Abstract
IL-17–producing CD27− γδ cells (γδ27− cells) are widely viewed as innate immune cells that make critical contributions to host protection and autoimmunity. However, factors that promote them over IFN-γ–producing γδ27+ cells are poorly elucidated. Moreover, although human IL-17–producing γδ cells are commonly implicated in inflammation, such cells themselves have proved difficult to isolate and characterize. Here, murine γδ27− T cells and thymocytes are shown to be rapidly and substantially expanded by IL-7 in vitro and in vivo. This selectivity owes in substantial part to the capacity of IL-7 to activate STAT3 in such cells. Additionally, IL-7 promotes strong responses of IL-17–producing γδ cells to TCR agonists, thus reemphasizing the cells’ adaptive and innate potentials. Moreover, human IL-17–producing γδ cells are also substantially expanded by IL-7 plus TCR agonists. Hence, IL-7 has a conserved potential to preferentially regulate IL-17–producing γδ cells, with both biological and clinical implications.
Keywords: lymphocytes, cytokines, proliferation
Studies of IL-17 intensified with the identification of a specific subset of CD4+ Th17 cells that upon activation primarily produces IL-17 as opposed to IFN-γ (Th1 cells), IL-4 (Th2 cells), or IL-10/TGFβ (Treg cells) (1–3). Th17 differentiation is regulated by transcription factors RORγt and STAT3, the latter in part explaining the promotion of Th17 differentiation by IL-6 and IL-23 (3). Paradoxically, more detailed studies of Th17 immunity have identified γδ T cells and/or innate-like lymphoid cells as critical initial producers of IL-17 (4, 5). At steady-state, γδ cells are only a minor subset of T lymphocytes, but upon infection by Listeria, Mycobacteria, or Plasmodium, or upon LPS administration, they expand and make critical contributions to host protection (5–9). They likewise underpin immunopathology in widely used models of inflammatory disease (10, 11). In humans, IL-17 protects against mucocutaneous candidiasis and is again implicated in autoimmune inflammation, including psoriasis, multiple sclerosis, and rheumatoid arthritis (12, 13). Hence, there is considerable interest in identifying factors that regulate IL-17–producing γδ cells in mice and humans.
Adding to this interest is the emergence of murine γδ cells as prime examples of thymic preprogramming, whereby functional distinctions between CD27+IFN-γ producers (γδ27+ cells) and CD27−IL-17–producing (γδ27−) cells are established by developmental cues that are largely uneludicated (8, 14). For example, γδ27− cells seem largely to arise from fetal thymocytes, requiring neither engagement of cognate ligand, nor RORγt or STAT3 that are both required for TCRαβ+ Th17 cell development (15). However, despite the dispensability of RORγt and STAT3 in development, most peripheral IL-17–producing γδ cells express RORγt and respond rapidly to IL-23 that signals via STAT3 (10). Such rapid responsiveness in the absence of TCR stimulation has led many to classify γδ27− cells as innate immune cells: Indeed, they generally respond poorly to concentrations of TCR agonists that would promote robust activation of γδ27+ cells (9). Nonetheless, assigning IL-17–producing γδ cells to innate immunity seems premature until more is known about what regulates the cells and how that might influence their response to TCR stimulation.
Although IL-17–producing γδ cells are likewise commonly evoked in human immune responses and immunopathologies, very little is known about these cells, because they have proved particularly hard to isolate and characterize (16). Thus, it seemed logical that by elucidating stimuli for murine γδ27− cells, one might identify the means to expand their human counterparts. This study identifies IL-7 as a profound and selective activator of IL-17–producing γδ cells in mouse and in human neonates.
Results
IL-7 Enriches for Lymph Node γδ27− Cells.
Lymph node (LN) γδ27+ cells appear like naïve conventional T cells, being primarily CD62L+ CD25− CD44lo ICOS−, whereas between 50% and 75% of γδ27− cells resemble activated T cells (CD62L− CD25+/− CD44hi ICOS+), although they are largely CD69− (Fig. 1A and Fig. S1A). As was reported (17), γδ27− cells also express higher levels of IL-7R than do γδ27+ cells (Fig. 1A). To determine whether this phenotype had functional implications, LN cells were cultured with IL-7 for 4 d. Over eight independent experiments, γδ27− cells were strikingly enriched ∼five- to sevenfold relative to γδ27+ cells and ∼6- to 10-fold relative to total LN cells, whereas αβT-cell numbers declined (Fig. 1B and Fig. S1 B and C). Essentially all γδ27− cells were TCRhi CD44hi, and now ∼70% expressed CD69 (Fig. 1 B and C). IL-7 also increased the proportion of γδ27+ cells expressing CD44 and CD69 (Fig. 1C), although their numbers declined ∼70% over 4 d, whereas absolute numbers of γδ27− cells increased three- to fourfold (Fig. 1D). Strikingly, this enrichment was for cells with IL-17–producing capacity, whose representation increased from ∼30% to ∼70% of the γδ27− subset (Fig. 1E). Consistent with this increase, IL-7 enriched for cells expressing RORγt protein but not for those expressing T-bet, a primary regulator of IFN-γ (Fig. S1D).
