Abstract
G protein-coupled receptors (GPCRs) compose the largest family of cell surface receptors and are the most common target of therapeutic drugs. The nonvisual arrestins, β-arrestin-1 and β-arrestin-2, are multifunctional scaffolding proteins that play critical roles in GPCR signaling. On binding of activated GPCRs at the plasma membrane, β-arrestins terminate G protein-dependent responses (desensitization) and stimulate β-arrestin–dependent signaling pathways. Alterations in the cellular complement of β-arrestin-1 and β-arrestin-2 occur in many human diseases, and their genetic ablation in mice has severe consequences. Surprisingly, however, the factors that control β-arrestin gene expression are poorly understood. We demonstrate that glucocorticoids differentially regulate β-arrestin-1 and β-arrestin-2 gene expression in multiple cell types. Glucocorticoids act via the glucocorticoid receptor (GR) to induce the synthesis of β-arrestin-1 and repress the expression of β-arrestin-2. Glucocorticoid-dependent regulation involves the recruitment of ligand-activated glucocorticoid receptors to conserved and functional glucocorticoid response elements in intron-1 of the β-arrestin-1 gene and intron-11 of the β-arrestin-2 gene. In human lung adenocarcinoma cells, the increased expression of β-arrestin-1 after glucocorticoid treatment impairs G protein-dependent activation of inositol phosphate signaling while enhancing β-arrestin-1–dependent stimulation of the MAPK pathway by protease activated receptor 1. These studies demonstrate that glucocorticoids redirect the signaling profile of GPCRs via alterations in β-arrestin gene expression, revealing a paradigm for cross-talk between nuclear and cell surface receptors and a mechanism by which glucocorticoids alter the clinical efficacy of GPCR-based drugs.
G protein-coupled receptors (GPCRs) regulate nearly every aspect of mammalian physiology and are targeted by ∼40% of prescription drugs (1). On agonist stimulation, GPCRs classically activate heterotrimeric G proteins, which regulate various cellular effectors to elicit changes in the level of second messenger molecules. GPCR signaling is intimately controlled by a family of intracellular adaptor proteins known as arrestins (2, 3). The ubiquitously expressed nonvisual arrestins, β-arrestin-1 and β-arrestin-2, bind nearly all agonist-activated GPCRs at the plasma membrane and terminate G protein-dependent signaling (desensitization). In addition, the β-arrestins function as signal transducers themselves by coupling activated GPCRs to components of other signaling pathways, such as the MAPK cascade. The recent discovery of receptor ligands that preferentially activate G protein-dependent signaling over β-arrestin–dependent signaling, or vice versa, holds promise for the development of drugs with improved therapeutic efficacy and/or reduced side effects (4).
The two β-arrestin isoforms are highly homologous but display a number of unique properties (2, 5). They differ in their cellular and subcellular distribution, affinity for GPCRs, interactions with other signaling proteins, and signaling responses. Given their central role in GPCR signaling, changes in the expression of β-arrestin-1 and β-arrestin-2 may have significant physiological and pathological consequences. Genetic deletion of both nonvisual arrestins in mice is embryonic lethal, and mice lacking a single isoform develop normally but exhibit marked differences in their response to pathological and pharmacological challenges (6). In addition, β-arrestin levels are altered in many human diseases, including Parkinson’s disease with dementia (7), multiple sclerosis (8), type 2 diabetes (9), coronary artery disease (10), and various cancers (11). Surprisingly, however, very little is known about the cellular factors that control β-arrestin gene expression.
Glucocorticoids are primary stress hormones that regulate a plethora of biological processes and function to maintain homeostasis (12). Because of their powerful anti-inflammatory and immunosuppressive actions, synthetic glucocorticoids are one of the most widely prescribed drugs worldwide, serving as mainstays in the treatment of acute and chronic inflammatory diseases, autoimmune diseases, organ transplant rejection, and cancers of the lymphoid system (13). Both the physiological and pharmacological actions of glucocorticoids are mediated by the glucocorticoid receptor (GR), a member of the nuclear receptor superfamily of ligand-dependent transcription factors. GR enhances or represses transcription of target genes by binding specific elements of DNA, termed glucocorticoid response elements (GREs), and recruiting coactivators or corepressors that modulate the activity of RNA polymerase II.
In this study, we show that glucocorticoids regulate β-arrestin gene expression in multiple cell types. Glucocorticoids enhance the expression of β-arrestin-1 and repress the expression of β-arrestin-2, with both effects accomplished at the transcriptional level by the binding of GR to intragenic GREs. Moreover, these glucocorticoid-mediated changes in β-arrestin expression shift the balance of protease-activated receptor 1 (PAR1) signaling to favor β-arrestin–dependent responses. Our findings reveal a unique means of cross-talk between nuclear and cell surface receptors and define a mechanism through which glucocorticoids can regulate the clinical efficacy of GPCR-targeted drugs.
Results
Glucocorticoids Differentially Regulate β-Arrestin-1 and β-Arrestin-2 Gene Expression.
