Abstract
Variability in opioid analgesia has been attributed to many factors. For example, genetic variability of the μ-opioid receptor (MOR)-encoding gene introduces variability in MOR function and endogenous opioid neurotransmission. Emerging evidence suggests that personality trait related to the experience of reward is linked to endogenous opioid neurotransmission. We hypothesized that opioid-induced behavioral analgesia would be predicted by the trait reward responsiveness (RWR) and the response of the brain reward circuitry to noxious stimuli at baseline before opioid administration. In healthy volunteers using functional magnetic resonance imaging and the μ-opioid agonist remifentanil, we found that the magnitude of behavioral opioid analgesia is positively correlated with the trait RWR and predicted by the neuronal response to painful noxious stimuli before infusion in key structures of the reward circuitry, such as the orbitofrontal cortex, nucleus accumbens, and the ventral tegmental area. These findings highlight the role of the brain reward circuitry in the expression of behavioral opioid analgesia. We also show a positive correlation between behavioral opioid analgesia and opioid-induced suppression of neuronal responses to noxious stimuli in key structures of the descending pain modulatory system (amygdala, periaqueductal gray, and rostral–ventromedial medulla), as well as the hippocampus. Further, these activity changes were predicted by the preinfusion period neuronal response to noxious stimuli within the ventral tegmentum. These results support the notion of future imaging-based subject-stratification paradigms that can guide therapeutic decisions.
Keywords: functional MRI, functional imaging, endophenotype
Opioids are the mainstay of moderate to severe pain management, but there is considerable variation in the analgesic response leading to inadequate analgesia for many patients (1). This variability has been attributed to many factors including the genetic variability of the μ-opioid receptor (MOR)-encoding gene that introduces variability in MOR function and endogenous opioid neurotransmission (2). Endogenous opioid neurotransmission mediates exogenous opioid analgesia (3), increases in response to noxious stimuli reducing the unpleasantness of noxious stimuli (4), enhances the pleasantness of rewarding stimuli (5), and influences the responsiveness of an individual to rewards (6). Therefore, endogenous opioids play a central role not only in mediating exogenous opioid analgesia, but also in endogenous modulation of pain perception and reward processing.
Reward is not one simple construct, and there are many different types of reward such as monetary gain, palatable food, mood enhancing drugs, and social reward. An increasingly well-identified set of brain regions consisting of the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), the ventral striatum, ventral pallidum, and the midbrain tegmentum as well as the prefrontal cortex, amygdala and the hippocampus are differentially involved in processing these many types of rewards (7). Further, activation in these brain regions are differentially involved in processing different aspects of reward such as prediction of (8, 9), anticipation of (10), and magnitude of (11) reward. Analgesia is rewarding and indeed human imaging studies demonstrate the role of the accumbens in anticipation of analgesia where the reward was placebo analgesia (12) and in predicting analgesia where the reward was offset analgesia (13). Crucially, several of these structures, both in humans (14, 15) and in animals (16–18), also contain MORs and play a role in opioid-induced behavioral analgesia.
These combined observations strongly suggest that behavioral opioid analgesia depends on endogenous opioid neurotransmission and involves components of the reward network. As reward responsiveness of an individual is also influenced by endogenous opioid neurotransmission in key structures of the reward network, we hypothesized that opioid-induced behavioral analgesia would be predicted by trait reward responsiveness (RWR) and activity in parts of the reward circuitry to noxious stimulation at baseline before opioid infusion. These relationships have not been determined to date, yet could have translational relevance, as measures that better stratify patients as positive responders to therapeutic interventions are increasingly being sought.
We induced opioid analgesia to noxious heat stimuli using an i.v. infusion of the short-acting MOR agonist, remifentanil, in a large group of healthy volunteers. We measured the neuronal response [blood oxygen level dependent (BOLD) response] to these stimuli before the infusion and during the infusion using the noninvasive technique of functional magnetic resonance imaging (fMRI). We used the reward responsiveness subscale of Carver and White’s behavioral inhibition system/behavioral activation system (BIS/BAS) scale (19) to measure the individual’s response to rewarding stimuli.
