Abstract
Widespread use of the endocrine disrupting chemical bisphenol A (BPA) in consumer products has resulted in nearly continuous human exposure. In rodents, low-dose exposures have been reported to adversely affect two distinct stages of oogenesis in the developing ovary: the events of prophase at the onset of meiosis in the fetal ovary and the formation of follicles in the perinatal ovary. Because these effects could influence the reproductive longevity and success of the exposed individual, we conducted studies in the rhesus monkey to determine whether BPA induces similar disturbances in the developing primate ovary. The routes and levels of human exposure are unclear; hence, two different exposure protocols were used: single daily oral doses and continuous exposure via subdermal implant. Our analyses of second trimester fetuses exposed at the time of meiotic onset suggest that, as in mice, BPA induces subtle disturbances in the prophase events that set the stage for chromosome segregation at the first meiotic division. Our analyses of third-trimester fetuses exposed to single daily oral doses during the time of follicle formation revealed an increase in multioocyte follicles analogous to that reported in rodents. However, two unique phenotypes were evident in continuously exposed animals: persistent unenclosed oocytes in the medullary region and small, nongrowing oocytes in secondary and antral follicles. Because effects on both stages of oogenesis were elicited using doses that yield circulating levels of BPA analogous to those reported in humans, these findings raise concerns for human reproductive health.
Bisphenol A (BPA) is a synthetic chemical that has endocrine disrupting properties. Owing to its high-volume production and widespread use in consumer products, including canned foods, pressure-printed receipts, dental sealants, and plastic products, humans are exposed to BPA on a daily basis. During the past 15 y, adverse effects of low-dose exposures have been reported in hundreds of studies of experimental animals (reviewed in refs. 1 and 2), and human studies reporting adverse effects are steadily increasing (reviewed in ref. 3).
Environmental exposures that affect the developing reproductive tract or influence gamete production may compromise fertility and, consequently, findings from studies of rodents exposed to BPA are of great concern. Fetal and neonatal BPA exposures reportedly affect the developing reproductive tract of both males and females, several distinct stages of oogenesis in the developing ovary, testosterone levels and sperm counts in the adult male, and the fertility of females exposed in utero (reviewed in refs. 4–7).
Despite growing evidence of harm, the relevance of findings from rodent studies has been challenged on the grounds that differences in BPA metabolism may result in different responses in rodents and humans to the same doses of BPA (reviewed in ref. 8). Recent pharmacokinetic studies, however, have provided direct evidence that, despite differences in metabolism, the pharmacokinetics are extraordinarily similar in rodents, nonhuman primates, and humans (9). Nevertheless, given the seriousness of the possible effects of BPA on the reproductive potential of the female, studies in an animal model with greater similarity to the human are clearly warranted.
The rhesus monkey has been recognized as a superior model for human reproductive physiology for many years (10, 11). As a model for developmental toxicology it has distinct advantages (12) because pregnancy in primates and rodents differs in several important respects, including placentation (13), placental protein products (14), and fetal adrenal function (15). Of particular relevance for studies of endocrine disrupting chemicals, the levels of estrogen maintained throughout pregnancy in rhesus females are similar to those in humans (16–18), which is not the case in mice.
Previous studies in mice suggest that low-level BPA exposure disrupts oogenesis at multiple stages. It disturbs the behavior of chromosomes at the onset of meiosis in the fetal ovary, disrupts the packaging of meiotically arrested oocytes into follicles in the newborn ovary, and affects the final stages of oocyte maturation in the adult ovary [(19–21), reviewed in ref. 5)]. In the present study, we investigated whether defects in the fetal stages of oogenesis might also be apparent in BPA-exposed rhesus females.
The studies presented here were conducted in conjunction with pharmacokinetic studies of female rhesus monkeys (9). Initial studies using single oral doses of 400 μg⋅kg−1⋅d−1 administered to nonpregnant females demonstrated rapid conjugation (inactivation) of BPA, with peak serum levels of 2–5 ng/mL attained 1–2 h after ingestion and a rapid decline thereafter. Because the dose was high (∼8 times the current FDA “safe” dose) but peak levels closely approximated levels observed in human studies (reviewed in refs. 22 and 23), we concluded that human exposure must be significantly higher than assumed, not primarily restricted to oral routes, and nearly constant (9). Thus, in addition to using an oral dosing strategy, we developed a protocol using the implantation of controlled-release Silastic capsules to achieve sustained low-level exposure.
The timing of exposure using both protocols was designed to mimic the developmental windows that elicited effects in the ovary in mice: an early exposure during the second trimester of pregnancy [gestational day (GD) 50–100], when germ cell differentiation and meiotic entry occur, and a late exposure during the third trimester (GD 100–term), when follicle formation takes place. We report here the results of meiotic studies of BPA-exposed and control animals from the early exposure cohort and studies of follicle morphology and the first wave of follicle growth from the late exposure cohort. Our results confirm previous findings in rodents, indicating that the early stages of oocyte development in the rhesus monkey are vulnerable to disturbance by BPA and suggesting that fetal exposures may adversely affect the reproductive potential of adult female primates, including humans.
