Bisphenol A (BPA) is a chemical used in the production of epoxy resins and polycarbonate plastics found in many household items (1). Human exposure is so ubiquitous that 0.2–20 ng/mL of BPA (2) is circulating in the body at any given time. Research has shown evidence that exposure to BPA is associated with problems such as anxiety (3), short-term memory loss (4), obesity (5), diabetes (6), and cardiovascular disease (7). However, critics of these studies have pointed out, among other things, that many of these studies were done in nonprimate laboratory animals whose physiology may differ from humans (8). In PNAS, Hunt et al. (9) provide evidence in the rhesus macaque monkey (Macaca mulatta) that prolonged maternal exposure to BPA has a direct influence on the process of meiosis in the fetal ovaries of a primate, the consequences of which are not seen for a generation. It is thus fair to say that BPA may be viewed as the only known aneugen, a chemical whose ingestion can produce oocytes, and thus embryos, with the wrong number of chromosomes (a condition known as aneuploidy) by disrupting the meiotic process. As aptly noted in 2003 by Hunt et al.: “BPA, a man-made substance with estrogenic properties, induces both a dramatic increase in congression failure and meiotic aneuploidy” (10).
Meiosis is the process by which a single diploid cell produces four haploid cells, each carrying one copy of each chromosome. In males, all four of these haploid cells become sperm, whereas, in females, only one of these cells becomes the oocyte. During meiosis, homologous chromosomes must pair along their lengths, recombine with each other to form crossovers, and then segregate (11). Pairing and recombination occur during prophase, the earliest stage of meiosis. During early prophase, double-strand breaks are induced, which can be repaired as a crossover event in which large regions of DNA are exchanged between homologous chromosomes. Crossovers mature into chiasmata that physically link homologous chromosomes together and ensure the proper segregation of homologues at the first meiotic division. The accurate execution and completion of this “genetic ballet” is a critical first step in producing healthy, viable offspring, demonstrated by the low threshold for allowing meiotic errors to continue to term.
Hunt et al. had previously shown that exposing female mice to low doses of BPA led to high levels of meiotic failure and serious perturbations in meiotic chromosome segregation (10). A subsequent study of female meiosis in mouse oocytes exposed to BPA in utero revealed high levels of defects in the intimate association (i.e., synapsis) that characterizes properly paired chromosomes and an increase in meiotic recombination (12). In adult females, the consequences of these defects could be observed in eggs and embryos carrying the wrong number of chromosomes. Such aneuploid eggs and embryos result from the failure of homologous chromosomes to segregate properly at meiosis and, in a great many organisms, have been shown to be the consequence of aberrant pairing, synapsis, and recombination during meiotic prophase. Notably, Susiarjo et al. were able to generate similar defects by disrupting one of the two genes known to encode estrogen receptors, strengthening the idea that BPA acts on the oocyte by interfering with one or more aspects of estrogen signaling (12).
The suggestion that such effects of BPA on meiosis might be limited to mice or rodents was disproved by Allard and Colaiácovo (13), who demonstrated that BPA exposure also interferes with meiotic prophase in Caenorhabditis elegans at internal concentrations similar to those used on mice. In addition to decreased brood size and embryonic lethality, Allard and Colaiácovo (13) observed defects in synapsis similar to those seen in the mouse (10), as well as defects in the recombination process. They also saw a delay in the ability of the oocyte to form paired chromosomes connected by chiasmata (known as bivalents). Finally, they showed that BPA produced these defects via an antiestrogenic effect on the C. elegans germline (13). Despite these observations, the question remained whether primates were really different—perhaps in some way immune to the effects of BPA?
Hunt et al. (9) show two potentially damaging consequences to the process of meiosis in rhesus macaque from continuous or single daily dose exposure to levels of BPA lower than what most humans encounter. First, the oocytes of fetuses carried by mothers in the single daily dose cohort showed a higher number of recombination nodules as evidenced by MutL homologue 1 (MLH1) staining (9). MLH1 functions during meiosis to facilitate crossing over and mismatch repair, and the quantification of MLH1 foci following immunolocalization is an accepted way to measure the number of recombination events during meiosis. The increase in the number of MLH1 foci in BPA-treated rhesus monkeys suggests that, during meiosis, a higher number of double-strand breaks were created or a greater percentage of double-strand breaks were repaired as crossover events. The consequences of increased meiotic recombination in primates are unclear, but there is substantial reason to suggest that altering the number and distribution of crossovers may affect chromosome segregation (14). Second, rhesus macaque fetuses continuously exposed to BPA showed a significantly higher number of oocytes with abnormal centromere associations as detected by staining of SYCP3 (an antibody to an essential structural component of the synaptonemal complex) and CREST (a kinetochore antibody used to identify centromeres) (9) (Fig. 1D, 2). These abnormal associations may reflect nonhomologous centromeric associations, which can lead to meiotic segregation defects.
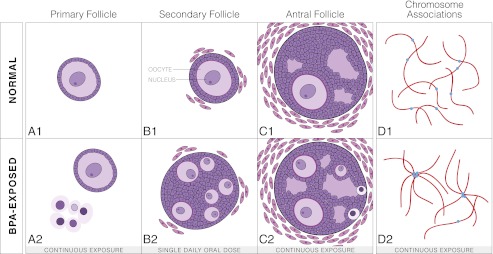
Fig. 1.