Results
Twenty-five subjects (mean age, 30 y; age range, 21–46 y; 11 females) attended two visits where moderately painful heat stimuli were delivered via a contact thermode on the right forearm, as blocks of 10 stimuli before (preinfusion) and during a 40-min infusion of μ-opioid agonist (remifentanil) or saline (control visit). The main experimental design is previously published (20). The experimental design is outlined in Fig. S1. Remifentanil induced a significant reduction (P < 0.01) in heat pain intensity ratings (Fig. 1) and a significant increase in mental and physical sedation (Fig. S2). The increases in tranquility and sociability scores were not statistically significant. The opioid-induced changes in psychophysical variables were defined as [v opioid(infusion − preinfusion)] − [v saline(infusion – preinfusion)], where v is the psychophysical variable. A negative value indicates a reduction. See SI Results for details of opioid-induced changes in mental and physical sedation, tranquility, sociability, and cardiorespiratory variables.
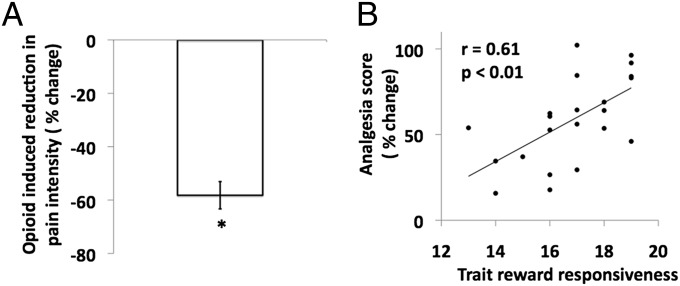
Fig. 1.
Opioid-induced reduction in pain intensity and behavioral analgesia. (A) Y axis shows the mean opioid-induced reduction in pain intensity (percentage of change) of heat noxious stimuli. The opioid-induced reduction in pain intensity is defined as [v opioid(infusion−preinfusion)] − [v saline(infusion–preinfusion)], where v is the pain intensity. Error bars indicate SEM (one-sample t test; *P < 0.01). (B) Scatter plot of significant positive correlation (r = 0.61; P = 0.002) between the opioid-induced behavioral analgesia as percentage of change in pain intensity (y axis) and the trait reward responsivness score (x axis). To depict analgesia as a positive value, it is defined as [v opioid(preinfusion−infusion)] − [v saline(preinfusion−infusion)], where v is pain intensity. Pearson’s r and P values are shown in the scatter plot.
Main Psychophysical Finding.
As hypothesized, the magnitude of behavioral analgesia correlated positively with trait RWR (r = 0.61; P < 0.01) (Fig. 1). To express opioid-induced behavioral analgesia as a positive value, we defined it as [v opioid(preinfusion−infusion)] − [v saline(preinfusion−infusion)], where v is the pain intensity. This effect is driven by the remifentanil infusion and not by the saline infusion (Fig. S3).
The opioid-induced changes in sedation, tranquility, and sociability did not show a significant correlation with either opioid-induced behavioral analgesia or trait RWR.
We used a perception-locked stimulus in all subjects for both visits by adjusting the temperature of the contact-thermode stimulus to achieve a subjective rating of moderate pain. The stimulus temperature was kept constant between baseline and the subsequent remifentanil/saline block for an individual, but the temperature was not the same for every subject. However, there was no significant difference between the group mean temperatures used for the two visits. The mean temperature (±SD) used was 50.8 °C (±1.7) during the saline visit and 50.6 °C (±1.9) during the remifentanil visit. Importantly, there was no relationship between the temperature used and the magnitude of analgesia subsequently produced (Fig. S4).
BOLD Signal Change to Noxious Stimuli During the Preinfusion Period.
There are no significant differences in the neuronal response (BOLD signal change) to noxious stimuli between the remifentanil and saline preinfusion periods anywhere in the brain. This is consistent with preinfusion period psychophysical data that show no difference between the two visits (SI Results).