Results
The results presented here represent one of several studies of the same cohort of females, among them pharmacokinetic studies (9) and studies of other organ systems, including the mammary gland (24). The early and late exposure protocols were designed to mimic the developmental windows reported to elicit effects on oogenesis in mice (reviewed in ref. 5). For both exposures, we examined effects resulting from single daily oral doses of BPA consumed by the mother and of continuous low-level exposure delivered via maternal subdermal implant.
For animals receiving oral doses, mean levels of bioactive (unconjugated) BPA in maternal serum close to the time of ovarian tissue collection were 0.51 ± 0.20 ng/mL for second-trimester pregnancies and 0.31 ± 0.13 ng/mL for third-trimester pregnancies. These values were obtained 4 h after administration of the last maternal oral dose; in nonpregnant females, this time point was closest to the average level during the 24 h following a single oral dose (9). For animals in the implant exposure group, mean levels of bioactive BPA in maternal blood at the time of ovary collection were 0.45 ± 0.23 ng/mL for second-trimester fetuses and 0.90 ± 0.13 ng/mL for third-trimester fetuses; these values represent the sustained levels achieved using the implant paradigm and are in close agreement with levels reported in human maternal serum (reviewed in ref. 23).
BPA Exposure Induces Changes in Meiotic Chromosome Behavior.
In mice, defects in meiotic prophase were observed in oocytes from fetuses exposed from 11 to 18 d of gestation, a developmental window that includes mitotic germ cell proliferation, entry into meiosis, and progression of most oocytes to pachytene (19). In the rhesus monkey, oocytes initiate meiosis during the second trimester and reach mid prophase around 100 d of gestation (25). Accordingly, we initiated BPA exposure at 50 d of gestation and removed fetuses by cesarean section after 50 d of exposure.
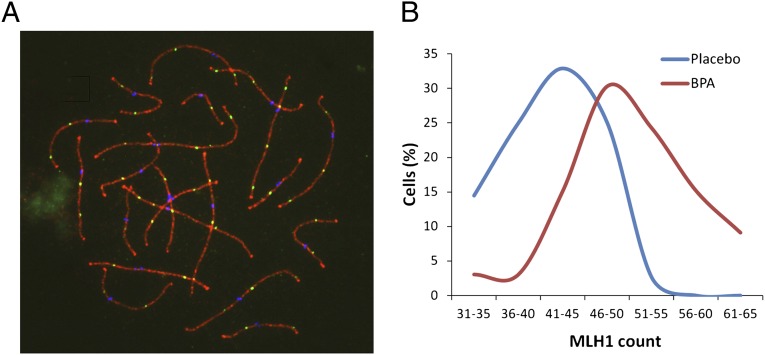
Exposure coinciding with the time of meiotic entry in the fetal mouse ovary increased synaptic defects and the number of recombination events between homologous chromosomes during meiotic prophase. To determine whether BPA exposure induced similar meiotic aberrations in the monkey, surface spread preparations were made from fetal ovaries at the end of the early exposure and pachytene cells from control and treated ovaries were analyzed. An immunofluorescence staining protocol using antibodies to SYCP3, a component of the lateral element of the synaptonemal complex (SC); MLH1, a component of late recombination nodules; and CREST antiserum to detect centromeres allowed us to analyze synapsis and recombination in the same cells.
To assess recombination levels, the number of MLH1 foci on the SCs of pachytene cells was counted. Because MLH1 foci correlate with almost all crossovers, counts of MLH1 foci in pachytene cells are frequently used to assess recombination levels (26). Technical difficulties associated with slide preparation on the first set of animals diminished our ability to obtain cells suitable for MLH1 staining and, as a result, counts could only be obtained on two BPA-exposed and one control animal from the group given a single daily oral dose. Although MLH1 counts were higher in both exposed animals than in the single control, the limited data precluded meaningful analysis. A sufficient number of pachytene cells for analysis were available, however, from animals exposed continuously to BPA via Silastic implants. Consistent with previous findings in mice (19), a highly significant increase in mean MLH1 values per cell was observed in oocytes from exposed compared with placebo-treated fetuses (50.4 ± 7.0 for 33 cells from exposed females and 42.2 ± 5.9 for 70 cells from controls; t = 6.8; P < 0.001; Fig. 1).
Fig. 1.
Recombination is increased in BPA-exposed females. (A) Example of pachytene oocyte used to obtain MLH1 counts. Triple immunostaining with antibodies to SYCP3 (red), MLH1 (green), and CREST (blue) allowed detection of synaptonemal complex, sites of recombination, and centromeres, respectively. (B) Comparison of MLH1 counts from 33 cells from fetuses continuously exposed to BPA (red curve) and 70 from placebo-treated controls (blue curve). Mean values were highly significantly different (50.4 ± 7.0 and 42.2 ± 5.9 for BPA-exposed and placebo, respectively; t = 6.8; P < 0.001).