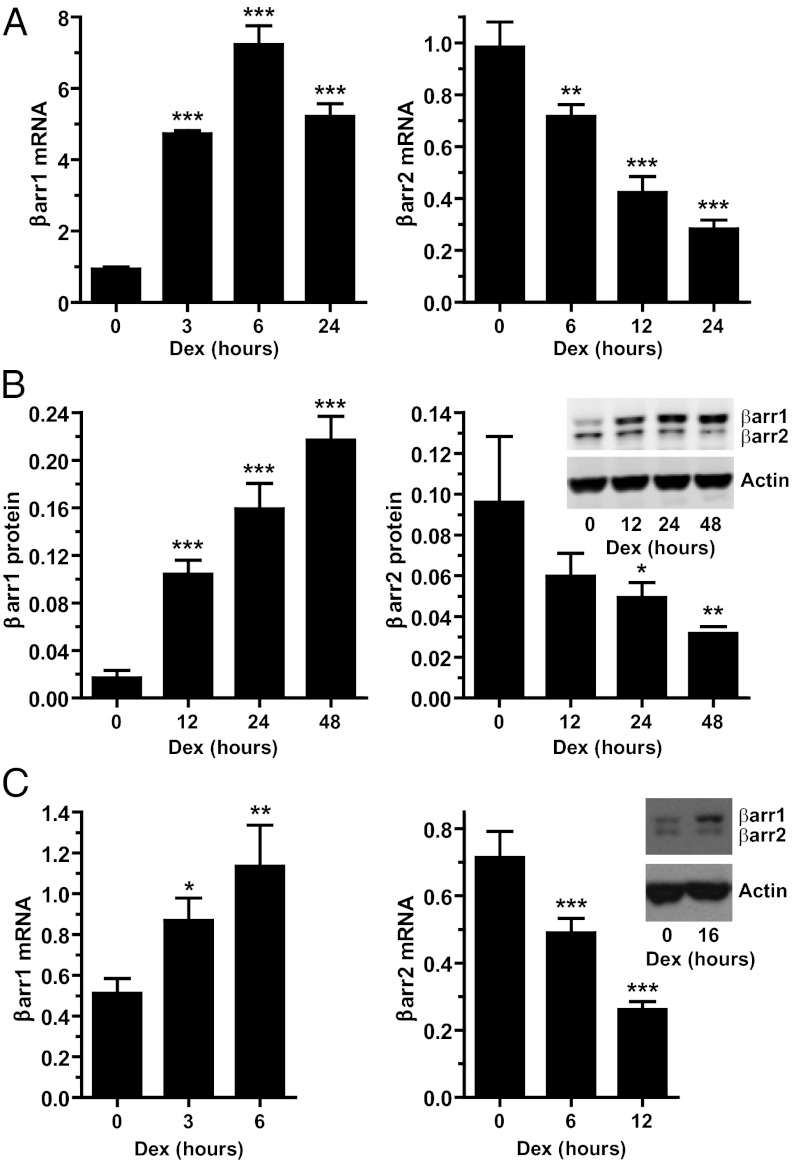
We investigated whether glucocorticoids regulate the expression of the β-arrestin genes by treating human lung adenocarcinoma cells (A549) with the synthetic glucocorticoid dexamethasone (Dex). Dex rapidly induced the expression of β-arrestin-1 mRNA, which reached a maximum of 7.7-fold over basal levels after 6 h of hormone exposure and remained elevated over the 24-h treatment period (Fig. 1A). In contrast, glucocorticoids repressed the expression of β-arrestin-2. A 12-h exposure to Dex resulted in a 57.0% decrease in β-arrestin-2 mRNA, and a 24-h exposure led to a 71.2% reduction (Fig. 1A). The EC50 for the Dex-mediated induction of β-arrestin-1 mRNA was 3.1 nM, and the IC50 for the Dex-mediated repression of β-arrestin-2 mRNA was 5.0 nM (Fig. S1A), concentrations in agreement with the Kd value for Dex binding to GR and commonly achieved in patients receiving glucocorticoid therapy. The glucocorticoid-mediated alterations in β-arrestin-1 and β-arrestin-2 mRNA led to corresponding changes in protein levels. Dex treatment of A549 cells resulted in a time-dependent up-regulation of β-arrestin-1 and down-regulation of β-arrestin-2 protein (Fig. 1B).
Fig. 1.
Gluocorticoids differentially regulate β-arrestin-1 and β-arrestin-2 gene expression. (A) A549 cells were treated with 100 nM Dex for the indicated times, and β-arrestin-1 and β-arrestin-2 mRNA were analyzed by RT-PCR. (B) A549 cells were treated with 100 nM Dex for the indicated times, and β-arrestin-1 and β-arrestin-2 protein were evaluated by immunoblot analysis. Shown are the quantitation of β-arrestin-1 and β-arrestin-2 normalized to β-actin and a representative immunoblot. (C) U2OS cells stably expressing GRs were treated with 10 nM Dex for the indicated times, and β-arrestin-1 and β-arrestin-2 were evaluated by RT-PCR and immunoblot analysis. Data represent mean ± SD from three or four independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
The regulation of β-arrestin gene expression also occurred with the natural glucocorticoids cortisol in humans and corticosterone in rodents, which increased β-arrestin-1 and decreased β-arrestin-2 expression at both the mRNA and protein levels in A549 cells (Fig. S1B). β-arrestin-1 and β-arrestin-2 expression was altered by glucocorticoids in other cell types as well. Dex treatment of human osteosarcoma cells stably expressing GRs resulted in the up-regulation of β-arrestin-1 and down-regulation of β-arrestin-2 (Fig. 1C). In addition, the expression of β-arrestin-1 mRNA and protein was increased by glucocorticoids in mouse embryonic fibroblasts and in vivo in mouse spleen (Fig. S2). These data demonstrate that the β-arrestin-1 and β-arrestin-2 genes are differentially regulated in multiple cell types by glucocorticoids.
