Abstract
Schindler/Kanzaki disease is an inherited metabolic disease with no current treatment options. This neurologic disease results from a defect in the lysosomal α-N-acetylgalactosaminidase (α-NAGAL) enzyme. In this report, we show evidence that the iminosugar DGJNAc can inhibit, stabilize, and chaperone human α-NAGAL both in vitro and in vivo. We demonstrate that a related iminosugar DGJ (currently in phase III clinical trials for another metabolic disorder, Fabry disease) can also chaperone human α-NAGAL in Schindler/Kanzaki disease. The 1.4- and 1.5-Å crystal structures of human α-NAGAL complexes reveal the different binding modes of iminosugars compared with glycosides. We show how differences in two functional groups result in >9 kcal/mol of additional binding energy and explain the molecular interactions responsible for the unexpectedly high affinity of the pharmacological chaperones. These results open two avenues for treatment of Schindler/Kanzaki disease and elucidate the atomic basis for pharmacological chaperoning in the entire family of lysosomal storage diseases.
Keywords: glycosidase, protein folding, structural biology, X-ray crystallography, E.C. 3.2.1.49
The lysosome is an acidic organelle containing dozens of enzymes that catalyze the breakdown of cellular metabolites. One lysosomal enzyme is α-N-acetylgalactosaminidase (α-NAGAL) (E.C. 3.2.1.49), a hydrolase that catalyzes removal of terminal α-linked N-acetylgalactosamine (α-GalNAc) and (less efficiently) galactose monosaccharides (1). Substrates for human α-NAGAL include glycans found in O-linked glycosylation, blood group A antigens, and others with α-GalNAc glycosides. In humans, mutations in the NAGA gene that codes for α-NAGAL lead to a loss of enzymatic activity in the lysosome and the subsequent accumulation of substrates, which eventually results in the autosomal recessive Schindler/Kanzaki disease (2–4). Schindler/Kanzaki disease belongs to the lysosomal storage disease family, which includes such diverse diseases as Tay-Sachs, Sandhoff, Gaucher, and Fabry diseases. Lysosomal storage diseases are characterized by the accumulation of substrates in the absence of a functional enzyme, leading to progressive deterioration in patients. Schindler/Kanzaki disease presents a wide range of symptoms in patients but is primarily characterized by neuronal pathologies (5–11). In humans, the NAGA gene is most closely related to the GLA gene, which codes for α-galactosidase (α-GAL A), and mutations in GLA lead to Fabry disease (12–14).
There are three principal treatment strategies currently approved or in clinical trials for lysosomal storage diseases. The first is enzyme replacement therapy, where the missing enzymatic activity is provided by regular injections of enzyme purified from recombinant sources. This therapy has been approved for the treatment of Fabry, Gaucher, and Pompe diseases and mucopolysaccharidosis-I, -II, and -VI (15). A second strategy is substrate reduction therapy, where an inhibitor of an enzyme upstream in a biosynthetic pathway leads to reduced substrate accumulation (16). A third approach is pharmacological chaperone therapy, where the mutant enzyme is stabilized by the addition of a small-molecule chaperone. This strategy has been proposed for Gaucher and Pompe diseases and is currently in phase III clinical trials for Fabry disease (17, 18). Approximately 50% of Fabry disease mutations lead to defects in the folding or stability of the enzyme, and this subset responds to pharmacological chaperone in cellular studies (18). Additionally, the pharmacological chaperone strategy allows the possibility of treatment of lysosomal storage diseases with neurological manifestations, because small-molecule chaperones can potentially cross the blood–brain barrier, whereas macromolecular enzymes are typically unable to cross into the brain. To prevent the occurrence of lysosomal storage diseases, a modest 5–15% threshold of enzymatic activity may be sufficient (17, 19–21).
The most promising pharmacological chaperone in the clinic is the iminosugar analog of galactose, 1-deoxygalactonojirimycin (DGJ), used for the treatment of Fabry disease (22). DGJ can bind and stabilize α-GAL A at neutral pH in the endoplasmic reticulum (ER), allowing it to traffic to the lysosome, where it then dissociates at low pH (18, 22–28). Paradoxically, the addition of a small-molecule competitive inhibitor of an enzyme leads to an increase in the amount of activity of the enzyme, which slows the progression of the disease.
In this report, we sought specific pharmacological chaperones for the human α-NAGAL enzyme. We found that DGJ can bind, inhibit, and chaperone α-NAGAL nearly as well as it chaperones α-GAL A. Because DGJ has promiscuity for a number of lysosomal enzymes, we tested a related molecule with improved specificity for α-NAGAL. We synthesized an iminosugar, 2-acetamido-1,2-dideoxy-d-galactonojirimycin (DGJNAc), specific for α-NAGAL (29, 30), and we examined its ability to bind, inhibit, and chaperone human α-NAGAL. We performed enzyme kinetics experiments to measure the binding and inhibitory properties of the two compounds on human α-NAGAL. We show that DGJNAc and DGJ are able to protect human α-NAGAL from proteolytic degradation. We determined high-resolution crystal structures of the complexes of DGJNAc and DGJ bound to human α-NAGAL at 1.4 and 1.5 Å, respectively, and a glucose-soaked structure with an empty active site at 1.6 Å. We then tested the ability of DGJNAc and DGJ to chaperone α-NAGAL in cellular assays. Overall, our experiments reveal the atomic basis for the tight binding of iminosugars to lysosomal glycosidases. We show that both DGJNAc and DGJ are suitable pharmacological chaperones for α-NAGAL, and the potency of DGJNAc in vitro is ∼10-fold greater. We propose DGJNAc and DGJ as potential pharmacological chaperones for Schindler/Kanzaki disease patients.