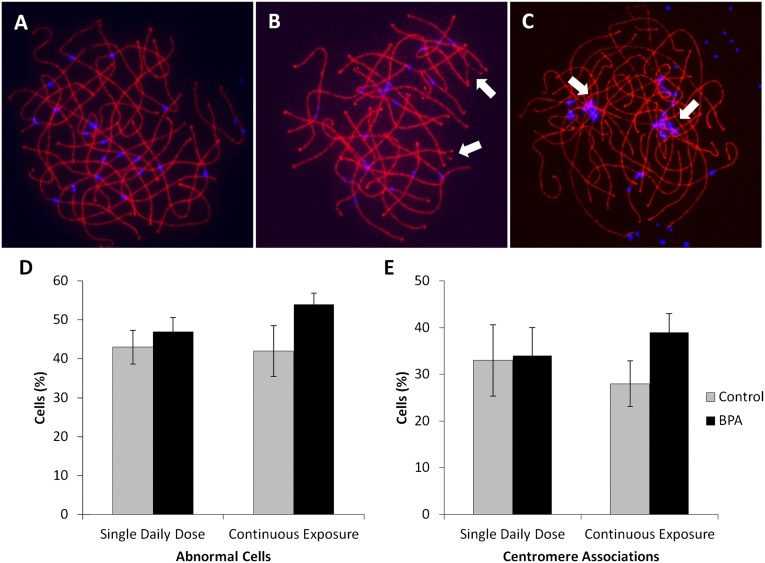
In the mouse, BPA induced synaptic defects (i.e., defects involving one or several chromosome pairs in an otherwise normal pachytene cell) and end-to-end associations that almost exclusively involved centromeric regions of nonhomologous chromosomes (19). To determine whether BPA exposure in the rhesus monkey induced similar defects, cells judged to be at pachytene on the basis of SYCP3 staining were scored. As in the human female (27), the incidence of synaptic defects in control females was high compared with that in the mouse, with over one-third of cells displaying some type of synaptic defect (Fig. 2). Against this high background, a slight decrease in the proportion of normal pachytene cells was evident in fetuses from females given a single daily oral dose and those continuously exposed, but the difference between placebo and exposed fetuses did not reach significance for either exposure (Fig. 2D). Scoring of nonhomologous associations was complicated by the fact that, unlike mouse chromosomes in which centromeres are located close to the telomeres and can be unambiguously identified as regions of dense chromatin at one end of the SC, centromeres are interstitially placed on rhesus chromosomes. Thus, rather than scoring end-to-end associations, we scored the number and extent of centromere associations in pachytene cells. No obvious difference between exposed and control animals was observed in the group given a single daily oral dose (Fig. 2E); however, a significant increase in oocytes with associations was observed in the continuous exposure group (χ2 = 11.8; P < 0.01). In addition, the extent of the associations was markedly different, with an increase in cells with associations involving five or more chromosomes in exposed fetuses (Fig. S1).
Fig. 2.
Comparison of meiotic defects in BPA-exposed and placebo-treated fetuses. (A–C) Images of pachytene cells. (A) normal, (B) cell with two synaptic defects (arrows), (C) cell with associations involving at least four centromeres. Cells were immunostained with an antibody to SYCP3 to detect the synaptonemal complex (red) and CREST (blue) to visualize centromeres. (D) Frequency of synaptic defects in single daily oral and continuously exposed fetuses. For both exposures, synaptic defects were slightly, although not significantly, increased (χ2 = 0.3, P = 0.9 and χ2 = 3.6, P = 0.17 for single and continuous, respectively) in BPA-exposed fetuses (black bars) compared with placebo controls (gray bars). Values represent mean ± SEM; data represent 118 cells from four females given a single daily oral dose compared with 135 cells from controls, and 105 cells from six continuously exposed females compared with 83 cells from two controls. (E) Comparison of centromere associations in oocytes from BPA-exposed (black bars) and placebo-treated (gray bars) fetuses. Bars represent mean ± SEM. The number of cells with associations was not significantly different between animals receiving single daily oral BPA doses and controls (χ2 = 0.5; P = 0.9) but was significantly increased in fetuses continuously exposed to BPA (χ2 = 11.8; P = 0.01).
BPA Influences Follicle Formation.
In mice, a variety of estrogenic exposures (including BPA) disrupt follicle formation, causing an increase in multioocyte follicles (reviewed in ref. 28). In the rhesus monkey, follicle formation occurs during the third trimester of pregnancy (25). Thus, neonates from females exposed to BPA from GD 100–term were used to assess effects of BPA on follicle formation.