(A–C) 1, Representation of the development of a follicle in both a normal fetus and a BPA-exposed fetus. (A–C) 1, Progression of a normal follicle. (A) 1, Development of a normal, single-oocycte primary follicle. (B) 1, A normal, single-oocyte secondary follicle with the oocyte and nucleus labeled. (C) 1, A normal antral follicle. (A–C) 2, Progression of a BPA-exposed follicle. (A) 2, A normal primary follicle alongside a “nest” or grouping of small, unenclosed oocytes with pyknotic nuclei. (B) 2, A multioocyte secondary follicle with oocytes of similar sizes. (C) 2, A multioocyte antral follicle with three developing oocytes and two small, nongrowing oocytes trapped at the edge of the follicle. (D) 1, Normally distributed chromosomes with centromeres not associated during pachytene of prophase I of meiosis I. Blue dots represent centromeres and the red line represents SYCP3 staining, a marker for the synaptonemal complex. Synaptonemal complex holds homologous chromosomes together until dissolved during diplotene. 2, In oocytes of fetuses from mothers continuously exposed to BPA a higher number of associated centromeres during pachytene were detected than in controls, indicating an association between nonhomologous chromosomes.
Stepping back from chromosome synapsis and segregation and examining fetal ovarian development, Hunt et al. (9) demonstrate a strong influence of BPA on follicular development and growth within the rhesus macaque ovary. BPA exposure during follicle development has been shown to be disruptive in many species. For example, Rivera et al. showed in lambs that BPA exposure during follicular development increased the number of multioocyte follicles (15), and Rodriguez et al. found a smaller pool of primordial follicles in rats exposed to BPA (16). Despite this, the impact BPA has on follicular development and growth in the fetal ovary remained unexplored in primates.
Hunt et al. (9) observed three key abnormal morphologies in the BPA-exposed rhesus monkeys. First, many small, unenclosed oocytes [referred to as “nests” by Hunt et al. (9)] were found in the ovaries of continuously exposed females but not in controls. The nuclei of many cells within these nests appeared pyknotic (Fig. 1A, 2). Second, although continuously exposed females did not show a significantly higher number of follicles with two to five oocytes compared with controls, they did exhibit a significantly higher number of follicles with greater than five oocytes (Fig. 1C, 2). There was also a notable difference in size among the oocytes within these follicles. Third, ovaries in which there were a significantly higher number of multioocyte follicles present (Fig. 1B, 2) were found in offspring from mothers receiving a single daily dose of BPA, similar to findings from Rivera et al. (15). These findings together suggest that the same estrogenic disruption seen in other species is present in primates, and importantly show that continuous exposure, as humans face daily, leads to even more extreme follicular defects.
This study (9) is not the first to link gestational BPA exposure with later consequences for the offspring. For example, Tharp et al. linked gestational BPA exposure to advanced mammary gland development in female rhesus monkeys (17). Separately, Jang et al. linked gestational BPA exposure to decreased hippocampal neurogenesis in later generations of mice (18), which could affect learning and memory in these offspring.
Can we now talk about human meiotic errors, such as trisomy 21, and point to BPA as a contributing factor? The evidence provided by Hunt et al. (9) allows us to suspect, but perhaps not indict. One of the most important lessons to remember when considering studies such as this is the multifactorial nature of the response of human beings to an environmental stimulus. A complicated mix of genetic and environmental factors drive the progression of disease in one person and not another. Still, what we gain here is insight into a possible environmental influence on the etiology of aneuploidy driven by errors and delays in the meiotic program.
Acknowledgments
We thank Angela Seat for preparing Fig. 1.
Footnotes
The authors declare no conflict of interest.
See companion article on page 17525.
References
- 1.Kang J-H, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2-3):79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50(1):85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Tian D, Hong X, Chen L, Xie L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology. 2011;61(4):565–573. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 6.Batista TM, et al. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS ONE. 2012;7(3):e33814. doi: 10.1371/journal.pone.0033814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melzer D, et al. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS ONE. 2012;7(8):e43378. doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengstler JG, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41(4):263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt PA, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci USA. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt PA, et al. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13(7):546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 11.Page SL, Hawley RS. Chromosome choreography: The meiotic ballet. Science. 2003;301(5634):785–789. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- 12.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3(1):e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allard P, Colaiácovo MP. Mechanistic insights into the action of bisphenol A on the germline using C. elegans. Cell Cycle. 2011;10(2):183–184. doi: 10.4161/cc.10.2.14478. [DOI] [PubMed] [Google Scholar]
- 14.Lamb NE, et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet. 1996;14(4):400–405. doi: 10.1038/ng1296-400. [DOI] [PubMed] [Google Scholar]
- 15.Rivera OE, Varayoud J, Rodríguez HA, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32(3):304–312. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez HA, Santambrosio N, Santamaría CG, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30(4):550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Tharp AP, et al. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci USA. 2012;109(21):8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang YJ, et al. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296(1-3):73–82. doi: 10.1016/j.tox.2012.03.007. [DOI] [PubMed] [Google Scholar]