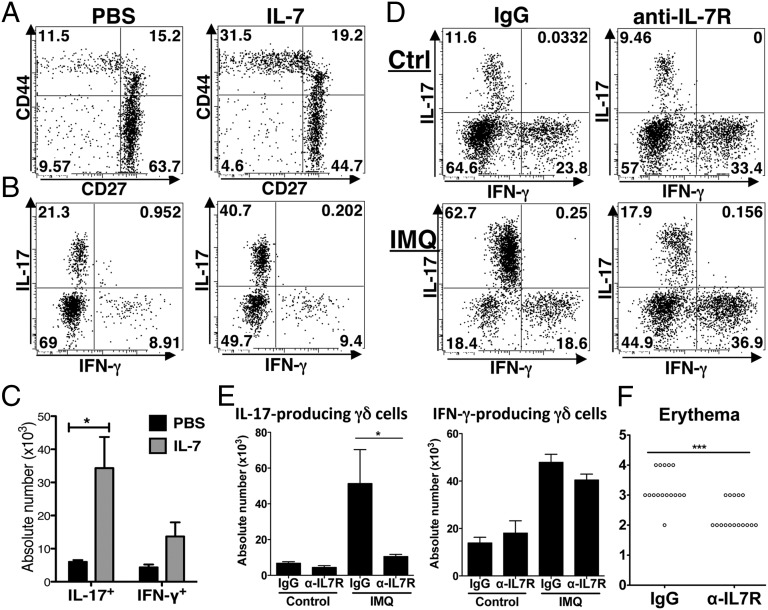
Fig. 1.
IL-7 enriches for IL-17–competent γδ T cells. (A) γδ T cells from lymph nodes (LN) of adult mice, stained for CD44, CD69, IL-7R, and CD27. (B) Representative plots (from n = 8 experiments) of total LN cells, ex vivo (Left) and after 4-d culture in vitro with IL-7 (Right). (C) Surface staining of γδ T cells after 4-d culture in vitro with IL-7. (D) Absolute numbers of CD44lo and CD44hi γδ27+ cells and CD44lo and CD44hi γδ27− cells from LN cells cultured as in B. Error bars are SEM from n = 8 experiments: *P < 0.05, ***P < 0.0005. (E) Intracellular staining for IL-17 in γδ T cells from LN cells cultured as in B and then activated with PMA + ionomycin.
To probe the generality of these observations, we investigated cells from the peritoneal cavity, known to harbor IL-17–producing γδ T cells (18, 19). Ex vivo almost all γδ cells were CD44hi (Fig. S1E), and they were enriched after 4 d in IL-7, compared with total cells (Fig. S1 E and F). However, whereas IL-7 maintained γδ27+ cell numbers in vitro relative to culture in medium alone, absolute numbers of γδ27− cells were again increased: ∼ninefold relative to medium alone, and ∼fourfold relative to numbers harvested ex vivo (Fig. S1G). Among these cells, the proportion of IL-17 producers was again increased (Fig. S1H). Thus, IL-7 preferentially enriches for IL-17–competent γδ T cells from two distinct anatomical sources.
To examine whether the same effects would be achieved in vivo, mice were administered recombinant IL-7 three times over 5 d and then examined on day 7. There was a conspicuous enrichment of CD44hi γδ27− cells, with absolute numbers of LN γδ cells competent to make IL-17 upon activation increasing >fivefold, compared with two- to threefold increases in IFN-γ–competent cells (Fig. 2 A–C). Note that before and after IL-7 treatment, few γδ cells coproduced IL-17 and IFN-γ, consistent with developmental preprogramming (8).
Fig. 2.
IL-7 enriches in vivo for IL-17–competent γδ T cells. (A) Representative plots (n = 3) of γδ T cells from total adult LNs from mice treated with PBS (Left) and IL-7 (Right). For all plots, numbers indicate percent of cells in relevant quadrant. (B) Intracellular staining for IL-17 and IFN-γ in gated γδ T cells, from mice treated as in A, and then activated in vitro with PMA + ionomycin. (C) Absolute numbers of IL-17+ and IFN-γ+ γδ T cells from mice treated as in B: Error bars are SEM from n = 3 mice; *P < 0.05. (D) Mice were treated with Vaseline (Ctrl; Upper) or imiquimod (IMQ; Lower) and anti–IL-7R Ab (Right) or isotype control (Left). Intracellular staining for IL-17 and IFN-γ in γδ T cells from LN cells, activated with PMA + ionomycin. (E) Absolute numbers of IL-17+ and IFN-γ+ γδ cells obtained as described in D (error bars are SEM from two experiments, n = 8 mice in total, *P < 0.05). (F) Score for skin erythema from mice treated with IMQ as in D (from 4 experiments, n = 16 mice in total, P < 0.005).
To test whether IL-7 is required for the expansion of IL17–producing γδ cells in vivo, we examined mice treated epicutaneously with imiquimod (IMQ) in which the development of acute psoriaform lesions is largely attributable to expansion of IL-17–producing γδ cells in the skin and skin-draining LNs (11, 20). Indeed, such lesions are comparable in WT and αβ T-cell–deficient mice but dramatically reduced in TCRδ−/− mice (11, 20). Administration of anti–IL-7R antibody almost completely blocked the enrichment (∼10-fold) in IL-17+ γδ cells in the skin-draining LNs of mice administered IMQ versus vaseline but did not significantly limit the two- to threefold expansion of IFN-γ+ γδ cells (Fig. 2 D and E). Skin erythema scores, which compose a highly reproducible marker of IMQ-induced pathology, were significantly reduced in anti–IL-7R–treated animals (Fig. 2F), as was epidermal thickening that is associated with dermal IL-17–producing γδ cell expansion (11, 20). That some reddening nonetheless occurred most likely reflects widely acknowledged nonimmunological effects of IMQ (20).