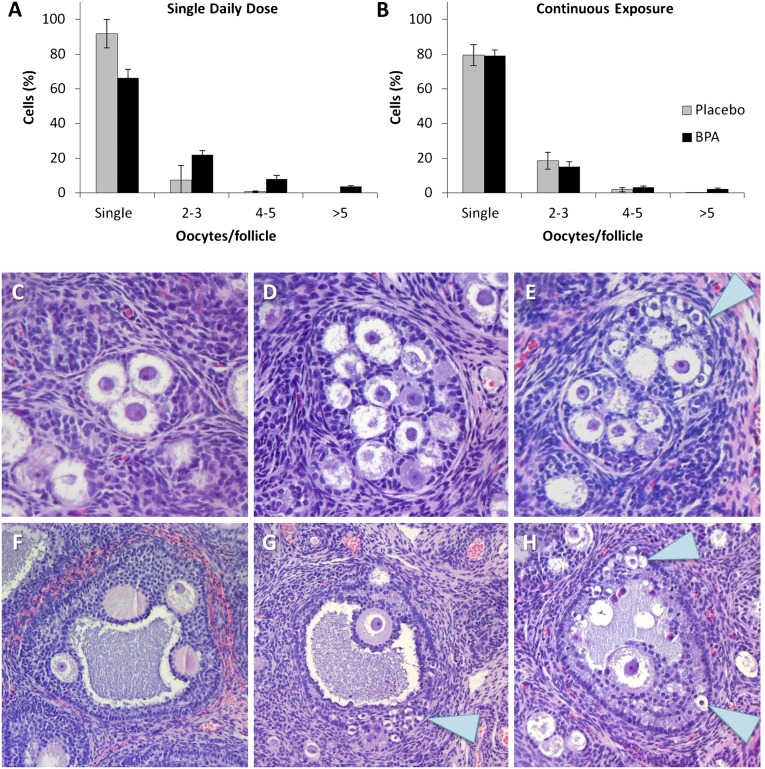
To test the hypothesis that BPA exposure in utero disrupts follicle formation, we compared the incidence of multioocyte follicles in serial sections of ovaries from exposed and control females. It is inherently difficult to obtain accurate counts of total primordial follicles (29). This, coupled with the density of primordial follicles in the cortical region of the rhesus ovary, made it impractical to count single and multioocyte primordial follicles. Thus, we focused on growing follicles, counting secondary and antral follicles in the medullary region of the ovary, and scoring the number of oocytes in each follicle (Fig. 3). A comparison of the proportion of secondary and antral follicles containing a single, two to three, four to five, or more than five oocytes revealed a highly significant difference between control and exposed females given a single daily oral dose (Fig. 3A; χ2 = 71.7; P < 0.001), with the most noticeable difference in categories with the greatest number of oocytes (four to five and more than five).
Fig. 3.
Multioocyte follicles in BPA-exposed and control fetuses. (A and B) Secondary and antral follicles in the medullary region were counted and scored for the number of oocytes contained (data are shown as means ± SEM). For daily oral exposures (A), the difference between exposed and controls in the proportion of follicles with one, two to three, four to five, or more than 5 oocytes was highly significantly different (χ2 = 71.7; P < 0.001). For continuous exposures (B), the overall distribution was not significantly different; however, considered alone, the proportion of follicles with more than five oocytes was significantly enriched in exposed females (χ2 = 17.2; P < 0.01). (C–H) Examples of multioocyte follicles from exposed females. (C) Multioocyte secondary follicle containing three oocytes; this type of follicle was common in exposed and control ovaries. (D and E) Multioocyte secondary follicles containing many oocytes and oocytes of variable sizes (E, arrow). (F and G) Multioocyte antral follicles; in two (G and H), small, apparently nongrowing oocytes are evident at the edge of the follicle (arrows).
In females exposed continuously, the overall distribution of follicles containing from one to more than five oocytes was not significantly different between exposed and control females (Fig. 3B). However, as in the group given a single oral dose, the proportion of follicles with either four to five or more than five oocytes was increased; indeed, considered alone, the proportion of follicles with more than five oocytes was highly significantly increased in the exposed females (χ2 = 17.2; P < 0.01).