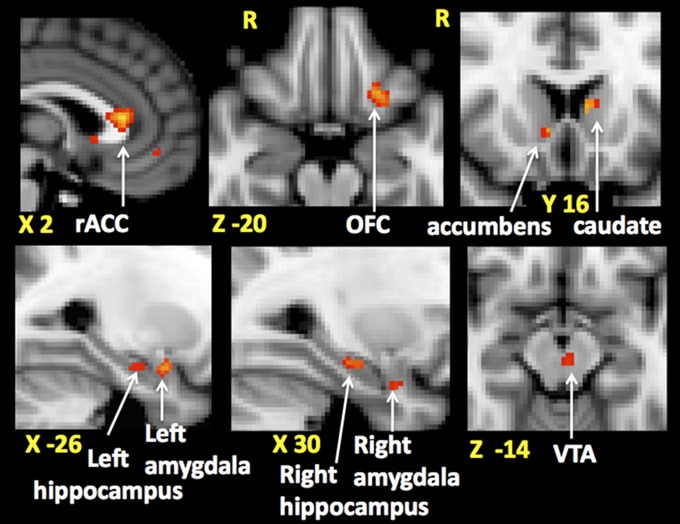
The average neuronal response to noxious stimuli during the preinfusion period for each subject was generated by averaging the two preinfusion period functional scans. Using these maps in a model with a regressor for the analgesia score, we asked whether the magnitude of analgesia is predicted by the preinfusion period neuronal response anywhere in the brain (whole brain analysis) and in nine reward-processing areas of the brain (hypothesis-based directed searches). This revealed that the higher the preinfusion period brain activity to noxious stimuli in reward-related areas of the brain the greater the subsequent behavioral analgesia. These baseline, preinfusion active brain areas were: the left orbitofrontal cortex (OFC) and rostral anterior cingulate cortex (rACC) identified using whole brain analysis, bilateral hippocampi, bilateral amygdala, the left caudate, the right nucleus accumbens (NAc), and ventral tegmental area (VTA) (Fig. 2) identified using directed searches (masks shown in Fig. S5 and localization of VTA activity shown in Fig. S6). This relationship is illustrated with scatter plots in Fig. S7.
Fig. 2.
Areas of the brain where preinfusion period neuronal response to noxious stimuli predict opioid-induced behavioral analgesia. Behavioral analgesia (percentage of change in pain intensity) is defined as [v opioid(preinfusion−infusion)] − [v saline(preinfusion−infusion)], where v is pain intensity. Clusters of voxels in the rostral anterior cingulate cortex (rACC), left orbitofrontal cortex (OFC), right nucleus acumbens, left caudate (in the Upper row from Left to Right), left amygdala and hippocampus, right amygdala and hippocampus, ventral tegmental area (VTA), (Lower row, Left to Right) are shown. Montreal Neurological Institute coordinates are denoted in millimeters below each slice.
Further, we also found a positive correlation between the preinfusion period brain activity (extracted percentage of BOLD) from voxels that predicted behavioral analgesia and subjects’ trait RWR scores. This relationship is statistically significant in all areas except for the VTA and left hippocampus (SI Results).
To determine whether any of these brain regions predict analgesia independent of that predicted from RWR, we performed a partial correlation between the brain activities identified at baseline as predictive of behavioral analgesia after accounting for RWR score. This revealed that the preinfusion period brain activity significantly predicted the behavioral analgesia score independent of RWR in the following brain areas: VTA (r = 0.507; P = 0.016), left caudate (r = 0.563; P = 0.017), left OFC (r = 0.448; P = 0.037), and rACC (r = 0.505; P = 0.017). By combining all brain regions identified at baseline as predictive of behavioral analgesia independent of RWR, the significance was r = 0.571; P = 0.005.
Opioid-Induced Changes in Neuronal Response to Noxious Stimuli.
For each subject, the brain activity map representing the opioid-induced changes was generated using the functional scans obtained from the two preinfusion periods and the two infusion periods. Opioid-induced changes are defined as [v opioid(preinfusion−infusion)] − [v saline(preinfusion–infusion)], where v is the neuronal response evoked by noxious stimuli. These maps were used in a model containing a regressor for the group mean of the opioid-induced changes in brain activity and another regressor for the analgesia score. This identified brain areas with opioid-induced changes in activity and brain areas where the change in activity specifically correlated with the analgesia score. Using hypothesis-based directed searches we specifically asked whether nine reward-related brain regions that predicted the analgesia magnitude during the preinfusion period influenced the expression of behavioral analgesia during the infusion.
The opioid suppressed the neuronal response to noxious stimuli in bilateral insular, bilateral basal ganglia, and the anterior cingulate cortex (ACC) (whole brain analysis) (Fig. S8).