Mechanism of Enrichment.
Directly ex vivo, few γδ27+ and γδ27− cells were dividing as judged by Ki67 staining, but after 4 d in IL-7, >90% of γδ27− cells were dividing compared with only ∼30% for γδ27+ cells (black versus gray lines; Fig. 3A). Furthermore, when cells were labeled ex vivo with a membrane-intercalating dye, carboxy-fluorescein diacetate succinimidyl ester (CFSE), γδ27− cells showed much greater dye dilution (by cell division) than did γδ27+ cells (Fig. 3B), and it was those dividing cells that accounted for almost all IL-17 production upon stimulation (Fig. 3C). Hence, IL-7 drives the preferential expansion of γδ27− cells with, by contrast, little evidence of selective survival: indeed, Bcl-2 mRNA whose up-regulation has been associated with antiapoptotic effects of IL-7 in T cells (21) was more strongly expressed by γδ27+ cells (Fig. S2A).
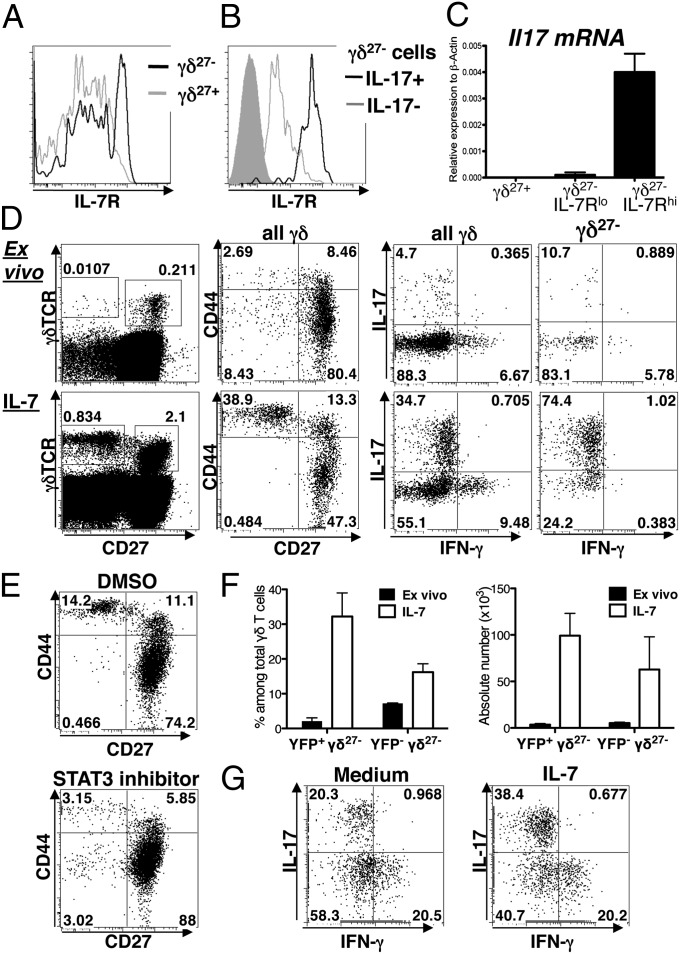
Fig. 3.
IL-7 promotes expansion of IL-17–competent γδ T cells via selective STAT3 activation. (A) Staining for Ki67 (cells in cycle) in gated γδ27− (Left) and γδ27+ (Right) LN cells ex vivo (gray line) and after 4-d culture with IL-7 (black line). Shaded histograms show Ki67 isotype staining. (B) Offset histograms of γδ27+ (red) and γδ27− (blue) LN cells labeled with CFSE and then cultured for 4 d with IL-7. Shaded gray area represents γδ T cells stained ex vivo. (C) CFSE-labeled LN cells were cultured for 4 d with IL-7, activated with PMA + ionomycin and stained for intracellular IL-17 and gated on γδ cells. (D) Flow cytometric detection of intracellular pSTAT5 and pSTAT3 in gated γδ27− and γδ27+ cells as labeled. Open and shaded areas indicate IL-7 treatment and controls, respectively. (E) Intracellular localization by ImageStream Flow Cytometry of pSTAT3 among two representative CD44hi γδ27− (Upper) and CD44lo γδ27+ (Lower) LN cells after 30-min culture with IL-7 (BF, Bright field). (F) LN cells preincubated with a specific STAT3 inhibitor and subsequently cultured with IL-7 for 72 h. Representative plots (from n = 3 experiments). (G) Staining for Ki67 and intracellular IL-17 in gated γδ T cells cultured as in F; open and shaded areas indicate STAT3 inhibitor preincubation and controls, respectively. For all plots, numbers indicate percent of cells in relevant gate or quadrant.