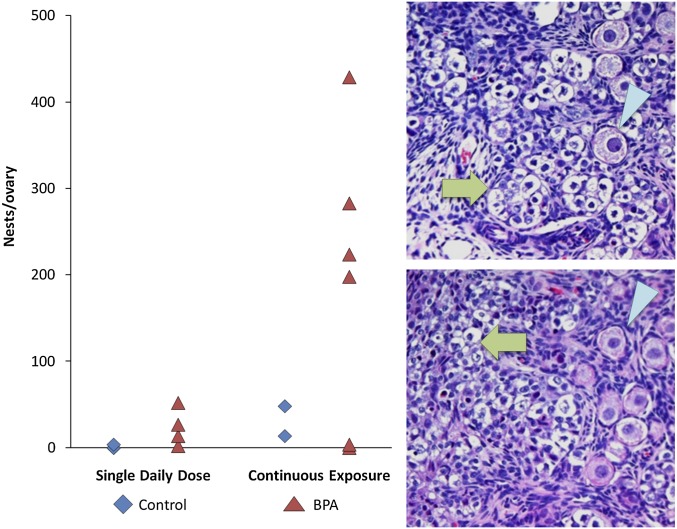
Although the continuous exposure treatment yielded only an increase in follicles containing a large number of oocytes, the emergence of two unique phenotypes in this group suggests a striking effect on follicle formation. First, in some growing follicles, oocytes markedly different in size were present (Fig. 3E). This was particularly striking in several large antral follicles in which small oocytes appeared trapped in the granulosa cell layer at the edge of the follicle (Fig. 3 G and H). Second, during the analysis of secondary and antral follicles, it became apparent that many small, unenclosed oocytes were present in the medullary region of ovaries from some females in the continuous exposure group. As shown in Fig. 4, these unenclosed oocytes were present in “nests.” When the slides were decoded, it was evident that four of the six continuously exposed animals exhibited an extraordinary number of nests.
Fig. 4.
Unenclosed oocytes persist in some BPA-exposed fetuses. The number of nests of small, unenclosed oocytes scored in the medullary region of each animal in the single daily oral (graph; left) and continuous exposure (graph; right) groups. Images show examples of “nests” of unenclosed oocytes (green arrows). Compared with normal primary follicles (arrowheads in Upper and Lower Right), unenclosed oocytes are small and, in many, nuclei appear pyknotic.
Importantly, the two phenotypes were correlated; antral follicles containing small, apparently nongrowing oocytes were restricted to females with a high frequency of unenclosed oocytes. These findings prompted us to rescore the animals given a daily oral dose. Although more nests were scored in ovaries of BPA-exposed females, this was not a strong phenotype in those with daily oral exposure (Fig. 4). Further, an analysis of antral follicles revealed only a single follicle containing oocytes of markedly disparate size; notably, this follicle was found in the female with the highest number of nests.
Discussion
Previous studies in mice have identified three stages of oogenesis that are adversely affected by BPA (reviewed in ref. 5). In primates, two of these (the onset of meiosis and follicle formation) occur during fetal development, and the current study was designed to determine whether maternal levels of BPA analogous to those reported in humans induce detectable effects in the fetal primate ovary.
Because current levels and routes of human exposure remain unclear, we assessed effects resulting from exposures of different durations. The administration of single daily oral doses of BPA has been reported to induce a wide range of effects on the developing rodent fetus, including both stages of oogenesis under investigation in the current study (19, 21, 30, 31). However, in both nonpregnant female monkeys and mice, the oral dose administered in our studies yields peak levels of bioactive BPA in the 3 to 4 ng/mL range within several hours of ingestion, followed by a rapid decline to low levels (9). Importantly, the average concentration of free BPA during the 24-h period (0.5 ng/mL) is considerably lower than the 2 ng/mL levels reported in numerous human studies (reviewed in ref. 9), suggesting that this exposure paradigm does not adequately mimic human exposure. Indeed, it has been argued that, in addition to oral exposures, humans must be exposed to significant amounts of BPA via non-oral routes and exposure must be nearly constant (23, 32). Thus, to assess the effect of constant, low-level exposure, we used controlled-release capsule implants to provide sustained, low-level BPA exposure.
Our results suggest that, as in mice, the fetal primate ovary is sensitive to BPA and exhibits alterations in both meiotic prophase and follicle formation. Given the physiological relevance of the rhesus model and the similarity between levels of bioactive (unconjugated) BPA in maternal serum of rhesus monkeys and those reported in humans (23), the finding that data from small groups of monkeys recapitulate many findings from studies in mice raises concerns about the reproductive impact of current levels of human exposure to BPA.
BPA Disrupts the Key Events of Meiotic Prophase.
We reported previously that fetal BPA exposure affects synapsis and recombination during meiotic prophase in the mouse (19). These events are critical for the segregation of homologous chromosomes at the first meiotic division, and subtle BPA-induced disturbances during prophase increase the incidence of chromosomally abnormal eggs produced by the mature female mouse (19). Similar prophase disturbances have been reported in Caenorhabditis elegans (33) and in human fetal ovaries exposed in vitro (34), and expression studies in the mouse have provided evidence of corresponding changes in gene expression (35).
Our studies of prophase oocytes, although largely limited to the group of continuously exposed females, provide evidence of similar defects, suggesting that BPA adversely affects prophase events in a wide range of eukaryotes, including primates. As in mice (19, 33), BPA exposure increased the levels of recombination in pachytene cells. However, although the same trends were observed, synaptic defects in BPA-exposed monkeys were considerably less pronounced than in the mouse. Several factors likely contribute to this difference. As in humans (27), analysis of pachytene-stage cells in the rhesus female is more complex than in the mouse, because the SCs are longer and a large number of synaptic defects are evident even in control females. This high background of synaptic defects almost certainly complicates efforts to discern an increase in synaptic defects in a limited sample size. However, centromeric associations between nonhomologous chromosomes were significantly increased in continuously exposed animals. The reason that similar defects were not apparent in the group given single daily oral exposure is not clear, although our studies in mice suggest that this phenotype is strongly dose-dependent.