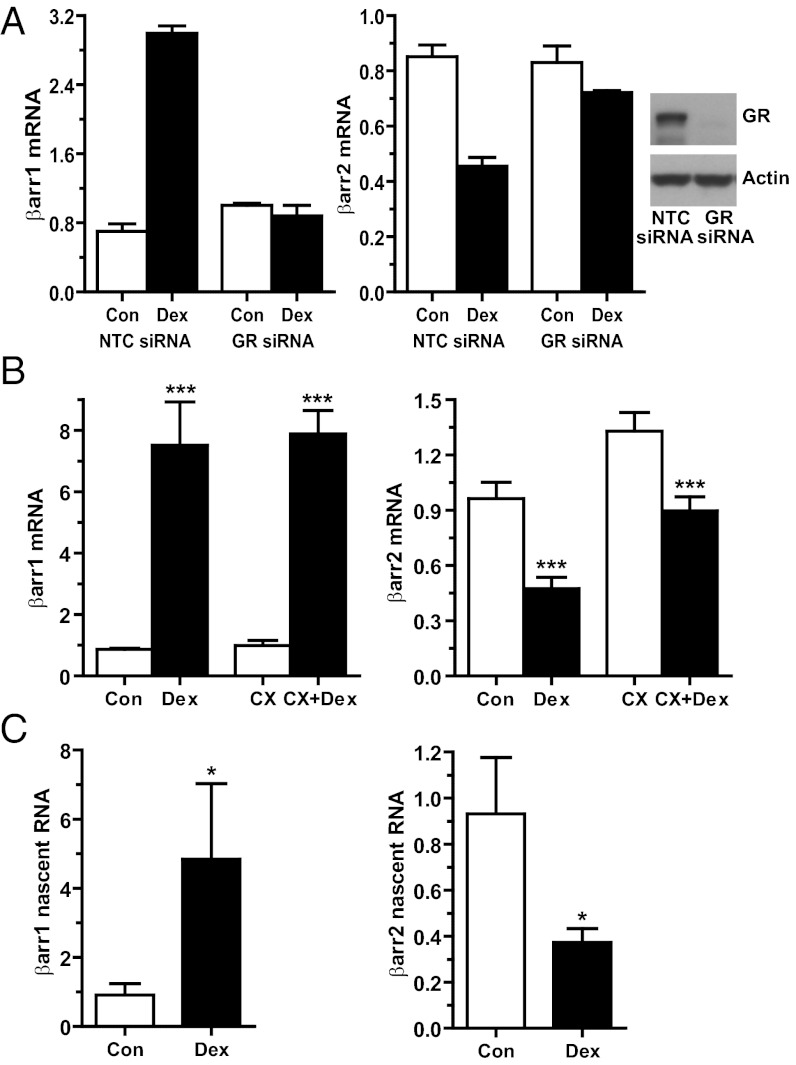
To define the molecular mechanisms underlying the glucocorticoid alteration in β-arrestin gene expression, we first investigated whether GRs are necessary for the regulatory event. Knockdown of GR in A549 cells by siRNA abolished the Dex-mediated increase in β-arrestin-1 expression and decrease in β-arrestin-2 expression (Fig. 2A). The ability of glucocorticoids to induce β-arrestin-1 and repress β-arrestin-2 was also prevented by cotreatment with the glucocorticoid antagonist RU486 (Fig. S3A). We used the protein synthesis inhibitor cycloheximide to determine whether the β-arrestin-1 and β-arrestin-2 genes were primary or secondary targets of glucocorticoids. In A549 cells pretreated with cycloheximide, Dex treatment still up-regulated β-arrestin-1 (Fig. 2B). The expression of β-arrestin-2 was modestly induced by cycloheximide (Fig. 2B), a phenomenon frequently observed with the use of protein synthesis inhibitors (14). Nevertheless, Dex still significantly down-regulated β-arrestin-2 mRNA in the cycloheximide-treated cells (Fig. 2B). Thus, the β-arrestin-1 and β-arrestin-2 genes are primary targets of GR regulation. We next examined whether GR altered transcription of the β-arrestin genes by measuring β-arrestin-1 and β-arrestin-2 nascent RNA with primers targeting intronic sequences. Dex treatment of A549 cells resulted in a 5.3-fold induction in β-arrestin-1 nascent RNA and a 60.0% reduction in β-arrestin-2 nascent RNA (Fig. 2C). Finally, glucocorticoid treatment had no major impact on the half-life of β-arrestin-1 or β-arrestin-2 mRNA (Fig. S3B). Collectively, these data indicate that the glucocorticoid-induced increase in β-arrestin-1 and decrease in β-arrestin-2 are accomplished by GRs acting directly on the β-arrestin genes to modulate their rates of transcription.
Fig. 2.
Glucocorticoid regulation of β-arrestin-1 and β-arrestin-2 gene expression requires GR and involves direct effects on transcription. (A) A549 cells transfected with NTC siRNA (Con, control) or GR siRNA were treated with 100 nM Dex for 4 h (β-arrestin-1) or 12 h (β-arrestin-2). (Left) RT-PCR analysis of β-arrestin-1 and β-arrestin-2 mRNA. Data represent mean ± SD from two independent experiments. (Right) Immunoblot of GR protein. (B) A549 cells pretreated for 1 h with vehicle or 10 μg/mL of cycloheximide (CX) were exposed to 100 nM Dex for 4 h (β-arrestin-1) or 12 h (β-arrestin-2), and β-arrestin-1 and β-arrestin-2 mRNA levels were analyzed by RT-PCR. (C) A549 cells were treated with 100 nM Dex for 3 h (β-arrestin-1) or 6 h (β-arrestin-2), and the levels of β-arrestin-1 and β-arrestin-2 nascent RNA were analyzed by RT-PCR. Data represent mean ± SD from three or four independent experiments. *P < 0.05; ***P < 0.001.
Glucocorticoid Regulation of β-Arrestin Gene Expression Involves GR Binding to Intragenic GREs.