IL-7 signals are primarily transduced by STAT5 and PI3-kinase (22–24). However, IL-7–dependent STAT5 phosphorylation was comparable among γδ27+ cells and γδ27− cells (Fig. 3D), failing to account for the differential effects of IL-7 on γδ27− cells. In fact, STAT5 activation antagonizes Th17 differentiation (25) in which regard the promotion of IL-17–producing cells by IL-7 seemed paradoxical. However, IL-7 may also activate STAT3 (22) which mediates the effects of cytokines known to promote IL-17–producing γδ T cells (10): indeed, after IL-7 stimulation γδ27− cells showed >threefold higher phospho-STAT3 expression than γδ27+ cells (Fig. 3D). This phosphorylation was largely limited to CD44hi cells, in which most pSTAT3 was nuclear, as illustrated by colocalization with propidium iodide (Fig. 3E and Fig. S2C). In Il17aCreR26ReYFP “fate mapping” mice, cells transcribing the Il17a locus induce cre that excises a stop codon, thereby irreversibly activating an enhanced yellow fluorescent protein (eYFP) gene in the rosa26 locus (26). LN eYFP+ γδ cells are CD44hi and RORγt+ (Fig. S2D), and after 30-min stimulation with IL-7, pSTAT3 was selectively expressed by eYFP+ γδ cells (Fig. S2E).
When LN cells were incubated for 3 d with IL-7 in the presence or absence of an inhibitor that blocks STAT3 phosphorylation but leaves STAT5 phosphorylation intact (Fig. S2F), γδ27+ cells were little affected, whereas the preferential enrichment of γδ27− cells was reduced by >50%, with a corresponding reduction in Ki67+ cells, and very severe attenuation of cells with IL-17–producing potential (Fig. 3 F and G and Fig. S2G). This effect was not attributable to any toxicity of the inhibitor; for example, γδ27− annexin-V profiles were equivalent with or without it (Fig. S2H). Correlating with the selective IL-7–mediated activation of STAT3 in γδ27− cells were very low levels of the STAT3 suppressor, SOCS3, relative to CD44lo γδ27+ cells (Fig. S2I). Interestingly, the minor CD44hiγδ27+ subset also expressed low levels of SOCS3, perhaps accounting for the maintenance of these cells in IL-7 compared with the loss of bulk γδ27+ cells (Fig. 1D).
IL-7 Enriches for γδ27− Thymocytes.
IL-17–producing γδ27− cells reportedly arise during fetal thymic development (4, 18), although they can easily be found in the thymus of adult mice, where they express conspicuously high levels of IL-7R (Fig. 4 A and B). High levels of Il17 mRNA were consistently detected only among IL-7Rhiγδ27− cells (Fig. 4C). IL-7 is absolutely required for γδ cell development, and culture with IL-7 fueled the survival and expansion of all adult γδ thymocytes, as shown by a time course (Fig. S3A). Nonetheless, there was again a strong enrichment for CD44hiIL-17–competent, TCRhi CD27− γδ cells, with such cells transitioning from the minority to the majority by comparison to IFN-γ−competent cells (Fig. 4D and Fig. S3A).
Fig. 4.
IL-7 enriches for IL-17–competent γδ thymocytes. (A and B) Histograms for IL-7R staining of: adult γδ27− (black line) and γδ27+ (gray line) thymocytes ex vivo (A); γδ27− cells expressing IL-17 (black line) or not expressing IL-17+ (gray line) after PMA + ionomycin activation (B). Gray shaded area is isotype control staining. (C) Il17 mRNA levels in sorted γδ27+, and IL-7Rlo and IL-7Rhi γδ27− thymocytes determined by real-time RT-PCR. (D) Total thymocytes from adult mice ex vivo or activated for 4 d in vitro with IL-7 (Left); CD44 and CD27 expression among gated γδ T cells (Center Left); Intracellular staining for IFN-γ and IL-17 in all γδ T cells (Center Right) and γδ27− cells (Right) after PMA + ionomycin activation. For all plots, numbers indicate percent of cells in relevant gate or quadrant. (E) Adult thymocytes preincubated with a specific STAT3 inhibitor (Lower) or vehicle control (Upper) and subsequently cultured with IL-7 for 72 h. Representative plots (from n = 3 experiments) of gated γδ T cells. (F) Percentage and absolute numbers of eYFP+ γδ27− and eYFP− γδ27− thymocytes from adult Il17aCreR26ReYFP mice ex vivo (black bars) or after culture with IL-7 for 4 d (open bars). (G) γδ cells stained for IL-17 and IFN-γ after 7-d FTOC from embryonic day 16.5 fetal thymus in the presence (Right) or absence (Left) of IL-7 (from n = 3 experiments with ≥3 thymic lobes per condition).
As for LN cells, CFSE labeling and Ki67 staining of thymocytes ex vivo showed that IL-7 primarily promoted proliferation of CD44hiCD27− thymocytes with IL-17 potential (Fig. S3 B and C): Indeed, after 4 d, >90% of CD44hiCD27− thymocytes were cycling with >98% of IL-17–competent cells found among these. Again there was little evidence for IL-7–mediated selective survival of γδ27− cells with Bcl-2 mRNA levels lower in γδ27− cells than in γδ27+ cells (Fig. S3D). The preferential expansion of CD44+γδ27− thymocytes was reduced ≥80% by the STAT3 inhibitor (Fig. 4E and Fig. S3E). Conspicuously, thymic γδ27− cells were not selectively enriched by other STAT5- (IL-2, IL-15, and IL-21) and STAT3- (IL-6) activating cytokines, either alone or added to suboptimal concentrations of IL-7. IL-2 activated all γδ thymocytes, but still enriched (almost twofold) for γδ27+ cells, whereas IL-15 primarily activated γδ27+ cells (Fig. S4). Thus, as for LN cells, IL-7 promotes preferential STAT3-dependent expansion of IL-17–competent γδ27− thymocytes.