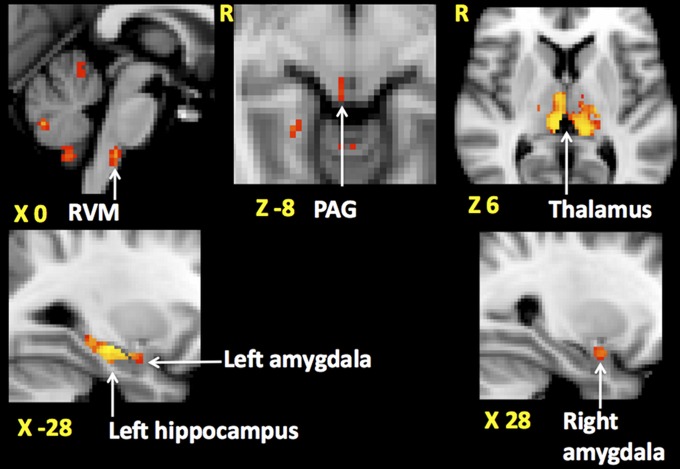
The more informative analysis exploring the relationship between opioid-induced neuronal response and opioid analgesic score revealed a significant positive correlation between the opioid-induced changes in brain activity and the magnitude of behavioral analgesia in the following areas: bilateral thalamus, periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) using whole brain analysis (localization of RVM shown in Fig. S6), left hippocampus, and bilateral amygdala (Fig. 3) using directed searches (masks shown in Fig. S5). This relationship is illustrated with scatter plots in Fig. S9. Interestingly, the opioid-induced changes in brain activity from voxels in these areas failed to show a significant correlation with trait RWR (SI Results).
Fig. 3.
Areas of the brain where opioid-induced changes in neuronal response to noxious stimuli show a significant positive correlation with opioid-induced behavioral analgesia. Opioid-induced changes in neuronal response and behavioral analgesia are defined as [v opioid(preinfusion−infusion)] − [v saline(preinfusion−infusion)], where v is the neuronal response or the pain intensity of noxious stimuli. A positive value for the change in neuronal response indicates an opioid-induced reduction. A positive correlation means the higher the behavioral analgesia the higher the opioid-induced suppression of the neuronal response. Clusters of voxels in rostral ventromedial medulla (RVM), periaqueductal gray (PAG), bilateral thalamus (in the Upper row from Left to Right), left amygdala and hippocampus and the right amygdala (Lower row, Left to Right) are shown. Montreal Neurological Institute coordinates are denoted in millimeters below each slice.
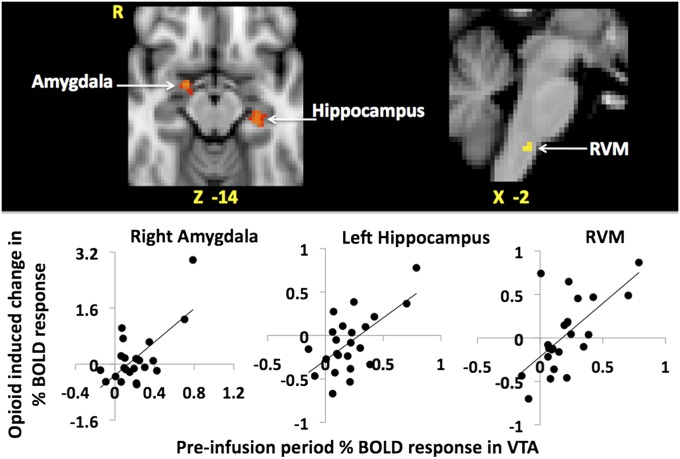
There was no significant correlation between the opioid-induced changes in brain activity and the magnitude of analgesia in the rACC, left OFC, left caudate nucleus, right NAc, and VTA, areas of the reward circuitry that predicted behavioral opioid analgesia in the preinfusion period. Rather it is possible that these areas drive brain activity changes during the opioid infusion within those specific brain regions whose opioid-induced change in neural activity does correlate with behavioral analgesia (i.e., bilateral amygdala, hippocampus, PAG, and RVM). To investigate this, we used the group mean maps of the opioid-induced changes in brain activity and a regressor for the neuronal (extracted percentage of BOLD) response to noxious stimuli during the preinfusion period from the voxels in the right NAc and VTA (key nuclei in the reward circuitry) that predicted the behavioral analgesia score. We limited our searches to the bilateral amygdala, left hippocampus, PAG, and RVM as they contributed to the expression of opioid analgesia in our study and are components of the descending pain modulatory system (DPMS) and reward circuitry (masks shown in Fig. S5). We found a significant positive correlation between the preinfusion period neuronal response to noxious stimuli in the VTA and the opioid-induced changes in the right amygdala, left hippocampus, and the RVM only (Fig. 4). The preinfusion period brain activity to noxious stimuli in the right NAc showed a significant positive correlation only with the opioid-induced changes in neural activity in the RVM.