Results
Inhibition of α-NAGAL by the Iminosugars DGJNAc and DGJ.
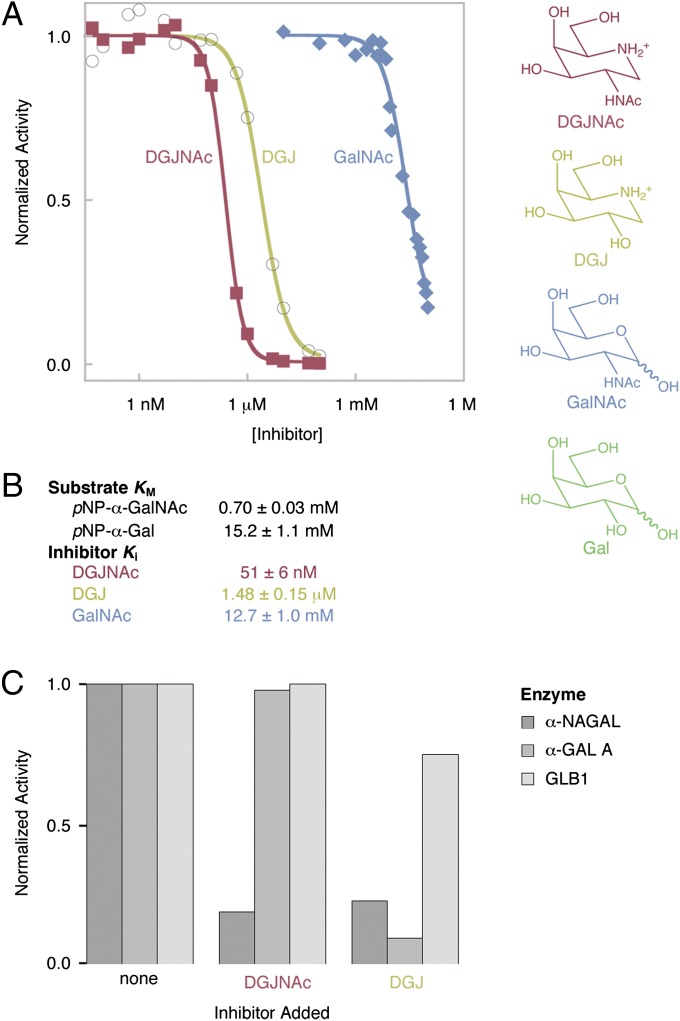
To test whether DGJNAc and DGJ would make suitable pharmacological chaperones for human α-NAGAL, we first measured their binding in enzymatic inhibition assays. As predicted from their similarity to the catalytic products of the α-NAGAL reaction, both DGJNAc and DGJ are inhibitors of α-NAGAL. DGJNAc is a tight binding inhibitor of α-NAGAL, with a Ki of 51 nM, whereas DGJ binds 29-fold weaker with a Ki of 1.5 μM (Fig. 1). We also tested the ability of catalytic products to inhibit human α-NAGAL: GalNAc binds 250,000-fold weaker with a Ki of 12.7 mM (Fig. 1), and galactose is a very weak inhibitor, where concentrations up to the assay limit of 100 mM did not inhibit the enzyme. By comparison, in the interaction exploited in clinical trials of pharmacological chaperone therapy for Fabry disease, DGJ has a Ki of 40 nM for α-GAL A. Because the affinities of DGJNAc for α-NAGAL and DGJ for α-GAL A are similar, DGJNAc has potency in the appropriate range for pharmacological chaperone therapy in Schindler/Kanzaki disease.
Fig. 1.
Enzymatic Inhibition of α-NAGAL by DGJ, DGJNAc, and GalNAc. (A) DGJNAc (red), DGJ (yellow), and GalNAc (blue) were tested for their ability to inhibit the α-NAGAL-catalyzed cleavage of pNP-α-GalNAc. (B) The measured IC50 values were converted into Ki values as described in the text. KM values for substrates are also shown. (C) Three enzymes with related activities (α-NAGAL, α-GAL A, and GLB1) were tested in enzymatic assays in the presence of no inhibitor (Left), 20 μM DGJNAc (Center), or 20 μM DGJ (Right). DGJNAc shows increased specificity over DGJ and inhibits only α-NAGAL.
To examine the specificity of DGJNAc and DGJ, we performed the inhibition assays with two lysosomal enzymes with closely related activities, α-GAL A and GLB1 (also known as β-galactosidase). DGJNAc is highly specific for α-NAGAL, whereas DGJ inhibits α-GAL A better than α-NAGAL and partially inhibits GLB1 (Fig. 1C), in agreement with previous enzymatic and crystallographic studies (18, 25, 27, 31–34).
Protease Protection Conferred by Iminosugar Binding.