Further evidence that IL-7 preferentially expands IL-17–competent thymocytes within the γδ27− subset was derived from the Il17aCreR26ReYFP mice. Ex vivo ∼1% of γδ thymocytes are eYFP+CD27−, whereas ∼8% are eYFP−CD27− (Fig. 4F), roughly consistent with ∼11% of γδ27− thymocytes producing IL-17 upon short-term activation (Fig. 4D, Upper). Conversely, 4 d in IL-7 increased eYFP+CD27− cell numbers >30-fold, making them the larger subset compared with eYFP−CD27− cells that had increased much less (Fig. 4F).
To demonstrate that developing γδ27− cells are a preferential target of IL-7, fetal thymocytes were examined because the fetal thymus will not be a target for peripheral T-cell recirculation. Supplemental IL-7 added to 7-d fetal thymic organ culture (FTOC) expanded absolute numbers of total γδ thymocytes by ∼fivefold, but again the impact was preferential for IL-17–competent γδ thymocytes whose representation was increased twofold over IFN-γ–producing γδ thymocytes (Fig. 4G). To verify that IL-7 preferentially activated γδ27− thymocytes rather than promoting the conversion of γδ27+ thymocytes to γδ27− cells, IL-7 was applied to purified CD44hiγδ27−, CD44hiγδ27+, or CD44loγδ27+ thymocytes. After a 4-d culture, CD44hiγδ27− cells appeared by microscopy to be highly activated, by contrast to the γδ27+ subsets. To normalize the number of cells in the cultures, purified subsets were admixed with thymocytes from age-matched TCRδ−/− mice. Strikingly, neither γδ27+ subset generated IL-17–competent cells over 4 d, whereas >70% of cells arising from only 5,000 CD44hi γδ27− were IL-17 competent (Fig. S5). Thus, IL-7 primarily expands cells with IL-17 competence rather than differentiating cells toward IL-17 de novo.
IL-7 Promotes Adaptive Potential to Produce IL-17.
IL-17–producing γδ cells are widely viewed as innate because they are rapidly activated by IL-1 and IL-23 alone and are relatively unresponsive to TCR agonists that strongly activate IFN-γ–producing γδ27+ cells (Fig. S6A) (9). However, in the presence of IL-7, TCR agonists promoted a >20-fold enrichment of γδ27− cells relative to LN cells, whereas γδ27+ cells were enriched by only three- to fourfold: By 4 d, ∼100% of γδ27− cells were CD69+CD44hiCD25+ICOS+ (Fig. 5 A and B and Fig. S6B). Compared with IL-7 alone, suboptimal concentrations of TCR agonists added to IL-7 increased γδ27− IL-17–competent cell numbers by an additional 40–50% (Fig. 5 C and D), whereas there was negligible synergy for γδ27+ cells, which instead responded very strongly to the combination of IL-15 + TCR agonists (Fig. S6 C and D).
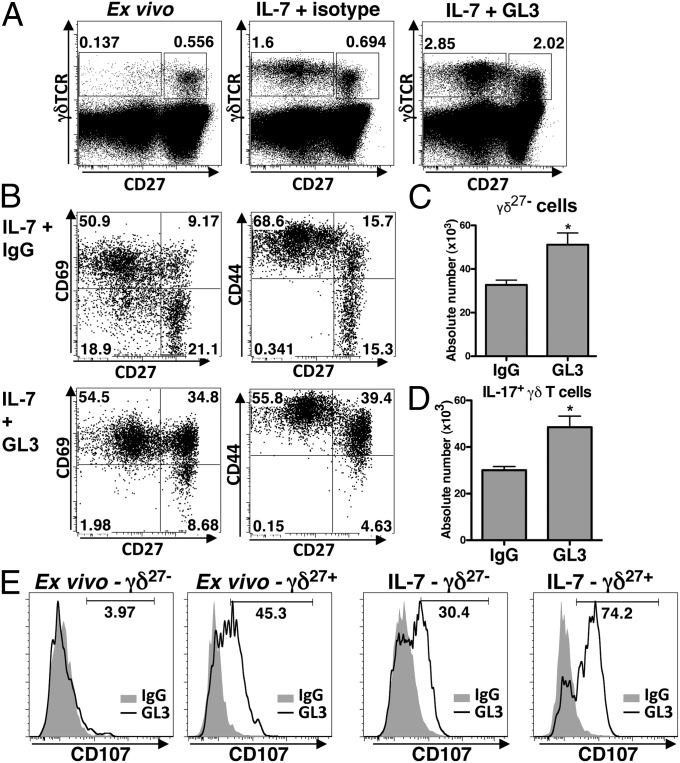
Fig. 5.
TCR agonists and IL-7 cooperatively promote IL-17–producing γδ cells. (A) Total LN cells stained for markers as indicated ex vivo (Left) and after 4-d culture with IL-7 + 1 μg/mL TCR-agonist antibody [GL3] (Right) or isotype control (Center). (B) LN γδ T cells stained for markers as indicated after 4-d culture with IL-7 and either TCR-agonist antibody (Lower) or isotype control (Upper). For all plots, numbers indicate percent of cells in relevant quadrants. (C) Absolute number of total γδ27− (Upper) or IL-17–expressing γδ27− (Lower) LN cells after culture in IL-7 with 1 μg/mL GL3 or IgG control, as in A. Error bars are SEM from n = 3 experiments; *P < 0.05. (E) Staining for CD107 in gated γδ27− (Left) and γδ27+ (Right) cells, activated for 6 h with 10 μg/mL TCR agonists (line) or isotype control (shaded area), from ex vivo LN (Left) and after 4-d culture with IL-7 (Right).