Fig. 4.
Areas of the brain where preinfusion period neuronal response to noxious stimuli in the ventral tegmental area (VTA) show a significant positive correlation with opioid-induced changes in neuronal response to noxious stimuli. Opioid-induced changes in neuronal (percentage of BOLD) response are defined as [v opioid(preinfusion−infusion)] − [v saline(preinfusion−infusion)], where v is the neuronal response to noxious stimuli. A positive value for the change in neuronal response indicates an opioid-induced reduction. A positive correlation means the higher the preinfusion period neuronal response in the VTA the higher the opioid-induced suppression of the neuronal response. Clusters of voxels in right amygdala, left hippocampus, and rostral ventromedial medulla (RVM) (in the Upper row from Left to Right), are shown. Montreal Neurological Institute coordinates are denoted in millimeters below each slice. The respective scatter plots below illustrates these relationships.
Discussion
To improve the understanding of factors contributing to the variability of the opioid analgesic response, we investigated the relationship between behavioral opioid analgesia and neural correlates of opioid-induced analgesia, trait RWR, and the responsiveness of the reward network to noxious stimuli at baseline as a likely predictive network.
Carver and White’s RWR scale measures individual differences in sensitivity to reward signals and the ability to respond positively when exposed to such signals (19). We used this scale in our cohort of subjects and found that those individuals with high RWR score experienced a higher magnitude of behavioral opioid analgesia, in this instance the reward being the analgesia. Our imaging results show that the magnitude of this reward i.e., analgesia is predicted by the preinfusion period neuronal response to a noxious stimulus in areas of the left OFC, rACC, bilateral hippocampi, bilateral amygdala, the left caudate, the right NAc, and VTA: areas that play a role in reward processing (7). This neuronal response was higher in individuals with a high RWR score. Human opioid receptor imaging studies indicate that several of these regions are components of the endogenous opioid system (14) and show increased activation in response to noxious stimuli (4) particularly in those individuals with higher scores on reward-related personality traits (21). Based on these findings, although our study is not a receptor imaging study, we believe that the neuronal response to noxious stimuli in a similar set of areas is reflecting the reactivity of the endogenous opioid neurotransmission. As exogenous opioids mediate behavioral analgesia via the endogenous opioid system (3) it is not surprising that the reactivity of this system is able to predict the magnitude of subsequent exogenous opioid-induced behavioral analgesia experienced by our subjects. As reward-related personality traits are underpinned by endogenous opioid neurotransmission, we believe that the RWR score in our subjects is a psychophysical measure of the reactivity of the areas of the endogenous opioid system that predicted opioid analgesia. Of these brain areas the baseline activity in the VTA, left caudate, rACC, and the left OFC predicted the behavioral analgesia score beyond RWR, illustrating the utility of fMRI rather than behaviorally based measures as potentially useful predictive metrics as has been shown in other domains (10).
Opioid-Induced Changes in Neuronal Response to Noxious Stimuli.
In line with previous studies, the opioid induced a significant suppression of the neuronal response to noxious heat stimuli in many areas of the cerebral pain network (22, 23). As this result is derived from a group of low, medium, and high opioid analgesic responders, the threshold criterion will not necessarily show significant changes in all pain-related brain regions.