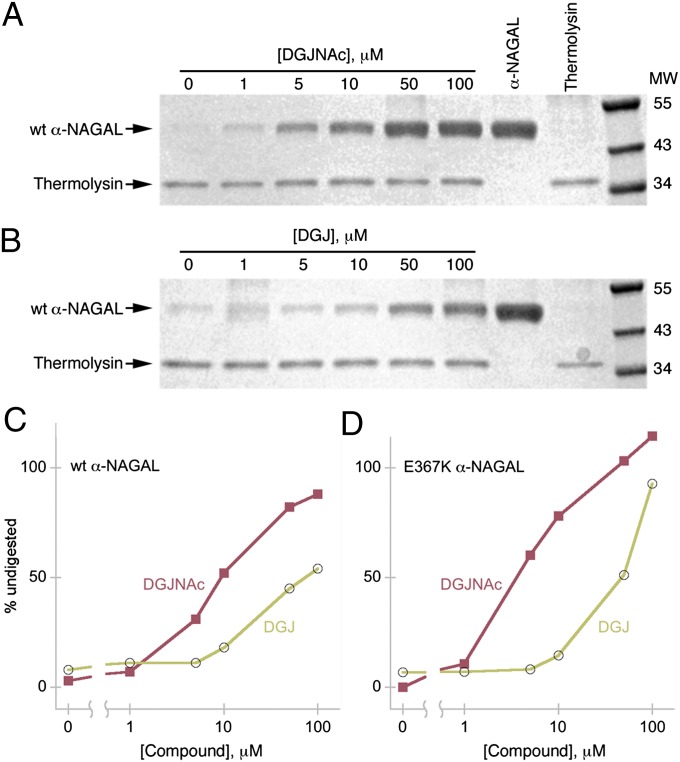
We tested whether DGJNAc and DGJ could limit the proteolytic degradation of α-NAGAL when exposed to limited thermolysin digestion (35). Native α-NAGAL protein is resistant to many proteases, including trypsin, thermolysin, and pepsin, but addition of denaturant allowed for digestion. α-NAGAL was preincubated with different concentrations of DGJNAc or DGJ, and then equilibrated in 4 M urea before limited digestion and SDS/PAGE, revealing the amount of undigested α-NAGAL. The digestions show that, at pH 7.2 (mimicking the pH in the ER), both DGJNAc and DGJ are effective pharmacological chaperones for human α-NAGAL, where both compounds protect α-NAGAL from proteolysis, with DGJNAc protecting at ∼10-fold lower concentrations (Fig. 2). Both wild-type and the Schindler/Kanzaki mutant E367K α-NAGAL behave similarly in the digestion assay.
Fig. 2.
DGJ and DGJNAc confer protection against protease digestion of α-NAGAL. (A and B) Wild-type (wt) α-NAGAL was preincubated with 0–100 μM DGJNAc (A) or DGJ (B) before thermolysin digestion and SDS/PAGE. The intensity of the undigested α-NAGAL band indicates compound-induced protection from proteolysis. (C and D) Densitometry plots of the wild-type (C) and E367K mutant (D) α-NAGAL bands at different compound concentrations show the higher potency of DGJNAc over DGJ.
Atomic Basis of Iminosugar Interaction with Glycoside Hydrolase Family 27 Enzymes.
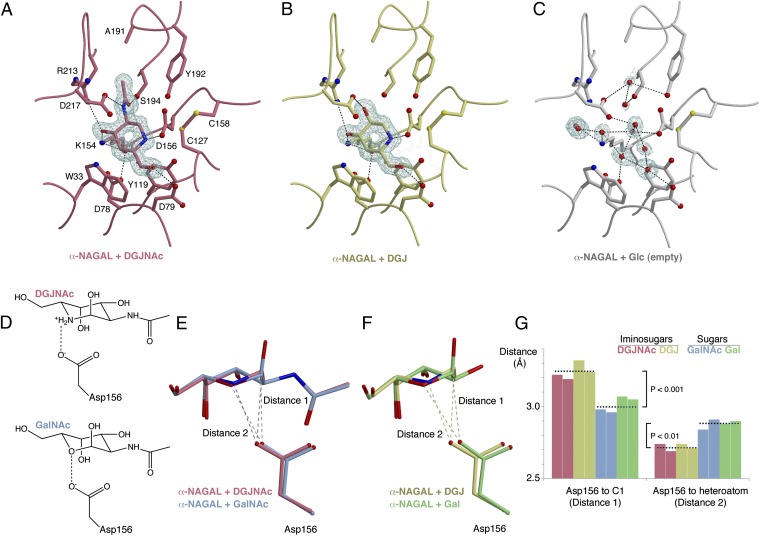
To investigate the atomic basis of DGJNAc and DGJ binding to α-NAGAL, we determined three crystal structures of α-NAGAL soaked with DGJNAc, DGJ, or glucose (Fig. 3 and Table S1). Comparison of the new structures with the previously determined complexes of α-NAGAL with GalNAc and Gal (36) reveals a highly favorable ionic interaction in the iminosugar complexes (Fig. 3D). In sugar complexes from this family of enzymes, the catalytic nucleophile is poised to attack C1 of the ligand (31, 33, 36–40). The distances from the nucleophile D156 to C1 of GalNAc (2.97 Å) and to C1 of Gal (3.06 Å) are shorter in the sugar complexes, where the syn lone pair of D156 points directly at C1 of the sugar, in position for nucleophilic attack. However, when an iminosugar is bound in the α-NAGAL active site, D156 shifts away from C1 (3.21 and 3.28 Å for DGJNAc and DGJ, respectively) and toward N5 of the ligand (Fig. 3 E–G). The DGJNAc and DGJ iminosugars bind more like each other than like the GalNAc and Gal sugars. When iminosugars are compared with sugars, the distances between D156 and the ligand shift by statistically significant amounts, with P < 0.01.
Fig. 3.
Pharmacological chaperone binding in the active site of human α-NAGAL. (A–C) σA-weighted 2Fo-Fc electron density maps of α-NAGAL soaked with DGJNAc, DGJ, and glucose respectively. A and B are contoured at 1.8σ around the ligand density and C at 1.5σ. (D) Schematic indicating the key interaction between Asp156 and DGJNAc or GalNAc. (E and F) Superposition of structures of iminosugar complexes comparing DGJNAc (red) and DGJ (yellow) with their sugar analogs GalNAc (blue) and Gal (green), and the location of the catalytic nucleophile Asp156. (G) Plots of the distances of Asp156 to the ligand C1 and heteroatom, indicating the different mode of binding of the iminosugars and sugars. The colors are as in E and F, the dashed line shows the mean of four grouped measurements, and brackets show P values from Student t tests of the paired data.