As a preface to killing target cells in response to TCR-mediated activation, T cells exocytose the contents of cytolytic granules in a process that involves movement to the cell surface of the protein CD107a (27). Hence, CD107a expression levels provided an additional assay for the impact of IL-7 on the response of γδ T cells to TCR stimulation. Strikingly, TCR agonists provoked surface up-regulation of CD107a by γδ27− cells only after the cells’ culture in IL-7, whereas γδ27+ cells up-regulated CD107a expression directly ex vivo, with no requirement for IL-7 (Fig. 5E). In sum, IL-7 selectively facilitates strong responses of IL-17–producing γδ cells to TCR stimulation whether measured by expansion, activation markers, or effector function.
IL-7 Reveals IL-17–Competent Human γδ Cells.
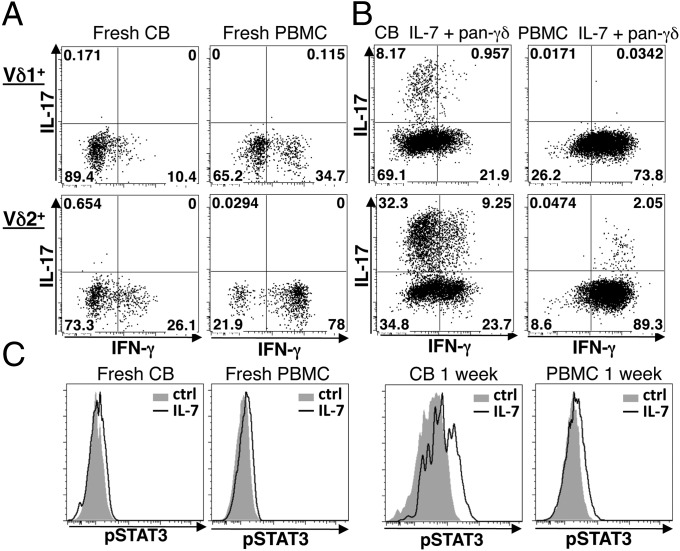
By contrast to mice, a substantive subset of IL-17–producing human γδ T cells has been hard to identify in healthy donors (16, 28). As reported (29), there was precocious production of IFN-γ by fresh human cord blood (CB) γδ cells and by adult TCRγδ+ peripheral blood mononuclear cells (PBMC) stimulated by PMA + ionomycin, but there was no obvious IL-17–producing subset (Fig. 6A). When PBMC were cultured for 1 wk with anti-TCRγδ + IL-7 and then activated for 6 h, IFN-γ monoproducers described ∼80% of cells; a small percentage coexpressed IL-17 and IFN-γ, but there was still no IL-17 monoproducer (Fig. 6B). However, when CB cells were likewise cultured, substantial fractions of Vδ2+ and Vδ1+ cells produced IL-17 with most being IL-17-monoproducers (Fig. 6B and Fig. S7A). Unsurprisingly, the percentages of γδ cells that were IL-17-competent varied with the source of CB from ∼15% to >40%, with higher representation always being among Vδ2+ cells: Indeed, IL-17–competent Vδ2+ cells sometimes outnumbered IFN-γ–competent cells (Fig. 6B and Fig. S7B). Comparing the main γδ cell subsets: 1-wk culture with IL7 + TCR agonists sustained absolute numbers of Vδ1+ cells but IL-17 monoproducers expanded by ∼50-fold, compared with no significant increase in IFN-γ–competent cells, whereas Vδ2+ cell numbers increased ∼10-fold within which IL-17 monoproducers expanded by >20-fold, compared with only a 10-fold increase in IFN-γ–competent cells. Hence, as in the mouse, the effects of IL-7 + TCR agonists are strongly biased toward IL-17 competence. Consistent with γδ cell activation, ∼100% lost the naïve T-cell marker CD45RA, up-regulated ICOS, and expressed CD161, a marker of human IL-17–producing T cells (30) (Fig. S7 B and C). When fresh or 1-wk cultures of CB or PBMC were freshly stimulated for 30 min with IL-7, all showed increased STAT5 phosphorylation, which did not therefore correlate with IL-17 competence. Conversely, STAT3 activation was measurable only in CB-derived Vδ2+ cells when cultured for 1 wk and then stimulated with IL-7 (Fig. 6C and Fig. S8A). Thus, as in mouse, IL-7–dependent STAT3 activation is highly selective.
Fig. 6.
Evocation of IL-17–producing γδ T cells from human cord blood. Representative plots from n ≥ 4 donors. (A and B) Intracellular staining for IFN-γ and IL-17 in Vδ1+ (Upper) and Vδ2+ (Lower) cells from CB and adult PBMC freshly isolated (A), or cultured for 1 wk (B) with IL-7 and pan γδTCR antibody: In each case, cells were prestimulated with PMA + ionomycin for 6 h. (C) pSTAT3 and pSTAT5 levels assessed by flow cytometry in gated Vδ2+ cells from fresh CB or adult PBMC (Left), or after 1 wk of culture with anti-CD3 + IL-2 (Right), in each case followed by 30-min IL-7 stimulation (open areas) or control (ctrl, shaded areas).