Therefore, to extract more meaningful results, we examined the relationship between the opioid-induced change in the neuronal response to noxious stimuli and the magnitude of behavioral analgesia. We observed a significant positive correlation between these two measures in areas of the DPMS, specifically the PAG, RVM, and amygdala. These areas of the DPMS receive nociceptive information from the periphery (24), contain MORs, and when activated by exogenous opioids contribute to opioid-induced behavioral analgesia (18, 25). The amygdala has direct projections to the PAG (26), which in turn projects to the RVM (27). PAG and RVM contain “on cells” and “off cells” (28, 29). In animals, on cells start firing while off cells stop firing in response to a noxious stimulus. Therefore, it is possible that the increased neuronal response to noxious stimuli in these areas observed in human imaging studies (30, 31) indicates the activity of the on cells. In our study, we define opioid-induced changes as [v opioid(preinfusion–infusion)] − [v saline(preinfusion−infusion)], where v is the neuronal response to noxious stimuli. A positive value indicates the magnitude of the opioid-induced reduction of the neuronal response. This means that the higher the analgesia the lower the activation of these areas in response to noxious stimulation. We believe this to represent the reduced nociceptive input to these supraspinal sites by the opioid-induced inhibition of the nociceptive transmission at the spinal dorsal horn. This would be achieved by the direct inhibitory action of opioids on the dorsal horn nociceptive transmission (32) and the indirect inhibitory action of opioids via activation of the descending inhibitory pathways (disinhibition of the off cells) or via suppression of the descending facilitatory pathways (inhibition of the on cells) (27).
Although the preinfusion period neuronal response to noxious stimuli in areas of the reward circuitry such as the rACC, OFC, NAc, caudate nucleus, and the VTA predicted opioid-induced behavioral analgesia, the opioid-induced brain activity in these structures did not correlate with the behavioral opioid analgesia reflecting a dissociation. It is possible that these structures influence the expression of behavioral opioid analgesia indirectly via other areas of the reward circuitry such as the amygdala and the hippocampus. Animal studies show that dopamine neurons of the VTA contribute to opioid-induced analgesia (17) and send efferents to both the hippocampus and the amygdala (33, 34). In keeping with these anatomical and functional links, we found that the preinfusion period neuronal response of the VTA that predicted the subsequent behavioral analgesia also significantly predicted the opioid-induced changes in neuronal activity in the right amygdala and the left hippocampus, areas that were shown to influence the expression of behavioral analgesia in our study. A similar relationship between the NAc and the hippocampus and the amygdala was not strong enough to survive statistical corrections. This is most likely because the NAc influences these structures indirectly via the pallidum and the VTA (35). Areas such as the rACC, a key area of the endogenous opioid system, could also be influencing the expression of opioid analgesia via its well-known functional links with the PAG and the RVM (15, 36).
Role of the Amygdala and the Hippocampus in Opioid Analgesia.
The hippocampus and the amygdala are components of the reward network with anatomical and functional links that regulate goal-directed behavior through the NAc (35). This functional congruity of the hippocampus, amygdala, and NAc is demonstrated in our results where the magnitude of a reward (behavioral opioid analgesia) is predicted by the neuronal response of these structures to an aversive stimulus.
Importantly, the opioid-induced changes in the brain activity in the amygdala and the hippocampus also show a significant positive correlation with the behavioral analgesia score, similar to that observed in the PAG and RVM. It is likely that the amygdala, rich in MORs, contributes to and influences the expression of behavioral opioid analgesia most probably via its connections with the PAG and RVM.
Based on preclinical studies, nociceptive processing in the hippocampus is thought to be intensity dependent (37). Consistent with these findings, human imaging studies have reported activation of the hippocampus in an intensity-dependent manner whether the change in perceived intensity of a noxious stimulus is due to increased stimulus intensity or anxiety (38, 39). Our finding that the higher the analgesia the higher the opioid-induced suppression of the neuronal response to noxious stimuli is in keeping with the intensity-dependent nature of nociceptive processing in the hippocampus.
Based on preclinical studies (16) it is also possible that in our subjects, opioids act directly on the hippocampus suppressing the neuronal response to noxious stimuli. Interestingly, the hippocampus contains neurons that are excited by noxious stimuli and neurons that are inhibited by noxious stimuli (40). Furthermore, pain and analgesic behaviors can both be produced by varying the frequency of the stimulating electrode placed in the hippocampus without changing the site of stimulation (41). This bidirectional response to nociceptive stimuli and the presence of endogenous opioid receptors make it possible for the hippocampus to perform a pain modulatory role similar to that of the amygdala, a structure that mediates exogenous opioid analgesia and has the ability to produce hyperalgesia or analgesia depending on the emotional context in which the nociceptive stimulus is perceived (42).