DGJNAc and DGJ Chaperoning of Human α-NAGAL in Cells.
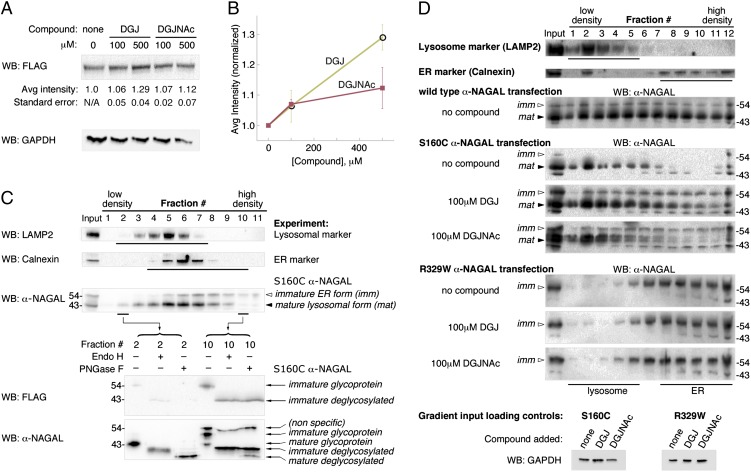
To examine the ability of DGJNAc and DGJ to chaperone wild-type α-NAGAL in vivo, we transfected human embryonic kidney (HEK 293T) cells with plasmids encoding FLAG-tagged human α-NAGAL in the presence or absence of the compounds. Immunoblots with anti-FLAG antibodies revealed higher levels of α-NAGAL protein expressed in cells cultured in the presence of DGJNAc or DGJ (Fig. 4 A and B). Despite the higher potency of DGJNAc in vitro, cells cultured with DGJNAc showed a lesser increase in α-NAGAL protein expression, possibly due to differential uptake of DGJ and DGJNAc in cells (30). Both DGJ and DGJNAc showed statistically significant increases in protein levels compared with no compound (100 μM DGJNAc and 500 μM DGJ have P < 0.05 in paired Student t tests). The ability of DGJ to chaperone wild type α-GAL A in cellular assays led to its eventual development as a clinical candidate (22).
Fig. 4.
Pharmacological chaperones can increase expression and lysosomal localization of human α-NAGAL in mammalian cells. (A and B) HEK 293T cells were transfected with FLAG-labeled human α-NAGAL and incubated in triplicate with 0, 100, or 500 μM DGJ or DGJNAc for 24 h. Anti-FLAG Western blots (WB) of lysates show increases in the amount of α-NAGAL expressed in the presence of DGJ and DGJNAc. α-NAGAL band intensities were normalized to expression of α-NAGAL in the absence of compound. Student t tests of paired data show P < 0.05 for 100 μM DGJNAc and 500 μM DGJ compared with no compound. Lysates were blotted with a GAPDH antibody as a loading control. (C) Subcellular fractionation of α-NAGAL shows mature and immature forms. Gradient separation of postnuclear supernatant of FLAG-labeled α-NAGAL-expressing 293T cell homogenate was followed by Western blotting with antibodies against LAMP2 (lysosomal marker), Calnexin (ER marker), FLAG, or α-NAGAL. α-NAGAL segregates into a larger form that colocalizes with the ER marker (white arrowhead, labeled imm) and a smaller form that colocalizes with the lysosomal marker (black arrowhead, labeled mat). Fractions 2 and 10 were analyzed by blotting with FLAG and α-NAGAL antibodies, showing that the C-terminal FLAG epitope is largely removed in the lysosome. The immature form is fully deglycosylated by Endo H and PNGase F, whereas the smaller mature form is fully deglycosylated by PNGase F but shows resistance to Endo H, characteristic of glycan processing in the Golgi. (D) DGJ and DGJNAc can increase the amount of mature α-NAGAL for select Schindler/Kanzaki mutations. Homogenates from HEK 293T cells stably expressing wild-type, S160C, or R329W α-NAGAL were subjected to subcellular fractionation and blotted with antibodies against α-NAGAL. Before fractionation, the gradient inputs were Western blotted with GAPDH (as a loading control). The S160C mutant shows increases in total protein and in lysosomal protein when DGJ or DGJNAc is added, restoring the wild-type protein distribution. In contrast, the R329W mutant does not respond and shows the same distribution of total and lysosomal α-NAGAL with and without compound added. The white and black arrowheads mark the immature and mature forms of α-NAGAL as in C.
To explore the effect of the compounds on mutant forms of α-NAGAL found in Schindler/Kanzaki disease patients, we performed subcellular fractionation assays to separate lysosomal and ER-resident forms of α-NAGAL (Fig. 4C). Overexpressed α-NAGAL shows two distinct bands when blotted with a polyclonal antibody. Subcellular fractionation revealed that the higher molecular weight band (Fig. 4C, white arrowhead) fractionates with the ER marker Calnexin, retains the C-terminal FLAG tag, and has no Endo H-resistant glycans, indicative of an immature ER protein. The lower molecular weight band (Fig. 4C, black arrowhead) fractionates with the lysosomal marker LAMP2, loses the C-terminal FLAG tag, and has Endo H-resistant glycans, indicative of a mature lysosomal protein.