CB Vδ2+ cells cultured for 1 wk with IL-7 + TCR agonist and stimulated for 2 h with PMA + ionomycin showed conspicuous reductions in RNA for T-bet, IL-2, and IFN-γ, with corresponding increases in IL-17A, IL-17F, and IL-22 RNAs (Fig. S8B). Indeed, a small number of IL-22–expressing cells was evoked, with the majority coproducing IL-17 (Fig. S8C). Again, these results were specifically due to IL-7, because IL-2 + TCR agonists skewed CB cells toward IFN-γ, with ∼10-fold fewer cells expressing IL-17 (Fig. S8D).
Discussion
This study identifies a pathway selectively activating the production of IL-17 by γδ T cells. It operates in vivo and is conserved in mice and humans. It broadens our perspectives on the biology of IL-7 and has clinical relevance, given that IL-7 is used to promote lymphocyte expansion in cancer patients and in bone marrow transplantation (31) and given that protocols are being sought to maintain and expand γδ cells for adoptive immunotherapy. Conversely, IL-7 blockade is being considered for inflammatory diseases.
Although a specific cytokine may not be essential for the development and survival of a particular lymphocyte subset, its capacity to regulate such cells is important. Such is the case for IL-7 and memory CD8+ αβ T cells, which are unaffected by IL-7 depletion but are nonetheless substantially expanded by IL-7, evoking an accumulation of CD44hiCD8+αβ T cells (32, 33). Likewise, IL-17–producing γδ cells may not depend on STAT3 (15), but they are rapidly responsive to IL-23 that signals via STAT3 (10). In this vein, this study identifies a capacity of IL-7 to activate STAT3 preferentially in γδ cells competent to produce IL-17, markedly expanding and activating such cells and promoting their functional responsiveness, for example, increased cytolytic potential. This activation is a selective role for IL-7 that for γδ cells has hitherto been regarded as a generic regulator of development and homeostasis for all γδ subsets (17, 34). This role of IL-7 offers parallels with its reported requirement to maintain TCRαβ+ Th17 cells, although a key difference is that the effects of IL-7 in that case were mediated by STAT5 (35), which seems paradoxical given that STAT5 can antagonize Th17 differentiation. By contrast, the role of IL-7 described here is in large part mediated by STAT3, emphasizing the importance of a relatively poorly understood phenomenon—namely, how different cytokines use different signaling pathways to effect specific roles.
Like the effects of IL-7 on αβ T cells (36, 37), IL-7–induced γδ27− expansion in vivo occurs in the periphery, but unlike for αβ T cells, this expansion does not require prior lymphodepletion, emphasizing the capacity of IL-7 to act selectively on γδ27− cells in physiologic situations. Indeed, blocking IL-7R almost completely abrogated the expansion of LN IL-17–producing γδ cells in response to IMQ, although it left the expansion of IFN-γ–producing cells largely untouched. Correspondingly blocking IL-7R inhibited the development of inflammatory lesions. Furthermore, increased IL-7 levels are detected in vivo after TLR activation (38) and in autoimmune diseases, such as rheumatoid arthritis (39), systemic juvenile rheumatoid arthritis (40), multiple sclerosis (41), and psoriasis (42), suggesting scenarios where potential dysregulation of IL-17-competent γδ cells should be investigated. Indeed, IL-17–producing γδ cells were recently described in psoriatic skin (11, 20, 43).
γδ cells are not confined to T-cell zones and may access reticular stromal cells that express IL-7 (44), potentially in the context of tissue-draining antigens. Thus, the capacity of IL-7 to promote γδ27− cell activation in response to TCR agonists provides an important perspective on cells commonly assigned to innate immunity. Additionally, we have shown that IL-7 selectively expands CD44hi γδ27− cells from the peritoneum, where CD44hi γδ T cells are strongly implicated in defense against bacterial infection (45).
Finally, IL-7 appears most abundant in neonatal mice, which is when lymphoid compartments are being filled according to homeostatic mechanisms and when murine IL-17–producing γδ cells, largely derived from fetal progenitors, are most abundant. Indeed, IL-7 may throughout life mobilize IL-17–producing γδ cells from a “self-renewing” pool set down in the fetus. It is also striking that the major evocation of human IL-17–producing γδ cells is from CB. Interestingly, two studies using cytokines established to promote Th17 cells used CB to evoke small numbers of IL-17–producing γδ cells (46, 47). γδ cells are functionally precocious relative to αβ T cells in mice and in humans, and one of their major biological contributions may be to protect neonates (29, 48, 49). Moreover, inflammatory immunopathologies may reflect inappropriate mobilization of cells laid down in the fetal/neonatal period. In pursuing this hypothesis, the major and selective potential of IL-7 needs now to be considered in immunoprotection and immunopathologies. This study also implies reciprocal selective roles of IL-2 and IL-15 in regulating IFN-γ–competent γδ cells, which may be germane to the resurgent clinical use of these reagents.
Materials and Methods
Cell preparation, flow cytometry, PCR, and cytokine measurements were performed as described in SI Materials and Methods.
Murine and Human Samples.