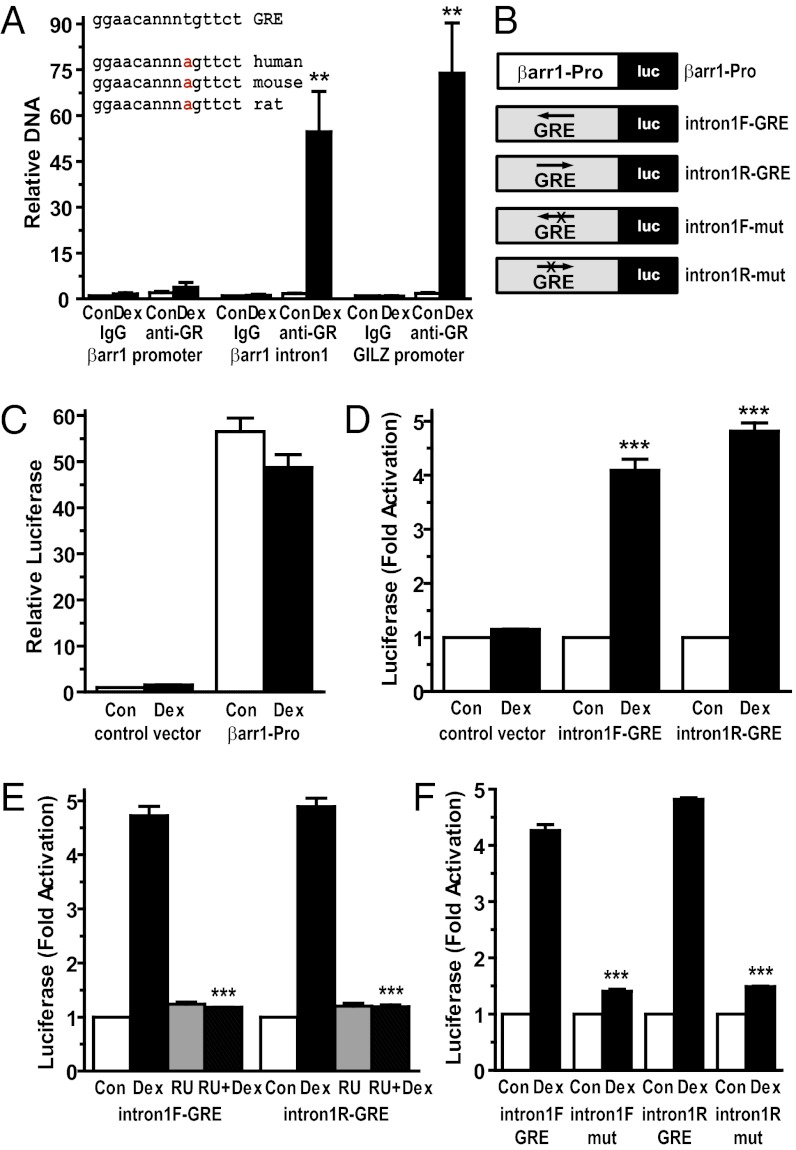
GR alters the transcription of many target genes by direct binding to specific sequences of DNA known as GREs (15). Sequence analysis of the β-arrestin-1 gene identified only one GRE, located in intron-1, that showed high homology to the consensus GRE and was conserved across species (Fig. 3A). We performed ChIP assays to investigate whether activated GRs were recruited to this intron-1 GRE in its native chromatin context. GR was strongly recruited to the β-arrestin-1 intron-1 GRE in A549 cells exposed to glucocorticoids for 90 min (a 31.9-fold enrichment over control levels) (Fig. 3A). A similar robust enrichment of activated GRs was observed at a functional GRE previously described in the distal promoter of the classic glucocorticoid-induced gene GILZ (Fig. 3A) (16). In contrast, GR was not significantly recruited to the β-arrestin-1 promoter (Fig. 3A).
Fig. 3.
Recruitment of activated GRs to a conserved and functional GRE in intron-1 of the β-arrestin-1 gene. (A) A549 cells were treated with vehicle (Con, control) or 100 nM Dex for 90 min, and ChIP assays were performed with equivalent amounts of IgG or anti-GR antibodies. Coimmunoprecipitated DNA was analyzed by quantitative RT-PCR using primers to the promoter of β-arrestin-1, the intron-1 GRE of β-arrestin-1, or a functional GRE previously identified in the promoter of the glucocorticoid-induced leucine zipper (GILZ) gene. Data are plotted as a function of input DNA and represent mean ± SEM for three independent experiments. (Inset) Sequence alignment of the consensus GRE and the GREs identified within intron-1 of the human, mouse, and rat β-arrestin-1 genes. (B) Schematic of β-arrestin-1 luciferase reporter plasmids used in this study. (C–F) A549 cells were transfected with the indicated luciferase reporter plasmids and treated for ∼18 h with vehicle, 100 nM Dex, 1 μM RU486 (RU), or both 1 μM RU486 and 100 nM Dex (RU + Dex). Cells were then harvested, and luciferase activity was measured. Data represent mean ± SD from three or four independent experiments. **P < 0.01; ***P < 0.001.
To assess whether the β-arrestin-1 intron-1 GRE functioned as a glucocorticoid-dependent enhancer in a heterologous system, we cloned an ∼500-bp fragment of intron-1 containing the GRE into a luciferase reporter in both the forward (GRE on the minus strand) and reverse (GRE on the plus strand) orientations (Fig. 3B). In addition, we cloned ∼1.7 kb of the β-arrestin-1 promoter into a luciferase reporter (Fig. 3B). A549 cells were transfected with the reporter plasmids, and luciferase activity was measured after an overnight treatment with vehicle or Dex. The β-arrestin-1 promoter stimulated luciferase expression to a 56.5-fold greater degree than the control vector; however, this activity was not significantly affected by glucocorticoid treatment (Fig. 3C). In contrast, glucocorticoids induced 4.1-fold and 4.8-fold increases in luciferase from reporters containing the intron-1 fragment in the forward and reverse orientations, respectively (Fig. 3D). The Dex-mediated increase in luciferase was abolished by cotreatment of cells with RU486 and by mutations that disrupted the intron-1 GRE (Fig. 3 E and F). Collectively, these results demonstrate that the GRE in intron-1 of the β-arrestin-1 gene binds activated GRs in cells and confers GR-dependent induction to a target gene.
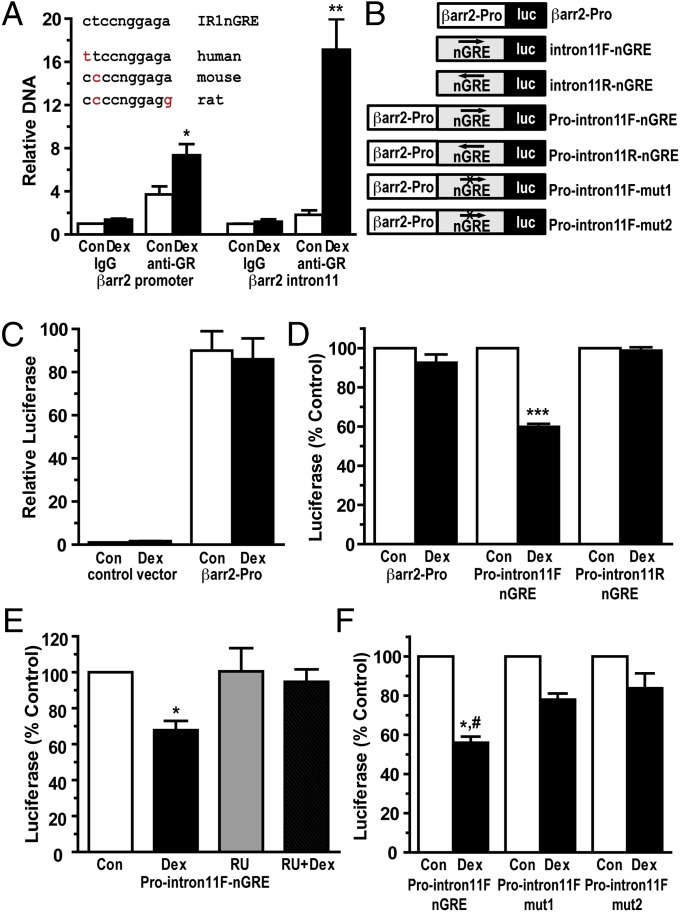
Our search of the β-arrestin-2 gene for GR-binding sites did not reveal a classic GRE; however, it did uncover five sites with high homology to the inverted repeat negative GRE (nGRE), which was recently reported to mediate glucocorticoid-dependent repression of many target genes (17). Two nGREs were located upstream of the transcription start site, and single nGREs were found within intron-1, intron-5, and intron-11. We focused our attention on the intron-11 nGRE, because this site was conserved across species and resided within a ∼800-bp region of DNA that was recently identified by ChIP-seq analysis as the only significant region of GR binding in the β-arrestin-2 gene (Fig. 4A) (18). We performed a ChIP assay on A549 cells treated with Dex for 90 min to verify recruitment of GRs to this intron-11 nGRE. In response to glucocorticoids, GR was strongly recruited to the β-arrestin-2 intron-11 nGRE (a 9.4-fold enrichment over control levels) (Fig. 4A). A small but significant (2.0-fold) enrichment of activated GRs was detected at the nGRE-containing β-arrestin-2 promoter (Fig. 4A).