DGJNAc and DGJ can restore wild-type cellular distribution to the S160C α-NAGAL, a Schindler/Kanzaki disease variant form. In the absence of pharmacological chaperone, cells expressing the S160C α-NAGAL mutant show little mature α-NAGAL (and almost no immature α-NAGAL, presumably due to ER-associated degradation). When DGJ or DGJNAc is added to the S160C α-NAGAL cells, the amounts of overall α-NAGAL and of mature lysosomal α-NAGAL increase relative to controls (Fig. 4D). Additionally, the cellular distribution of S160C α-NAGAL in the presence of DGJ or DGJNAc mimics that of wild-type α-NAGAL, suggesting that the S160C Schindler/Kanzaki mutant will respond to pharmacological chaperone therapy. In contrast, for the R329W Schindler/Kanzaki mutant, only immature α-NAGAL was detected, and there was response to neither DGJ nor DGJNAc (Fig. 4D). This observation is consistent with α-GAL A defects in Fabry disease, where ∼50% of the disease mutations respond to pharmacological chaperones (18).
Discussion
Since the initial structure of human α-NAGAL at 1.9-Å resolution (36), we have improved the resolution to 1.4 Å (among the highest resolution structures reported for human glycoproteins). The new structures provide unprecedented coordinate accuracy (with a maximum estimated coordinate error of 0.11 Å), revealing subtle changes in the enzyme upon pharmacological chaperone binding, and providing a structural basis for the much higher potency of iminosugars. The structural results presented here are applicable to the entire glycoside hydrolase family 27 (41) including α-NAGAL and α-GAL A, as well as other proteins that bind iminosugars.
The high-resolution structures provide an atomic basis for the tighter binding of the iminosugars, by highlighting the critical importance of the ion pair between the amine of DGJNAc/DGJ and the D156 carboxylate (Fig. 3 D–G). By revealing the interactions responsible for tight binding of ligands, the structures will aid in the design of high-affinity compounds for these and other lysosomal enzymes. We propose that future scaffolds for compounds that bind glycoside hydrolase active sites take advantage of a highly favorable ion pair with the nucleophilic carboxylate, leading to tight-binding inhibitors.
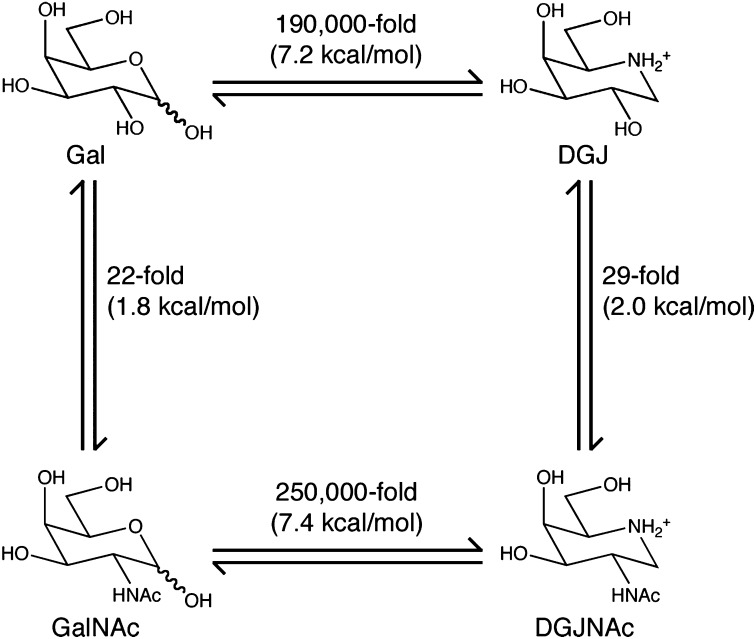
The structures and the enzyme inhibition data for the ligands allow us to construct a thermodynamic cycle for ligand binding to α-NAGAL (Fig. 5). Replacement of the ligand 2-OH by an N-acetyl group provides ∼2 kcal/mol of additional binding energy. Replacement of the ring oxygen in hexose sugars with nitrogen in iminosugars provides >7 kcal/mol of additional binding energy. The two substitutions taken together lead to >9 kcal/mol improved binding, or better than 106-fold improvement in binding affinity, a remarkable difference for highly similar compounds.
Fig. 5.
Thermodynamic cycle for binding of ligands to α-NAGAL. A ΔΔG representation of ligand binding to human α-NAGAL shows that the presence of an N-acetyl group of GalNAc and DGJNAc provides ∼2 kcal/mol of binding energy, whereas the ring nitrogen provides >7 kcal/mol. At the corners of the cycle, DGJNAc binds to α-NAGAL >106-fold tighter than galactose does.
Because of the similarity of pharmacological chaperones to natural ligands, they can have off-target binding. For example, 20 μM DGJ (a clinically relevant concentration for targeting α-GAL A in Fabry disease treatment) inhibits α-NAGAL by 70% and GLB1 by 25% in vitro (Fig. 1). In cellular assays, 10 μM DGJ inhibits both α-NAGAL by 63% and GLB1 by 25% (18), potentially affecting the degradation of substrates with terminal α-GalNAc and β-Gal glycosides. Thus, the potency and efficacy of DGJ for α-NAGAL are both encouraging and problematic pharmacologically. In Schindler/Kanzaki disease, DGJ may be useful as a pharmacological chaperone. However, the DGJ–α-NAGAL interaction also represents unwanted off-target binding of the DGJ–α-GAL A interaction central to pharmacological chaperoning for Fabry disease. The 1.6 μM Ki of DGJ for α-NAGAL is well below the therapeutic target concentration for treatment of Fabry disease, so off-target binding to α-NAGAL is expected. In contrast, DGJNAc contains an N-acetyl group that sterically precludes it from binding to α-GAL A and GLB1 (31–34), so it has improved selectivity for α-NAGAL.
