567-573.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine PE. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 11.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 12.Levine MI, Sackett MF. Results of BCG immunization in New York City. Am Rev Tuberc. 1946;53:517–532. doi: 10.1164/art.1946.53.6.517. [DOI] [PubMed] [Google Scholar]
- 13.Aronson JD. Protective vaccination against tuberculosis, with special reference to BCG vaccine. Minn Med. 1948;31:1336. [PubMed] [Google Scholar]
- 14.Ferguson RG, Simes AB. BCG vaccination of Indian infants in Saskatchewan. Tubercle. 1949;30:5–11. doi: 10.1016/s0041-3879(49)80055-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal SR, et al. BCG vaccination in tuberculous households. Am Rev Respir Dis. 1961;84:690–704. doi: 10.1164/arrd.1961.84.5P1.690. [DOI] [PubMed] [Google Scholar]
- 16.Velema JP, Alihonou EM, Gandaho T, Hounye FH. Childhood mortality among users and non-users of primary health care in a rural west African community. Int J Epidemiol. 1991;20:474–479. doi: 10.1093/ije/20.2.474. [DOI] [PubMed] [Google Scholar]
- 17.Niobey FM, et al. [Risk factors for death caused by pneumonia in children younger than 1 year old in a metropolitan region of southeastern Brazil. A case- control study] Rev Saude Publica. 1992;26:229–238. doi: 10.1590/s0034-89101992000400004. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: Follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321:1435–1438. doi: 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garly ML, et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–2790. doi: 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 20.Vaugelade J, Pinchinat S, Guiella G, Elguero E, Simondon F. Non-specific effects of vaccination on child survival: Prospective cohort study in Burkina Faso. BMJ. 2004;329:1309. doi: 10.1136/bmj.38261.496366.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood. 2008;111:1797–1804. doi: 10.1182/blood-2007-08-106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 24.Brooks MN, et al. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13:402–418. doi: 10.1111/j.1462-5822.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes Infect. 2007;9:1680–1687. doi: 10.1016/j.micinf.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Strutt TM, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564, 551p following 564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 28.Aaby P, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 29.Clark IA, Allison AC, Cox FE. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976;259:309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Minassian AM, et al. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis. 2012;205:1035–1042. doi: 10.1093/infdis/jis012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 33.Bistoni F, et al. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26:285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- 34.Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 35.Netea MG, et al. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.