Fig. 4.
Recruitment of activated GRs to a conserved and functional nGRE in intron-11 of the β-arrestin-2 gene. (A) A549 cells were treated with vehicle (Con, control) or 100 nM Dex for 90 min, and ChIP assays were performed with equivalent amounts of IgG or anti-GR antibodies. Coimmunoprecipitated DNA was analyzed by quantitative RT-PCR using primers to the promoter of β-arrestin-2 or to the intron-11 nGRE of β-arrestin-2. Data are plotted as a function of input DNA and represent mean ± SEM for three independent experiments. (Inset) Sequence alignment of the consensus IR1nGRE and the IR1nGREs identified within intron-11 of the human, mouse, and rat β-arrestin-2 genes. (B) Schematic of β-arrestin-2 luciferase reporter plasmids used in this study. (C–F) A549 cells were transfected with the indicated luciferase reporter plasmids and treated for ∼18 h with vehicle, 100 nM Dex, 1 μM RU486 (RU), or both 1 μM RU486 and 100 nM Dex (RU + Dex). Cells were then harvested, and luciferase activity was measured. Data represent mean ± SEM from between three and five independent experiments. *,#P < 0.05; **P < 0.01; ***P < 0.001.
To examine whether the β-arrestin-2 intron-11 nGRE functioned as a glucocorticoid-dependent repressor in a heterologous system, we cloned the ∼800-bp region of intron-11 containing the nGRE into a luciferase reporter in both the forward (nGRE on the plus strand) and reverse (nGRE on the minus strand) orientations (Fig. 4B). Approximately 1.0 kb of the β-arrestin-2 promoter was also cloned into a luciferase reporter (Fig. 4B). The reporter plasmids were transfected into A549 cells, and luciferase activity was measured after an ∼18-h incubation with vehicle or Dex. The β-arrestin-2 promoter enhanced luciferase expression by 90.0-fold over the control vector, but was unaffected by glucocorticoid treatment (Fig. 4C). The intron-11 nGRE-containing fragment by itself was inactive or stimulated luciferase expression only weakly (by 4-fold over the control vector), and its activity was insensitive to glucocorticoids (Fig. S4). We next investigated whether the intron-11 fragment could repress an active promoter by positioning the nGRE-containing DNA adjacent to the β-arrestin-2 promoter (Fig. 4B). In this configuration, the intron-11 fragment inhibited the ability of the β-arrestin-2 promoter to stimulate luciferase expression by 40.2% in the presence of glucocorticoids (Fig. 4D). Interestingly, this repression occurred only when the intron-11 fragment was in the forward orientation (nGRE on the plus strand) (Fig. 4D). The inhibition by glucocorticoids was reversed by cotreatment of cells with RU486 and by mutations that disrupt the intron-11 nGRE (Fig. 4 E and F). These results demonstrate that the nGRE within intron-11 of the β-arrestin-2 gene binds GRs in cells and confers GR-dependent repression to a target gene.
Glucocorticoid Regulation of β-Arrestin Gene Expression Redirects the Signaling Response of GPCRs.