BPA Disrupts Follicle Formation.
The formation of multioocyte follicles (MOFs) that contain more than a single oocyte has been reported in a wide variety of species (36–38). Although MOFs are common in some species, including the rhesus monkey (25, 38), they are relatively rare in rodents. MOFs, however, can be induced by a wide range of estrogenic exposures (reviewed in ref. 28). BPA exposure has been reported to increase the incidence of MOFs in the mouse (21, 39), rat (31), and lamb (30), and the second developmental window of exposure in our studies (GD 100–term) was designed to test the effect of BPA exposure on follicle formation in the fetal rhesus ovary.
A striking difference in the effects of single daily and continuous exposures on follicle formation was evident. Single daily exposure significantly increased multioocyte follicles, as reported in other species (21, 30, 31, 39). Further, as in rodents, the effect was evident not only as an increase in the number of MOFs, but also in the number of oocytes they contained (Fig. 3). Surprisingly, however, the number of MOFs was not significantly increased in continuously exposed animals, although the average number of oocytes contained in MOFs was higher. Because single daily and continuous exposure experiments were conducted during different breeding seasons, each group had a separate set of controls. Differences in the frequency of MOFs in the two control groups suggest that exposure to phytoestrogens, which fluctuate with growing conditions and are known to induce MOFs (40), likely differed. Thus, an effect of BPA on follicle formation in the continuous exposure group may not have been detected owing to the interference of phytoestrogens. However, we favor an alternate explanation: that disturbances in follicle formation in continuously exposed females manifest as a different phenotype, with a different underlying mechanism. Four of the six continuously exposed females exhibited a marked increase in small, unenclosed oocytes in the medullary region of the ovary. Although estrogenic exposures increase the incidence of unenclosed oocytes in the mouse, we believe that the number of these oocytes, their persistence, and their presence in the medullary region in close proximity to growing oocytes is unprecedented. That is, although unenclosed oocytes have been reported in studies conducted at the time of follicle formation (39), they have not been mentioned in studies of growing oocytes conducted on slightly older females (30, 31, 41), consistent with the expectation that unenclosed oocytes are eliminated by apoptosis. Thus, because we scored only the medullary region that contains the first wave of growing oocytes, the presence of a large number of unenclosed oocytes is surprising. Most previous studies have used single daily exposures (either oral or s.c. injection); thus, we postulate that this phenotype represents a more extreme effect that results from continuous BPA exposure around the time of follicle formation.
Follicle formation is complex and thought to involve programmed cell death as well as an intricate interaction between growth factor and other signaling pathways regulated by hormones and transcription factors (reviewed in ref. 28). However, a link between the oocyte cell cycle and follicle formation has also been suggested (ref. 42; reviewed in ref. 28), and delayed meiotic cell cycle progression in response to high levels of BPA has been reported (39). The oocyte is known to control the rate of follicle growth (43), and it seems plausible that oocyte enclosure may also depend on the developmental stage of the oocyte. Importantly, during the late exposure window used in our study, oocytes were transiting through the late stages of meiotic prophase and entering meiotic arrest. Thus, we hypothesize that the unique phenotypes encountered in continuously exposed females reflect a delay in meiotic progression that results in an increased number of oocytes that have not entered meiotic arrest by the time of follicle formation. If such oocytes are not yet ready for enclosure, multioocyte follicles may result from conflicting somatic and oocyte signals, causing the mistaken enclosure of “immature” oocytes. This mechanism provides an explanation for the size diversity in oocytes within multioocyte follicles and the presence of small, apparently nonresponsive oocytes in large antral follicles. That is, due to their immaturity, when such oocytes become enclosed in a multioocyte follicle that initiates growth during the first wave of folliculogenesis, they may be incapable of participating in the paracrine dialogue in the growing follicle, thus growing poorly or not at all.
Summary and Future Directions.
The inherent difficulty of primate studies necessitated the use of limited numbers of animals. Nevertheless, we observed significant differences between BPA-exposed and control females at two distinct stages of oogenesis. These results provide evidence in a primate model that circulating levels of bioactive BPA analogous to those reported in humans adversely affect egg development.
Importantly, changes induced during these early stages of oocyte development have the potential to affect female reproductive success and longevity. In the mouse, subtle changes induced by BPA during meiotic prophase lead to an age-independent increase in aneuploid eggs in the adult (19). Thus, the finding that BPA induces similar effects on female meiosis in the mouse, the nematode C. elegans (33), in cultured fragments of human fetal ovaries exposed in vitro (34), and now in the female rhesus monkey suggests that BPA induces prophase changes in a wide variety of eukaryotes. The implications for humans are troubling because the impact of these effects would not be manifested for a generation. Indeed, because exposure of the pregnant female affects the oocytes developing in the fetal ovary that will give rise to her grandchildren, establishing direct links in humans between BPA exposure and adverse reproductive outcomes will be virtually impossible.
