Abstract
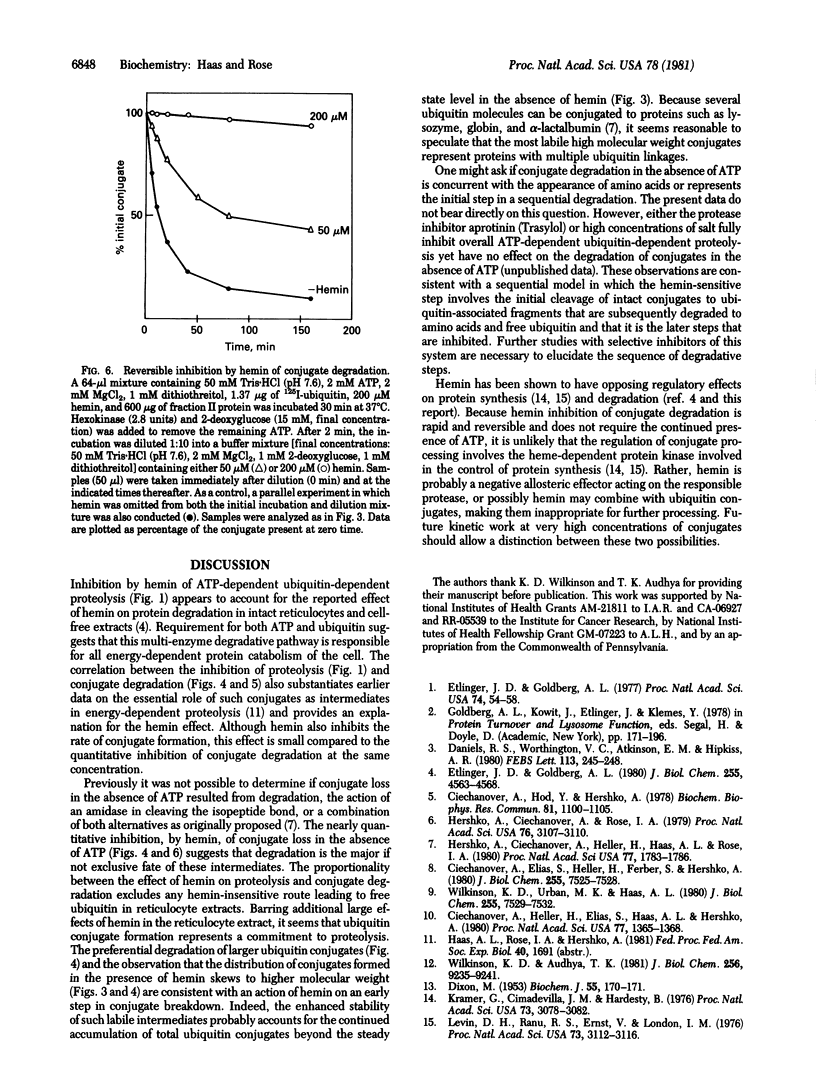
Hemin has been shown to inhibit specifically the energy-dependent degradation of normal and abnormal proteins in reticulocytes [Etlinger, J. D. & Goldberg, A. L. (1980) J. Biol. Chem. 255, 4563-4568]. The present work demonstrates that the action of hemin involves the multi-enzyme ATP-dependent ubiquitin-dependent proteolytic system exclusively. At a concentration of approximately 25 microM, hemin produces 50% inhibition of the degradation of 125I-labeled bovine serum albumin by this pathway. Hemin has no effect on the basal rate of proteolysis in the absence of either ATP or ubiquitin. At a concentration of hemin that gives complete inhibition of proteolysis, ATP-dependent formation of ubiquitin conjugates continues at about 50% of the control rate but the degradation of these ubiquitin conjugates is completely blocked. Inhibition of overall proteolysis and conjugate degradation are sensitive to hemin concentration to exactly the same extent. Hemin inhibition of conjugate breakdown results in the accumulation of higher molecular weight conjugates that are lost when hemin is removed by dilution. A model is proposed in which hemin acts as a negative allosteric effector in the initial step of a sequential degradative path by which intact ubiquitin conjugates are first cleaved to ubiquitin-associated fragments.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciechanover A., Elias S., Heller H., Ferber S., Hershko A. Characterization of the heat-stable polypeptide of the ATP-dependent proteolytic system from reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7525–7528. [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. S., Worthington V. C., Atkinson E. M., Hipkiss A. R. Proteolysis of puromycin-peptides in rabbit reticulocytes: detection of a high molecular weight oligopeptide proteolytic substrate. FEBS Lett. 1980 May 5;113(2):245–248. doi: 10.1016/0014-5793(80)80602-8. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. Control of protein degradation in reticulocytes and reticulocyte extracts by hemin. J Biol Chem. 1980 May 25;255(10):4563–4568. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Rose I. A. Resolution of the ATP-dependent proteolytic system from reticulocytes: a component that interacts with ATP. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3107–3110. doi: 10.1073/pnas.76.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K. D., Audhya T. K. Stimulation of ATP-dependent proteolysis requires ubiquitin with the COOH-terminal sequence Arg-Gly-Gly. J Biol Chem. 1981 Sep 10;256(17):9235–9241. [PubMed] [Google Scholar]
- Wilkinson K. D., Urban M. K., Haas A. L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7529–7532. [PubMed] [Google Scholar]