In contrast to traditional enzyme inhibitors used in the clinic, effective pharmacological chaperones must meet additional criteria (17). Traditional inhibitors simply bind the active site of their target with high potency, rendering the enzyme ineffective due to competitive inhibition. On the other hand, pharmacological chaperones must not only bind to the target enzyme with high potency, but they must also dissociate from the active site of the enzyme for catalysis to occur. Additionally, an effective pharmacological chaperone must also distribute efficiently to the ER, where folding of the target enzyme occurs.
DGJNAc, the iminosugar analog of GalNAc, has been proposed as a possible pharmacological chaperone for human α-NAGAL. DGJNAc was originally hypothesized to be an inhibitor of α-NAGAL by extension of observations on the Fabry disease equivalents (DGJ and α-GAL A) (17). However, a report of the specific synthesis and testing of the compound appeared only recently (29) [and not in a sometimes-cited earlier paper (42)]. Here, we describe biochemical, structural, and cellular studies on the interaction and chaperoning of human α-NAGAL by DGJNAc.
Our experiments point to DGJNAc and DGJ as reasonable candidates for pharmacological chaperones for Schindler/Kanzaki disease, as both compounds meet the affinity criterion suggested for developing new pharmacological chaperones. [A threshold of IC50 of <10 μM is recommended for promising pharmacological chaperone lead compounds (17).] Our protease protection studies show that both DGJNAc and DGJ are capable of reducing the amount of α-NAGAL digested by protease, indicating that they shift the folding equilibrium toward the folded state. In cellular assays, we show that DGJNAc and DGJ are able to increase the amount of wild-type α-NAGAL protein produced by mammalian cells. Additionally, we show that DGJNAc and DGJ are able to increase the amount of S160C α-NAGAL (a Schindler/Kanzaki disease variant form) delivered to the lysosome compared with controls. DGJNAc and DGJ increase cellular expression of α-NAGAL by 20–30%. This increase is comparable to the chaperoning seen with the compounds DGJ and isofagomine, which have been tested in clinical trials for Fabry and Gaucher diseases (18, 43).
Because the population of patients with Schindler/Kanzaki disease is extremely small, it is unlikely to be an active target for clinical development by the pharmaceutical industry, and the neurological manifestations of the disease make enzyme replacement therapy unsuitable. We suggest that DGJ (which has already succeeded in phase I clinical trials, demonstrating its safety in humans) might be repurposed for use as a pharmacological chaperone in the Schindler/Kanzaki disease patient population. Alternatively, DGJNAc (which has higher potency than DGJ for α-NAGAL in vitro but unexpectedly shows less efficacy in cellular experiments) might be adapted into a more effective pharmacological chaperone used in compassionate care for Schindler/Kanzaki disease. For example, iminosugar compounds for Gaucher disease were developed into clinical candidates by derivatization of the heterocyclic nitrogen with alkyl substituents (44, 45).
The role of α-NAGAL in cancer is controversial (46). It is well established that α-NAGAL in the serum is a biomarker for cancers including melanoma and that α-NAGAL activity is raised in patients with large tumor burdens (47). However, others have proposed a causal link between α-NAGAL and cancer progression: α-NAGAL in the serum is hypothesized to deglycosylate vitamin D binding protein, removing the latter’s ability to activate macrophages, leading to immunosuppression (48). The DGJNAc molecule we describe here, a specific inhibitor of α-NAGAL, allows for direct testing of this hypothesis. If the proposed mechanism of α-NAGAL exacerbating cancer progression proves to be correct, DGJNAc may then be useful in the treatment of cancer by reducing immunosuppression in patients.
In summary, the structures, results, and design principles reported here will improve therapeutic approaches toward protein folding diseases. We have synthesized and tested DGJNAc, a compound for the treatment of Schindler/Kanzaki disease, showing that it binds and chaperones human α-NAGAL both in vitro and in vivo. We have established that DGJ, a compound currently in clinical trials for Fabry disease, could be repurposed to treat Schindler/Kanzaki patients, who currently have no treatment options available to them. By characterizing the inhibitory properties of DGJNAc toward α-NAGAL, we describe a compound that can be used to directly test the role of α-NAGAL in immunosuppression of several cancers. If the role of α-NAGAL in immunosuppression is proven, DGJNAc, as a high-affinity inhibitor of α-NAGAL, might be useful as an anticancer compound.
Materials and Methods
For biochemistry and crystallography experiments, α-NAGAL was produced in Tn5 insect cells. Crystal structures of N201Q α-NAGAL, an engineered variant that produces high-quality crystals (36), were solved with DGJ bound, DGJNAc bound, or with an empty active site. DGJNAc was synthesized as described (29). For limited proteolysis studies, purified wild-type or E367K α-NAGAL was incubated overnight in 4 M urea, pH 7.2, in the presence and absence of DGJ or DGJNAc. Samples were digested briefly with thermolysin and analyzed by SDS/PAGE. Cellular studies used HEK 293T cells transfected with FLAG-tagged wild-type, S160C, or R329W α-NAGAL. Density gradient cellular fractionation was used to analyze the cellular distribution of wild-type and mutant forms of α-NAGAL, and the ability of DGJ and DGJNAc to rescue mutant forms of α-NAGAL. For complete methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Thomas Cleveland for cell culture suggestions and Daniel N. Hebert, Johan C. Sunryd, and Kristina M. Giorda for advice and assistance with mammalian cell culture. We thank Emily K. Schutsky and Yadilette Rivera-Colón for human GLB1. We gratefully acknowledge Jean Jankonic, Marc Allaire, and Vivian Stojanoff at the National Synchrotron Light Source X6A beam line, funded by the National Institute of General Medical Sciences, National Institutes of Health, under Agreement GM-0080. We thank PXRR beam line X25, funded by the Offices of Biological and Environmental Research and of Basic Energy Sciences of the Department of Energy, and from the National Center for Research Resources of the National Institutes of Health. This work was funded by National Institutes of Health Grant R01 DK76877 (to S.C.G.) and by National Science Foundation Integrative Graduate Education and Research Traineeship 0654128 (to N.E.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4DO4, 4DO5, and 4DO6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203924109/-/DCSupplemental.