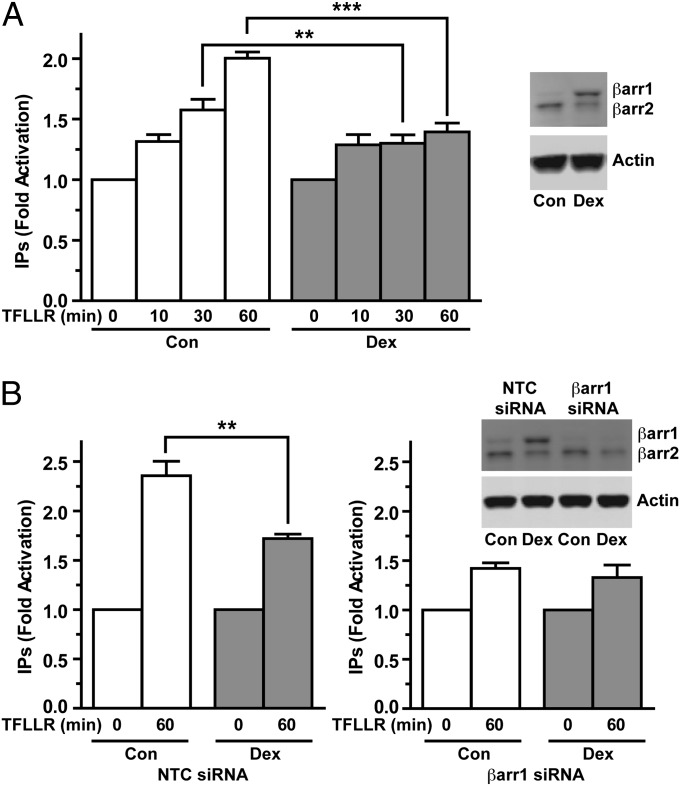
To determine whether the glucocorticoid-mediated changes in β-arrestin-1 and β-arrestin-2 gene expression alter GPCR activity, we investigated the signaling profile of PAR1. We chose PAR1 because it preferentially uses β-arrestin-1 both for desensitizing Gαq-mediated activation of phospholipase Cβ and for activating the MAPK pathway (19–21). In A549 cells, activation of endogenous PAR1 by the activating peptide TFLLR resulted in the dose-dependent stimulation of inositol phosphate and MAPK signaling (Fig. S5). We next compared the ability of PAR1 to couple to Gαq and stimulate the production of inositol phosphates in A549 cells pretreated with vehicle or Dex for 42 h. In control cells, addition of TFLLR for 10, 30, and 60 min resulted in a time-dependent increase in inositol phosphates that reached 1.3-, 1.6-, and 2.0-fold, respectively, over basal levels (Fig. 5A). In cells pretreated with Dex and exhibiting a 9.4-fold up-regulation of β-arrestin-1 (Fig. S6A), no difference in inositol phosphate production was seen after a short (10 min) incubation with TFLLR (Fig. 5A); however, inositol phosphate stimulation by PAR1 activation was reduced at the later 30-min (47.9% decrease) and 60-min (60.7% decrease) time points (Fig. 5A), a profile consistent with a greater degree of PAR1 desensitization. The reduced inositol phosphate signaling in the glucocorticoid-treated cells was not related to decreased PAR1 expression, given that Dex treatment induced a small transient rise in PAR1 mRNA and had no effect on PAR1 protein levels (Fig. S7). In addition, glucocorticoids did not appear to alter the conformation of PAR1 to diminish its signaling, given that the simultaneous treatment of A549 cells with both Dex and TFLLR for 1 h had no effect on PAR1 stimulation of inositol phosphates (Fig. S8).
Fig. 5.
Glucocorticoid-dependent up-regulation of β-arrestin-1 impairs PAR1 activation of inositol phosphate signaling. (A) A549 cells were pretreated with vehicle (Con, control) or 100 nM Dex for 42 h. Cells were then stimulated with the PAR1-activating peptide TFLLR (60 μM), and total [3H]inositol phosphates (IPs) were measured. Data were normalized to total lipids and plotted as fold increase over basal levels. (Right) Immunoblot of β-arrestin-1 and β-arrestin-2 from unlabeled cells processed in parallel. (B) A549 cells transfected with NTC siRNA or β-arrestin-1 siRNA were pretreated with vehicle or 100 nM Dex for 42 h. Cells were then stimulated with the PAR1-activating peptide TFLLR (60 μM) for 60 min, and [3H]inositol phosphates (IPs) were measured and analyzed as above. (Inset) Immunoblot of β-arrestin-1 and β-arrestin-2 from unlabeled cells processed in parallel. Quantitation of β-arrestin-1 and β-arrestin-2 levels is shown in Fig. S6. Data represent mean ± SD from three or four independent experiments. **P < 0.01; ***P < 0.001.
We next investigated whether the glucocorticoid-dependent increase in β-arrestin-1 was responsible for the impaired ability of PAR1 to couple to Gαq and stimulate inositol phosphate signaling. Consistent with elevated levels of β-arrestin-1 playing a role in this process, a greater amount of β-arrestin was observed to colocalize with TFLLR-activated FLAG-PAR1 at the plasma membrane in A549 cells pretreated with Dex for 42 h (Fig. S9). To more definitively assess the involvement of β-arrestin-1, we knocked down β-arrestin-1 using siRNA. In A549 cells transfected with nontargeting control (NTC) siRNA, TFLLR activation of PAR1 for 60 min stimulated a 2.4-fold increase in inositol phosphates in control cells, but only a 1.7-fold increase in cells pretreated with Dex for 42 h and exhibiting a 5.9-fold up-regulation in β-arrestin-1 (Fig. 5B and Fig. S6B). This 47.0% reduction in inositol phosphate production was similar to our findings in WT A549 cells treated with glucocorticoids (Fig. 5 A and B). In cells transfected with β-arrestin-1 siRNA, the basal expression of β-arrestin-1 was reduced, and the glucocorticoid up-regulation of β-arrestin-1 was abolished (Fig. 5B and Fig. S6B). These β-arrestin-1–deficient cells also exhibited a higher basal level of inositol phosphate, presumably reflecting increased activity of other GPCRs that are normally desensitized by β-arrestin-1. Nevertheless, TFLLR activation of PAR1 still resulted in a 1.4-fold induction of inositol phosphates in cells pretreated with vehicle. In contrast to our findings for WT and NTC siRNA-transfected cells, PAR1 stimulation of inositol phosphate was not repressed in the β-arrestin-1–deficient cells pretreated with glucocorticoids for 42 h, as a similar 1.3-fold increase was observed (Fig. 5B). These data indicate that the glucocorticoid-dependent increase in β-arrestin-1 gene expression underlies the impaired ability of PAR1 to couple to Gαq and stimulate inositol phosphate signaling.