Conclusion
Our results reveal that individuals with high reward responsiveness, a personality trait dependent on the endogenous opioid neurotransmission, experience more exogenous opioid-induced behavioral analgesia. The magnitude of this reward i.e., analgesia, was best predicted by the neural activity in the endogenous opioid-rich regions of the brain reward circuitry. Emerging evidence suggests that MOR polymorphism could contribute to variability in behavioral opioid analgesia by introducing variability of the MOR responsiveness to exogenous opioids. However, there is also an urgent need for endophenotypes that are simpler measurable markers that link behavior and genetics that underpin such behavior (43). It is possible that trait RWR and the neuronal response to noxious stimuli in the endogenous opioid-rich brain reward circuitry could be useful endophenotypes of behavioral opioid analgesia. As such, we have identified potentially useful measures to aid stratification of patients at baseline that are predictive of opioid-induced analgesia contributing to a personalized approach to opioid pharmacotherapy.
Methods
General details of the study methods are published elsewhere (20). The methods specific to the data presented here are given below and in SI Methods.
Study Procedure.
Thirty-three healthy subjects were recruited after obtaining written informed consent. Of these, 25 subjects completed the study. See SI Methods for details of excluded subjects. This research was approved by the Oxfordshire Research Ethics Committee B of the National Research Ethics Services (NRES).
During the two scanning visits the same cohort of subjects received an infusion of remifentanil during one of the visits and a saline infusion during the other (balanced for order), separated by at least 1 wk. They received a remifentanil infusion at an effect site concentration of 2 ng mL−1 for 30 min using a target-controlled infusion (TCI) pump, which delivers the desired effect site concentration controlling for the effects of subject demographics (Fig. S4 shows lack of influence of demographics on analgesia score). Total duration of the infusion was 40 min allowing 10 min to reach the steady effect site concentration. The subjects completed the BIS/BAS scale before scanning on the first visit.
fMRI Scanning and Stimulation Paradigm.
Functional imaging data were acquired using a 3T Varian-Siemens whole-body magnetic resonance scanner. See SI Methods for image acquisition details. Once the subject was in the scanner, we connected the infusion to an indwelling cannula in the left forearm and began physiological monitoring.
We used heat and punctate noxious stimuli. These were delivered separately in blocks of 10 stimuli before, during, and after the infusion with the heat stimulation blocks preceding the punctate stimulation blocks. The data from the postinfusion period and noxious punctate stimuli were used to investigate opioid withdrawal-induced hyperalgesia and are published elsewhere.
The temperature that delivered a moderately painful stimulus (5 on a numerical rating scale, NRS, where 0 corresponds to “no pain” and 10 to “severe pain”) was selected for each subject for each visit with the subject in the scanner but before starting the experiment. The same temperature was used for all stimuli during that visit for the individual subject.
After obtaining preinfusion period mood scores, functional scans began while delivering the noxious stimulation block, each consisting of 10 stimuli over ∼10 min. A visual analog scale (VAS) was used for recording perceived pain intensity of each stimulus. The infusion was commenced and the stimulation block was repeated during the infusion. Mood ratings were obtained before and after the stimulation block during the infusion period.
Analysis of Psychophysical Data.
Paired two-tailed t tests were used for comparison of baseline data from the two visits. A one-sample two-tailed t test was performed to evaluate whether the distribution of the opioid infusion-induced effects were significantly different from a mean of zero. For data that were nonnormally distributed, we used the Wilcoxon signed rank test.
Analysis of fMRI Data.
fMRI analyses of the heat functional scans were performed using FEAT (FMRI Expert Analysis Tool) version 5.98, part of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain software library; www.fmrib.ox.ac.uk/fsl (FSL). Statistical images were generated to identify significant brain activity evoked by noxious stimuli in each functional scan. These were then analyzed at higher levels to generate the necessary group statistical maps. For whole brain analyses we used mixed effects analysis and a cluster-based correction for multiple comparisons (Z score >2.3; P < 0.05). Where appropriate, we performed a priori hypothesis-driven directed searches using small volume correction with nonparametric permutation testing (5,000 permutations) (44) and threshold free cluster enhanced (TFCE) correction for multiple comparisons at P < 0.05 (45). All our directed searches were thresholded individually to yield a 5% false positive rate for each of the searches.