References
- 1.Dean KJ, Sung SS, Sweeley CC. The identification of α-galactosidase B from human liver as an α-N-acetylgalactosaminidase. Biochem Biophys Res Commun. 1977;77(4):1411–1417. doi: 10.1016/s0006-291x(77)80136-8. [DOI] [PubMed] [Google Scholar]
- 2.Desnick RJ, Schindler D. N-Acetylgalactosaminidase deficiency: Schindler disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th Ed. New York: McGraw–Hill; 2001. pp. 3483–3505. [Google Scholar]
- 3.van Diggelen OP, et al. Lysosomal α-N-acetylgalactosaminidase deficiency: A new inherited metabolic disease. Lancet. 1987;2(8562):804. doi: 10.1016/s0140-6736(87)92542-6. [DOI] [PubMed] [Google Scholar]
- 4.Kanzaki T, Yokota M, Mizuno N, Matsumoto Y, Hirabayashi Y. Novel lysosomal glycoaminoacid storage disease with angiokeratoma corporis diffusum. Lancet. 1989;1(8643):875–877. doi: 10.1016/s0140-6736(89)92867-5. [DOI] [PubMed] [Google Scholar]
- 5.van Diggelen OP, et al. α-N-acetylgalactosaminidase deficiency, a new lysosomal storage disorder. J Inherit Metab Dis. 1988;11(4):349–357. doi: 10.1007/BF01800424. [DOI] [PubMed] [Google Scholar]
- 6.Wang AM, Kanzaki T, Desnick RJ. The molecular lesion in the α-N-acetylgalactosaminidase gene that causes angiokeratoma corporis diffusum with glycopeptiduria. J Clin Invest. 1994;94(2):839–845. doi: 10.1172/JCI117404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang AM, Schindler D, Desnick R. Schindler disease: The molecular lesion in the α-N-acetylgalactosaminidase gene that causes an infantile neuroaxonal dystrophy. J Clin Invest. 1990;86(5):1752–1756. doi: 10.1172/JCI114901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keulemans JL, et al. Human α-N-acetylgalactosaminidase (α-NAGA) deficiency: New mutations and the paradox between genotype and phenotype. J Med Genet. 1996;33(6):458–464. doi: 10.1136/jmg.33.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodama K, et al. A new case of α-N-acetylgalactosaminidase deficiency with angiokeratoma corporis diffusum, with Ménière’s syndrome and without mental retardation. Br J Dermatol. 2001;144(2):363–368. doi: 10.1046/j.1365-2133.2001.04028.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakker HD, et al. Human α-N-acetylgalactosaminidase (α-NAGA) deficiency: No association with neuroaxonal dystrophy? Eur J Hum Genet. 2001;9(2):91–96. doi: 10.1038/sj.ejhg.5200598. [DOI] [PubMed] [Google Scholar]
- 11.Chabás A, Duque J, Gort L. A new infantile case of α-N-acetylgalactosaminidase deficiency. Cardiomyopathy as a presenting symptom. J Inherit Metab Dis. 2007;30(1):108. doi: 10.1007/s10545-006-0470-1. [DOI] [PubMed] [Google Scholar]
- 12.Desnick RJ, Ioannou YA, Eng CM. Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th Ed. New York: McGraw–Hill; 2001. pp. 3733–3774. [Google Scholar]
- 13.Garman SC. Structure-function relationships in α-galactosidase A. Acta Paediatr Suppl. 2007;96(455):6–16. doi: 10.1111/j.1651-2227.2007.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: From concept to clinics. EMBO Mol Med. 2009;1(5):268–279. doi: 10.1002/emmm.200900036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt FM, Jeyakumar M. Substrate reduction therapy. Acta Paediatr Suppl. 2008;97(457):88–93. doi: 10.1111/j.1651-2227.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Fan JQ. A counterintuitive approach to treat enzyme deficiencies: Use of enzyme inhibitors for restoring mutant enzyme activity. Biol Chem. 2008;389(1):1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin ER, et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis. 2009;32(3):424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 19.Leinekugel P, Michel S, Conzelmann E, Sandhoff K. Quantitative correlation between the residual activity of β-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum Genet. 1992;88(5):513–523. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- 20.Schueler UH, et al. Correlation between enzyme activity and substrate storage in a cell culture model system for Gaucher disease. J Inherit Metab Dis. 2004;27(5):649–658. doi: 10.1023/b:boli.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- 21.Ries M, et al. Pediatric Fabry disease. Pediatrics. 2005;115(3):e344–e355. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- 22.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5(1):112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 23.Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19(1):12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 24.Ishii S, et al. Mutant α-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: Biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J. 2007;406(2):285–295. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman RL, D’aquino JA, Ringe D, Petsko GA. Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability. Biochemistry. 2009;48(22):4816–4827. doi: 10.1021/bi9002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugawara K, et al. Molecular interaction of imino sugars with human α-galactosidase: Insight into the mechanism of complex formation and pharmacological chaperone action in Fabry disease. Mol Genet Metab. 