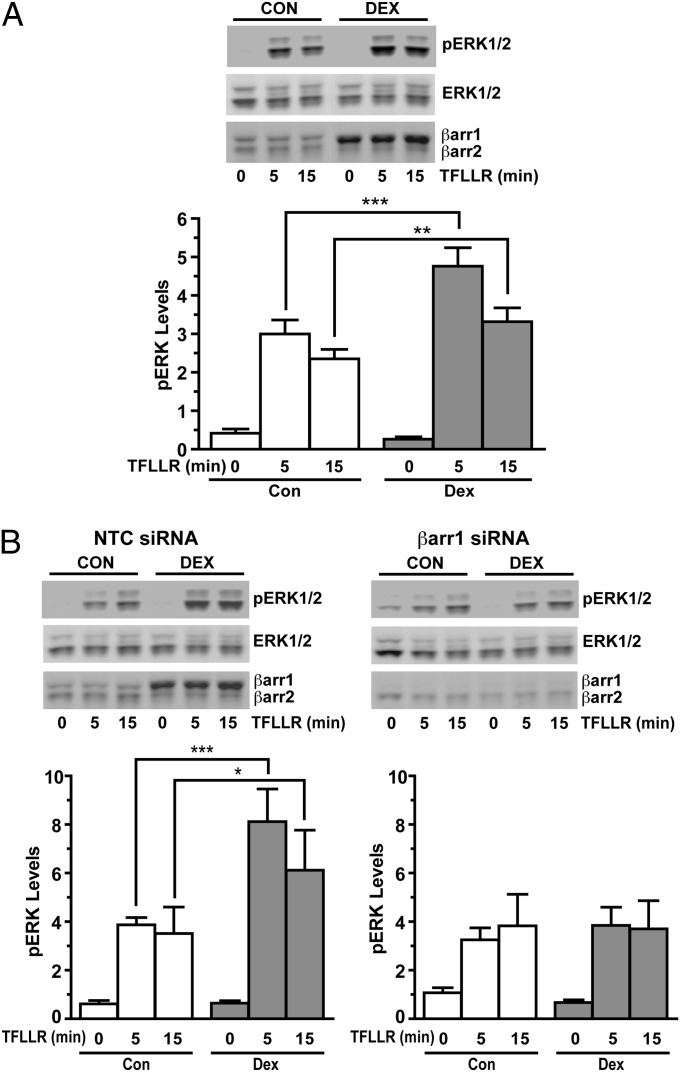
Whereas the binding of β-arrestin-1 to PAR1 shuts down G protein signaling (19, 21), it turns on PAR1 activation of the MAPK pathway (20). Therefore, we examined whether the up-regulation of β-arrestin-1 in glucocorticoid-treated cells enhances the ability of PAR1 to stimulate ERK phosphorylation. A549 cells were pretreated with vehicle or Dex for 48 h and then exposed to the PAR1-activating peptide TFLLR for 5 min and 15 min. In cells pretreated with vehicle, TFLLR activation of PAR1 resulted in a robust increase in phosphorylated ERK at both the 5-min and 15-min time points (Fig. 6A); however, in cells pretreated with Dex and showing a 4.4-fold increase in β-arrestin-1 (Fig. S6C), TFLLR activation of PAR1 produced a significantly greater stimulation of the MAPK pathway (Fig. 6A). The level of phosphorylated ERK at the 5-min and 15-min time points was increased by 159% and 141%, respectively, over the amount measured in cells cultured without glucocorticoids (Fig. 6A).
Fig. 6.
Glucocorticoid-dependent up-regulation in β-arrestin-1 enhances PAR1 activation of MAPK signaling. (A) A549 cells were pretreated with vehicle (Con, control) or 100 nM Dex for 48 h, then stimulated with the PAR1-activating peptide TFLLR (4 μM). (Upper) Immunoblots of phosphorylated ERK, total ERK, β-arrestin-1, and β-arrestin-2. (Lower) Quantitation of phosphorylated ERK normalized to total ERK. (B) A549 cells transfected with NTC siRNA or β-arrestin-1 siRNA were pretreated with vehicle or 100 nM Dex for 48 h, then stimulated with the PAR1-activating peptide TFLLR (4 μM). (Upper) Immunoblots of phosphorylated ERK, total ERK, β-arrestin-1, and β-arrestin-2. (Lower) Quantitation of phosphorylated ERK normalized to total ERK. Quantitation of β-arrestin-1 and β-arrestin-2 levels is shown in Fig. S6. Data represent mean ± SD from four independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether the enhanced stimulation of the MAPK pathway by activated PAR1 was mediated by the glucocorticoid-dependent increase in β-arrestin-1, we prevented the up-regulation of β-arrestin-1 by siRNA knockdown. In cells transfected with NTC siRNA, PAR1 stimulation of the MAPK pathway was greater in cells pretreated with Dex for 48 h and showing a 7.2-fold increase in β-arrestin-1 (Fig. 6B and Fig. S6D). Similar to our findings in WT A549 cells, the level of phosphorylated ERK at the 5-min and 15-min time points was increased by 210% and 174%, respectively, over the amount measured in cells not exposed to glucocorticoids. In marked contrast, the enhanced stimulation of ERK signaling by activated PAR1 was abolished in glucocorticoid-treated cells lacking the up-regulation of β-arrestin-1 (Fig. 6B and Fig. S6D). Collectively, these data indicate that the glucocorticoid-dependent increase in β-arrestin-1 expression is responsible for the enhanced ability of PAR1 to couple to and activate the MAPK pathway.
Discussion
In this study, we have shown that β-arrestin-1 and β-arrestin-2 are glucocorticoid-responsive target genes. We demonstrate in multiple cell types that glucocorticoids induce β-arrestin-1 and repress β-arrestin-2 expression through direct effects on transcription. The molecular nature of this regulation involves binding of activated GRs to a conserved and functional GRE and nGRE located within intron-1 of the β-arrestin-1 gene and intron-11 of the β-arrestin-2 gene, respectively. As a result of the glucocorticoid up-regulation of β-arrestin-1, the balance of PAR1 signaling is shifted away from G protein-dependent coupling (activation of phospholipase Cβ) toward more β-arrestin-1–dependent signaling (activation of ERK). These findings demonstrate a paradigm in which glucocorticoids modulate cellular responses to extracellular stimuli through the regulation of β-arrestin gene expression and consequent alterations in GPCR activity.
In addition to its classic role in platelet activation and the coagulation cascade, activated PAR1 promotes proinflammatory responses in many cell types (22). For example, PAR1 stimulates the release of numerous inflammatory mediators such as IL-8 and IL-6 in lung epithelial cells, and PAR1 activity in joints and the digestive tract has been implicated in the pathogenesis of arthritis and inflammatory bowel disease, respectively. Glucocorticoids are powerful anti-inflammatory agents, and the glucocorticoid-dependent up-regulation in β-arrestin-1 and redirection of PAR1 signaling may be a means by which glucocorticoids limit the proinflammatory response. In contrast to PAR1, which is desensitized primarily by β-arrestin-1 (19, 21), many GPCRs are preferentially desensitized by β-arrestin-2 (2). One member of this group with clinical relevance is the β2-adrenergic receptor (β2AR). Glucocorticoids are frequently used together with β2AR agonists in the treatment of obstructive airway diseases, such as asthma. The superior clinical efficacy of this combination therapy has led to the proposal that beneficial interactions occur between the GR and β2AR signaling pathways in the lung (23, 24). Our finding that glucocorticoids down-regulate β-arrestin-2 expression suggests that glucocorticoids might counteract β2AR desensitization. A reduction in β-arrestin-2 would allow agonist-bound β2AR to couple longer to Gαs proteins and promote a greater and/or more sustained relaxation response in airway smooth muscle cells. In lung epithelial cells, the glucocorticoid-dependent repression of β-arrestin-2 also might contribute to the clinical synergism by impairing the proinflammatory effects of β2AR agonists that require receptor coupling to the MAPK pathway through the scaffolding function of β-arrestin-2. Consistent with the beneficial effects of decreased β-arrestin-2 expression in the treatment of obstructive airway disease, genetic deletion of the β-arrestin-2 gene was found to prevent airway hyperresponsiveness in a mouse model of asthma (25).