To illustrate significant results from image analyses, we extracted the percentage of BOLD response. To define brain structures for small volume corrections, we used the Harvard Oxford Cortical and Subcortical Structural Atlas (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html). For correct identification of brainstem areas, we used the Duvernoy Brainstem Atlas (46). Details of how these areas were identified are in SI Methods.
Supplementary Material
Acknowledgments
This work was supported by Grants from the Medical Research Council of Great Britain and Northern Ireland, the National Institute for Health Research Oxford Biomedical Research Centre, and the Wellcome Trust (Oxford Centre for Functional MRI of the Brain) (to M.C.L., V.W., and I.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.L.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120201109/-/DCSupplemental.
References
- 1.Anonymous; Expert Working Group of the European Association for Palliative Care Morphine in cancer pain: Modes of administration. BMJ. 1996;312:823–826. [PMC free article] [PubMed] [Google Scholar]
- 2.Lötsch J, Geisslinger G. Are mu-opioid receptor polymorphisms important for clinical opioid therapy? Trends Mol Med. 2005;11:82–89. doi: 10.1016/j.molmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Dickenson AH, Kieffer B. 2006. Opiates: Basic mechanisms. Wall and Melzack's Textbook of Pain, eds McMahon SB, Koltzenburg M (Churchill Livingstone, New York), 5th Ed.
- 4.Zubieta JK, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 5.Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- 6.Schreckenberger M, et al. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J Nucl Med. 2008;49:1257–1261. doi: 10.2967/jnumed.108.050849. [DOI] [PubMed] [Google Scholar]
- 7.Haber SN, Knutson B. 2010. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26. [DOI] [PMC free article] [PubMed]
- 8.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 9.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yacubian J, et al. Subregions of the ventral striatum show preferential coding of reward magnitude and probability. Neuroimage. 2007;38:557–563. doi: 10.1016/j.neuroimage.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Scott DJ, et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones AK, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett. 1991;126:25–28. doi: 10.1016/0304-3940(91)90362-w. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 16.Favaroni Mendes LA, Menescal-de-Oliveira L. Role of cholinergic, opioidergic and GABAergic neurotransmission of the dorsal hippocampus in the modulation of nociception in guinea pigs. Life Sci. 2008;83:644–650. doi: 10.1016/j.lfs.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Franklin KB. Analgesia and the neural substrate of reward. Neurosci Biobehav Rev. 1989;13:149–154. doi: 10.1016/s0149-7634(89)80024-7. [DOI] [PubMed] [Google Scholar]
- 18.McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 19.Carver C. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scale. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 20.Wanigasekera V, Lee MC, Rogers R, Hu P, Tracey I. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J Neurosci. 2011;31:2835–2842. doi: 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–1134. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise RG, Williams P, Tracey I. Using fMRI to quantify the time dependence of remifentanil analgesia in the human brain. Neuropsychopharmacology. 2004;29:626–635. doi: 10.1038/sj.npp.1300364. [DOI] [PubMed] [Google Scholar]
- 23.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 25.Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: Activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res. 1988;460:281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 27.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 28.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinricher MM, Cheng ZF, Fields HL. Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J Neurosci. 1987;7:271–278. doi: 10.1523/JNEUROSCI.07-01-00271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunckley P, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwilym SE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 32.Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: A combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 34.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: A combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- 35.Sesack SR, Grace AA. Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna S. Nociceptive processing in the hippocampus and entorhinal cortex, neurophysiology and pharmacology. In: Schmidt RR, Willis WD, editors. Encyclopedia of Pain. New York: Springer; 2006. pp. 1369–1374. [Google Scholar]
- 38.Derbyshire SW, et al. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 39.Ploghaus A, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang XF, Xiao Y, Xu MY. Both endogenous and exogenous ACh plays antinociceptive role in the hippocampus CA1 of rats. J Neural Transm. 2008;115:1–6. doi: 10.1007/s00702-007-0808-3. [DOI] [PubMed] [Google Scholar]
- 41.Lico MC, Hoffmann A, Covian MR. Influence of some limbic structures upon somatic and autonomic manifestations of pain. Physiol Behav. 1974;12:805–811. doi: 10.1016/0031-9384(74)90017-1. [DOI] [PubMed] [Google Scholar]
- 42.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 43.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7:173–181. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- 44.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 46.Naidich TP, et al. Duvernoy's Atlas of the Human Brain Stem and Cerebellum. New York: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.