2009;96(4):233–238. doi: 10.1016/j.ymgme.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Guce AI, Clark NE, Rogich JJ, Garman SC. The molecular basis of pharmacological chaperoning in human α-galactosidase. Chem Biol. 2011;18(12):1521–1526. doi: 10.1016/j.chembiol.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin ER, et al. Co-administration with the pharmacological chaperone AT1001 increases recombinant human α-galactosidase A tissue uptake and improves substrate reduction in Fabry mice. Mol Ther. 2012;20(4):717–726. doi: 10.1038/mt.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best D, et al. Synthesis of 2-acetamido-1,2-dideoxy-d-galacto-nojirimycin [DGJNAc] from d-glucuronolactone: The first sub-micromolar inhibitor of α-N-acetylgalactosaminidases. Tetrahedron Lett. 2010;51(17):2222–2224. [Google Scholar]
- 30.Glawar AF, et al. Scalable syntheses of both enantiomers of DNJNAc and DGJNAc from glucuronolactone: The effect of N-alkylation on hexosaminidase inhibition. Chem Eur J. 2012;18(30):9341–9359. doi: 10.1002/chem.201200110. [DOI] [PubMed] [Google Scholar]
- 31.Garman SC, Hannick L, Zhu A, Garboczi DN. The 1.9 Å structure of α-N-acetylgalactosaminidase: Molecular basis of glycosidase deficiency diseases. Structure. 2002;10(3):425–434. doi: 10.1016/s0969-2126(02)00726-8. [DOI] [PubMed] [Google Scholar]
- 32.Garman SC, Garboczi DN. The molecular defect leading to Fabry disease: Structure of human α-galactosidase. J Mol Biol. 2004;337(2):319–335. doi: 10.1016/j.jmb.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Tomasic IB, Metcalf MC, Guce AI, Clark NE, Garman SC. Interconversion of the specificities of human lysosomal enzymes associated with Fabry and Schindler diseases. J Biol Chem. 2010;285(28):21560–21566. doi: 10.1074/jbc.M110.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohto U, et al. Crystal structure of human β-galactosidase: Structural basis of Gm1 gangliosidosis and morquio B diseases. J Biol Chem. 2012;287(3):1801–1812. doi: 10.1074/jbc.M111.293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park CW, Marqusee S. Pulse proteolysis: A simple method for quantitative determination of protein stability and ligand binding. Nat Methods. 2005;2(3):207–212. doi: 10.1038/nmeth740. [DOI] [PubMed] [Google Scholar]
- 36.Clark NE, Garman SC. The 1.9 Å structure of human α-N-acetylgalactosaminidase: The molecular basis of Schindler and Kanzaki diseases. J Mol Biol. 2009;393(2):435–447. doi: 10.1016/j.jmb.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto Z, Kaneko S, Momma M, Kobayashi H, Mizuno H. Crystal structure of rice α-galactosidase complexed with d-galactose. J Biol Chem. 2003;278(22):20313–20318. doi: 10.1074/jbc.M302292200. [DOI] [PubMed] [Google Scholar]
- 38.Golubev AM, et al. Crystal structure of α-galactosidase from Trichoderma reesei and its complex with galactose: Implications for catalytic mechanism. J Mol Biol. 2004;339(2):413–422. doi: 10.1016/j.jmb.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 39.Garman SC. Structural studies on α-GAL and α-NAGAL: The atomic basis of Fabry and Schindler diseases. Biocat Biotrans. 2006;24(1/2):129–136. [Google Scholar]
- 40.Guce AI, et al. Catalytic mechanism of human α-galactosidase. J Biol Chem. 2010;285(6):3625–3632. doi: 10.1074/jbc.M109.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantarel BL, et al. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichikawa Y, Igarashi Y, Ichikawa M, Suhara Y. 1-N-Iminosugars: Potent and selective inhibitors of β-glycosidases. J Am Chem Soc. 1998;120(13):3007–3018. [Google Scholar]
- 43.Benito JM, García Fernández JM, Ortiz Mellet C. Pharmacological chaperone therapy for Gaucher disease: A patent review. Expert Opin Ther Pat. 2011;21(6):885–903. doi: 10.1517/13543776.2011.569162. [DOI] [PubMed] [Google Scholar]
- 44.Brumshtein B, et al. Crystal structures of complexes of N-butyl- and N-nonyl-deoxynojirimycin bound to acid β-glucosidase: Insights into the mechanism of chemical chaperone action in Gaucher disease. J Biol Chem. 2007;282(39):29052–29058. doi: 10.1074/jbc.M705005200. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, et al. α-1-C-octyl-1-deoxynojirimycin as a pharmacological chaperone for Gaucher disease. Bioorg Med Chem. 2006;14(23):7736–7744. doi: 10.1016/j.bmc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Rehder DS, Nelson RW, Borges CR. Glycosylation status of vitamin D binding protein in cancer patients. Protein Sci. 2009;18(10):2036–2042. doi: 10.1002/pro.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greco M, et al. Serum proteomic profile of cutaneous malignant melanoma and relation to cancer progression: Association to tumor derived α-N-acetylgalactosaminidase activity. Cancer Lett. 2009;283(2):222–229. doi: 10.1016/j.canlet.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Gregory KJ, et al. Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells. PLoS One. 2010;5(10):e13428. doi: 10.1371/journal.pone.0013428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.