Similarly, our studies of females exposed during late gestation add to the compelling evidence that exposure to exogenous estrogens—including BPA—adversely affects follicle formation (reviewed in ref. 28). The impact of these changes on female reproduction remains unclear, but the presence of multioocyte follicles has been correlated with diminished egg quality (37, 38). Importantly, it seems likely that the extensive failure in oocyte packaging we observed in continuously exposed females would reduce the total pool of oocytes, thereby shortening the reproductive lifespan of the exposed female. Although several studies have demonstrated that BPA exposure impairs reproductive performance (7), and others have suggested a decrease in the total pool of primordial follicles in BPA-exposed females (30, 31), the reproductive lifespan of exposed females has not been evaluated. Our findings, coupled with the nearly ubiquitous exposure of humans to this chemical, underscore the urgency for such studies.
Finally, the differences in follicle formation defects we observed between single daily oral and continuous exposure groups adds to the growing concern that experimental studies using single daily oral exposures may not provide accurate models. Indeed, given that human exposure is nearly continuous and likely occurs by both oral and non-oral routes, the use of single daily oral exposures may underestimate the effects of BPA on the developing fetus. Thus, obtaining a clear understanding of the sources, routes, duration, and level of human BPA exposure in different populations is essential.
Materials and Methods
Animals.
The adult female rhesus macaques (Macaca mulatta) used in these studies have been described previously (9). All procedures for maintenance and handling of pregnant adult females were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. Animals were maintained at the California National Primate Research Center (CNPRC) and housed individually in stainless steel cages with a 0600–1800 hours light cycle and at a controlled temperature of 25–27 °C. Purina Monkey Chow and water delivered via rigid PVC pipes and a “lixit” device were provided for ad libitum consumption. Supplements consisting of seasonal produce, seeds, and cereal were offered as environmental enrichment. Females aged 6–13 y were naturally mated according to standard CNPRC procedures. Gravid females were sonographically screened (44) by 40 d of gestation to identify female fetuses. Screening accuracy was 100%; all fetuses were confirmed to be female at tissue collection.
BPA Administration.
Two treatment protocols were used. The first cohort was given small pieces of fruit containing 400 μg/kg body weight of deuterated BPA (dBPA; CDN Isotopes) or vehicle control once per day. A second cohort received continuous dBPA exposure via Silastic tubing implants demonstrated to produce serum levels ranging from 2.2 to 3.3 ng/mL of unconjuated dBPA in nonpregnant test females. Each cohort was further subdivided into early and late treatment groups. Early treatment animals were dosed from GD 50–100 (cohort 1: n = 5 treated, 6 control; cohort 2: n = 6 treated, 2 control) and fetuses were removed by cesarean section at the end of the treatment period. Late-treatment animals were dosed from GD 100–term (cohort 1: n = 6 treated, 6 control; cohort 2: n = 6 treated, 2 control). Levels of bioactive BPA in maternal serum were measured using isotope-dilution liquid chromatography-mass spectrometry as described previously (9).
Meiotic Analyses.
To obtain pachytene oocytes for meiotic studies, surface spread preparations (45) were made from early-treatment ovaries and immunostained with antibodies to SYCP3 and MLH1 for the analysis of synapsis and recombination as described previously (19), and with CREST antiserum to facilitate centromere location. Cells judged to be at pachytene on the basis of SYCP3 staining were scored for synaptic defects using criteria described previously (19). Cells with defects included those with small gaps, bubbles, or forks evident on one or several SCs in an otherwise normal cell or with a single major defect (e.g., synaptic failure or synapsis between nonhomologous chromosomes). All scoring was done by multiple observers who were blinded with respect to the status of individual animals. In addition, a second blinded analysis of centromeres was conducted; centromere associations were scored if three or more centromeres from nonhomologous chromosomes were tightly clustered (separated by two or fewer centromere signal domains).
Analysis of Follicle Formation.
To characterize follicle formation, ovaries from fetuses in the late-treatment group were embedded in paraffin, serially sectioned (5 μm), and stained with hematoxylin and eosin. To score growing follicles, every tenth section throughout the medullary region was selected for scoring. Each section was imaged, a line of demarcation was drawn around the medullary region, and growing follicles were staged and counted by two blinded observers. Follicles were scored as secondary if they contained two or more granulosa cell layers but no evidence of an antrum and as antral follicles if a partially or fully formed antrum was present. Numbers of oocytes per follicle were recorded, and follicles were counted only if the section contained the nucleus of at least one oocyte. Because clusters of unenclosed oocytes were evident in some animals, all slides were rescored in blinded fashion to count “nests,” defined as discrete groups of unenclosed follicles.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grants ES016770 (to C.A.V. and P.A.H.) and ES013527 (to P.A.H.); Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD21341 to T.H., and CNPRC Base Grant (National Institutes of Health) OD011107 (RR00169).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 17315.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207854109/-/DCSupplemental.