For methods related to animals and human samples, see SI Materials and Methods. In some experiments, mouse in vivo i.p. injections included PBS or recombinant mouse IL-7 [rmIL-7 (R&D Systems), 5 μg per mouse every 2 d for 1 wk]. In other experiments, a daily dose of 50 mg of Imiquimod (5% IMQ cream; Meda AB) or control cream (Vaseline) was applied to shaved backs of mice for 3 d. Anti–IL-7R (clone A7R34) or rat IgG control treatment was performed by i.p. injection (1 mg per mouse) on days −1 and +2 relative to IMQ application. A7R34 was obtained from Biolegend or, for some experiments, we made A7R34 from hybridoma (50).
Cell Culture.
Cells were incubated for 1, 2, 3, or 4 d with IL-2 (100 U/mL; Immunotools), IL-7, IL-6, IL-15, and IL-21 (all 20 ng/mL; R&D Systems). Where indicated, anti-TCRγδ (GL3: 1 or 10 μg/mL), IgG1κ isotype control, and anti-CD107a/b antibodies (1D4B, M3/84; Biolegend) were also added. After culture, dead cells were removed by Ficoll-Hypaque centrifugation (GE Healthcare). In some experiments, cells were preincubated with STAT3 inhibitor VII (Calbiochem) for 1 h before addition of IL-7. For human studies, cells were cultured for 1 wk with IL-7 (20 ng/mL; R&D Systems) or IL-2 (100 U/mL; Immunotools) in wells coated with pan anti-γδTCR (1 μg/mL, IMMU510; Beckman).
ImageStream Acquisition and Analysis.
Samples (4 × 107 cells per mL in 60 μL of wash buffer with 1 μg/mL PI) were acquired on a 5-laser 6-Channel ISx Imaging Flow Cytometer with 40× magnification controlled by INSPIRE software and fully ASSIST calibrated (Amnis).
Supplementary Material
Acknowledgments
We thank B. Silva-Santos and J. Ribot (Lisbon), G. Turchinovich and M. Wencker [London Research Institute (LRI) and Cancer Research UK (CRUK)], and Susan John (King’s College London; KCL) for advice, reagents, and assistance; Warren Leonard (National Institutes of Health) for access to data prepublication; D. Bonnet and colleagues (LRI, CRUK) for cord blood; and the Flow Cytometry Laboratories of CRUK, KCL, and the Comprehensive Biomedical Research Centre (cBRC), especially S. Purewal (CRUK), for work with Image Stream. We acknowledge grants from the Wellcome Trust (to A.C.H. and to D.J.P.); the Medical Research Council (MRC) for Programme and MRC Transplantation Centre grants to A.C.H. (co-principal investigator) and for core support (to A.J.P.); CRUK; and the Marie Curie Programme for support of M-L.M.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204327109/-/DCSupplemental.
References
- 1.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177(7):4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 6.Umemura M, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 7.Hamada S, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181(5):3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribot JC, et al. Cutting edge: Adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185(11):6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 13.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: Antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata K, et al. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood. 2011;118(3):586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 16.Caccamo N, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118(1):129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 17.Baccala R, et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174(8):4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 18.Shibata K, et al. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181(9):5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 19.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7(2):140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantelyushin S, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98(5):1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- 22.Lin JX, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2(4):331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 23.Pallard C, et al. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10(5):525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 24.Barata JT, et al. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200(5):659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1-2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 28.DeBarros A, Chaves-Ferreira M, d’Orey F, Ribot JC, Silva-Santos B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur J Immunol. 2011;41(1):195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons DL, et al. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39(7):1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 30.Maggi L, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40(8):2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 31.Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: Preclinical and clinical studies. J Intern Med. 2009;266(2):141–153. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieper WC, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195(12):1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.French JD, Roark CL, Born WK, O’brien RL. gammadelta T cell homeostasis is established in competition with alphabeta T cells and NK cells. Proc Natl Acad Sci USA. 2005;102(41):14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16(2):191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 36.Komschlies KL, et al. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J Immunol. 1994;152(12):5776–5784. [PubMed] [Google Scholar]
- 37.Geiselhart LA, et al. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166(5):3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 38.Sawa Y, et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30(3):447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 39.van Roon JA, et al. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52(6):1700–1710. doi: 10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
- 40.De Benedetti F, et al. Elevated circulating interleukin-7 levels in patients with systemic juvenile rheumatoid arthritis. J Rheumatol. 1995;22(8):1581–1585. [PubMed] [Google Scholar]
- 41.Haas J, Korporal M, Schwarz A, Balint B, Wildemann B. The interleukin-7 receptor α chain contributes to altered homeostasis of regulatory T cells in multiple sclerosis. Eur J Immunol. 2011;41(3):845–853. doi: 10.1002/eji.201041139. [DOI] [PubMed] [Google Scholar]
- 42.Bonifati C, et al. Increased interleukin-7 concentrations in lesional skin and in the sera of patients with plaque-type psoriasis. Clin Immunol Immunopathol. 1997;83(1):41–44. doi: 10.1006/clin.1996.4313. [DOI] [PubMed] [Google Scholar]
- 43.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 45.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 46.Moens E, et al. IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. J Leukoc Biol. 2011;89(5):743–752. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 47.Ness-Schwickerath KJ, Morita CT. Regulation and function of IL-17A- and IL-22-producing γδ T cells. Cell Mol Life Sci. 2011;68(14):2371–2390. doi: 10.1007/s00018-011-0700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayday AC. [gamma][delta] cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 49.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198(9):1403–1414. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudo T, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.