Abstract
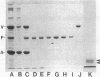
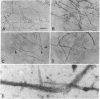
Fimbrin is a cytoskeletal protein associated with microfilaments in microvilli, microspikes, stereocilia, membrane ruffles, and cell--substratum attachment sites. Fimbrin purified from intestinal epithelial cell brush borders was found to be a monomeric protein of molecular weight 68,000. In a sedimentation assay, fimbrin bound to F-actin in a salt-dependent manner, with binding being optimal in 30 mM KCl and inhibited in greater than 100 mM KCl. In 50 mM KCl, which allows efficient polymerization of actin, the interaction was stabilized by the presence of polyethylene glycol. Under these conditions, binding was unaffected by the inclusion of up to 5 mM Ca2+ but was inhibited by greater than 0.5 mM Mg2+. Electron microscopy revealed that fimbrin crosslinked F-actin into relatively straight bundles with shorter bundles being formed at high fimbrin-to-actin ratios. The results suggest that fimbrin crosslinks F-actin in such a way as to confer some rigidity on the bundle formed. This proposed function for fimbrin is consistent with its in vivo localization in straight, highly organized, microfilament bundles such as microvilli, microspikes, and stereocilia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher A., Weber K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol. 1980 Jul;86(1):335–340. doi: 10.1083/jcb.86.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Purification of microvilli and an analysis of the protein components of the microfilament core bundle. Exp Cell Res. 1978 Oct 15;116(2):397–407. doi: 10.1016/0014-4827(78)90463-9. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Flock A., Flock B., Murray E. Studies on the sensory hairs of receptor cells in the inner ear. Acta Otolaryngol. 1977 Jan-Feb;83(1-2):85–91. doi: 10.3109/00016487709128817. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. L., Mooseker M. S., Graves T. A. Brush-border calmodulin. A major component of the isolated microvillus core. J Cell Biol. 1980 Jun;85(3):916–923. doi: 10.1083/jcb.85.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Graves T. A., Wharton K. A., Falco N., Howe C. L. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol. 1980 Dec;87(3 Pt 1):809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Amos L. A. Structure of actin filament bundles from microvilli of sea urchin eggs. J Mol Biol. 1979 Apr 5;129(2):319–331. doi: 10.1016/0022-2836(79)90285-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106–1110. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]