References
- 1.Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Richter CA, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun JM, Hauser R. Bisphenol A and children’s health. Curr Opin Pediatr. 2011;23:233–239. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol Cell Endocrinol. 2006;254-255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PA, Hassold TJ. Human female meiosis: What makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabaton NJ, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor JA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wimsatt WA. Some comparative aspects of implantation. Biol Reprod. 1975;12:1–40. doi: 10.1095/biolreprod12.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Mårtensson L. The pregnant rabbit, guinea pig, sheep and rhesus monkey as models in reproductive physiology. Eur J Obstet Gynecol Reprod Biol. 1984;18:169–182. doi: 10.1016/0028-2243(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 12.Chellman GJ, et al. Developmental and reproductive toxicology studies in nonhuman primates. Birth Defects Res B Dev Reprod Toxicol. 2009;86:446–462. doi: 10.1002/bdrb.20216. [DOI] [PubMed] [Google Scholar]
- 13.Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth IA. Comparative aspects of placental lactogens: Structure and function. Exp Clin Endocrinol. 1994;102:244–251. doi: 10.1055/s-0029-1211288. [DOI] [PubMed] [Google Scholar]
- 15.Abbott DH, Bird IM. Nonhuman primates as models for human adrenal androgen production: Function and dysfunction. Rev Endocr Metab Disord. 2009;10:33–42. doi: 10.1007/s11154-008-9099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellinwood WE, Stanczyk FZ, Lazur JJ, Novy MJ. Dynamics of steroid biosynthesis during the luteal-placental shift in rhesus monkeys. J Clin Endocrinol Metab. 1989;69:348–355. doi: 10.1210/jcem-69-2-348. [DOI] [PubMed] [Google Scholar]
- 17.Hein PR, Schatorjé JS, Frencken HJ, Segers MF, Thomas CM. Serum hormone levels in pregnant cynomolgus monkeys. J Med Primatol. 1989;18:133–142. [PubMed] [Google Scholar]
- 18.Weiss G, Butler WR, Hotchkiss J, Dierschke DJ, Knobil E. Periparturitional serum concentrations of prolactin, the gonadotropins, and the gonadal hormones in the rhesus monkey. Proc Soc Exp Biol Med. 1976;151:113–116. doi: 10.3181/00379727-151-39155. [DOI] [PubMed] [Google Scholar]
- 19.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt PA, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16:107–116. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tharp AP, et al. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci USA. 2012;109:8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Wagenen G, Simpson ME. Embryology of the Ovary and Testis in Homo sapiens and Macaca mulatta. Yale Univ Press, New Haven, CT; 1965. [Google Scholar]
- 26.Anderson LK, Reeves A, Webb LM, Ashley T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics. 1999;151:1569–1579. doi: 10.1093/genetics/151.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tease C, Hartshorne G, Hultén M. Altered patterns of meiotic recombination in human fetal oocytes with asynapsis and/or synaptonemal complex fragmentation at pachytene. Reprod Biomed Online. 2006;13:88–95. doi: 10.1016/s1472-6483(10)62020-2. [DOI] [PubMed] [Google Scholar]
- 28.Pepling ME. Follicular assembly: Mechanisms of action. Reproduction. 2012;143:139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 29.Tilly JL. Ovarian follicle counts—Not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera OE, Varayoud J, Rodríguez HA, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–312. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez HA, Santambrosio N, Santamaría CG, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30:550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ Health Perspect. 2010;118:1051–1054. doi: 10.1289/ehp.0901717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allard P, Colaiácovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci USA. 2010;107:20405–20410. doi: 10.1073/pnas.1010386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brieño-Enríquez MA, et al. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod. 2011;26:2807–2818. doi: 10.1093/humrep/der249. [DOI] [PubMed] [Google Scholar]
- 35.Lawson C, et al. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod. 2011;84:79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillette LJ, Jr, Moore BC. Environmental contaminants, fertility, and multioocytic follicles: A lesson from wildlife? Semin Reprod Med. 2006;24:134–141. doi: 10.1055/s-2006-944419. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 38.Telfer E, Gosden RG. A quantitative cytological study of polyovular follicles in mammalian ovaries with particular reference to the domestic bitch (Canis familiaris) J Reprod Fertil. 1987;81:137–147. doi: 10.1530/jrf.0.0810137. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HQ, et al. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39:5651–5657. doi: 10.1007/s11033-011-1372-3. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson WN, et al. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–1889. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: Exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 42.Paredes A, et al. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology. 2005;146:5267–5277. doi: 10.1210/en.2005-0965. [DOI] [PubMed] [Google Scholar]
- 43.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 45.Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–68. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.