In summary, we have shown that glucocorticoids directly regulate the expression of the β-arrestin-1 and β-arrestin-2 genes and thereby redirect the signaling profile of GPCRs. Of current therapeutic interest is the development of GPCR ligands that specifically activate G protein-dependent signaling pathways and not β-arrestin–dependent responses, and vice versa (4, 26). The hope is that these selective or biased agonists will promote therapeutically beneficial signaling while curtailing cellular responses that adversely affect the patient. Our data indicate that glucocorticoids can shift the balance of G protein- and β-arrestin–dependent responses for a given GPCR via changes in β-arrestin gene expression. Thus, certain combinations of glucocorticoids and GPCR agonists may be more therapeutically beneficial than the receptor agonist alone.
Materials and Methods
Quantitative RT-PCR Analysis.
The abundance of individual mRNAs was determined using a Taqman one-step RT-PCR procedure with a 7900HT sequence detection system (Applied Biosystems).
Immunoblot Analysis.
Membranes with equivalent amounts of protein were incubated with primary antibodies to β-arrestin-1, β-arrestin-2, GR, phosphorylated ERK, total ERK, PAR1, or β-actin and developed using the LI-COR Odyssey Imaging System.
ChIP Assays.
A549 cells were fixed in 1% paraformaldehyde, and DNA was sheared by sonication. Protein–DNA complexes were immunoprecipitated with equivalent amounts of IgG or anti-GR antibodies. Quantitative RT-PCR was performed on both input and immunoprecipitated DNA.
Luciferase Assays.
A549 cells were transfected with firefly and Renilla luciferase reporters, and luciferase activity was measured using the Dual Luciferase Reporter Assay (Promega).
MAPK Assays.
A549 cells were pretreated with vehicle or 100 nM Dex for 48 h. During the last ∼18 h of this preincubation, cells were cultured in serum-free medium. The PAR1-activating peptide TFLLR was added, lysates were prepared, and phosphorylated ERK and total ERK were quantified by immunoblot analysis.
Inositol Phosphate Assays.
A549 cells were pretreated with vehicle or 100 nM Dex for 42 h. During the last ∼18 h of this preincubation, cells were labeled with 2.5 μCi/mL of [myo-3H]inositol (Perkin-Elmer) in serum- and inositol phosphate-free DMEM. The PAR1-activating peptide TFLLR was added to the cells, and [3H]inositol phosphates were separated by anion-exchange chromatography and quantified by scintillation counting.
Statistical Analysis.
The t test or one-way ANOVA with Tukey’s post hoc analysis was used to determine statistical significance (P < 0.05). All statistical analyses were performed using GraphPad Prism software. All experimental procedures are described in more detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Robert J. Lefkowitz (Duke University) for providing the mouse embryonic fibroblasts and Dr. JoAnn Trejo (University of California at San Diego) for providing the FLAG-PAR1 expression vector. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209411109/-/DCSupplemental.
References
- 1.Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- 2.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 3.Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell LM, Gesty-Palmer D. Beyond desensitization: Physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bychkov ER, Gurevich VV, Joyce JN, Benovic JL, Gurevich EV. Arrestins and two receptor kinases are upregulated in Parkinson’s disease with dementia. Neurobiol Aging. 2008;29:379–396. doi: 10.1016/j.neurobiolaging.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 9.Luan B, et al. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 10.Archacki SR, et al. Identification of new genes differentially expressed in coronary artery disease by expression profiling. Physiol Genomics. 2003;15:65–74. doi: 10.1152/physiolgenomics.00181.2002. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, et al. Elevated β-arrestin1 expression correlated with risk stratification in acute lymphoblastic leukemia. Int J Hematol. 2011;93:494–501. doi: 10.1007/s12185-011-0824-9. [DOI] [PubMed] [Google Scholar]
- 12.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 14.Hershko DD, Robb BW, Wray CJ, Luo GJ, Hasselgren PO. Superinduction of IL-6 by cycloheximide is associated with mRNA stabilization and sustained activation of p38 map kinase and NF-kappaB in cultured caco-2 cells. J Cell Biochem. 2004;91:951–961. doi: 10.1002/jcb.20014. [DOI] [PubMed] [Google Scholar]
- 15.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JC, et al. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA. 2004;101:15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surjit M, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Reddy TE, et al. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19:2163–2171. doi: 10.1101/gr.097022.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CH, Paing MM, Trejo J. Termination of protease-activated receptor-1 signaling by beta-arrestins is independent of receptor phosphorylation. J Biol Chem. 2004;279:10020–10031. doi: 10.1074/jbc.M310590200. [DOI] [PubMed] [Google Scholar]
- 20.Kuo FT, Lu TL, Fu HW. Opposing effects of beta-arrestin1 and beta-arrestin2 on activation and degradation of Src induced by protease-activated receptor 1. Cell Signal. 2006;18:1914–1923. doi: 10.1016/j.cellsig.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. Beta-arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 22.Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol Ther. 2009;123:292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ. Biochemical basis of asthma therapy. J Biol Chem. 2011;286:32899–32905. doi: 10.1074/jbc.R110.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M, Michel MC. How can 1 + 1 = 3? β2-adrenergic and glucocorticoid receptor agonist synergism in obstructive airway diseases. Mol Pharmacol. 2011;80:955–958. doi: 10.1124/mol.111.075481. [DOI] [PubMed] [Google Scholar]
- 25.Walker JK, et al. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.