Abstract
Patients with Down syndrome (trisomy 21, T21) have hematologic abnormalities throughout life. Newborns frequently exhibit abnormal blood counts and a clonal preleukemia. Human T21 fetal livers contain expanded erythro-megakaryocytic precursors with enhanced proliferative capacity. The impact of T21 on the earliest stages of embryonic hematopoiesis is unknown and nearly impossible to examine in human subjects. We modeled T21 yolk sac hematopoiesis using human induced pluripotent stem cells (iPSCs). Blood progenitor populations generated from T21 iPSCs were present at normal frequency and proliferated normally. However, their developmental potential was altered with enhanced erythropoiesis and reduced myelopoiesis, but normal megakaryocyte production. These abnormalities overlap with those of T21 fetal livers, but also reflect important differences. Our studies show that T21 confers distinct developmental stage- and species-specific hematopoietic defects. More generally, we illustrate how iPSCs can provide insight into early stages of normal and pathological human development.
Down syndrome (DS, trisomy 21, T21) affects many tissues, including blood (1). Many DS neonates exhibit erythrocytosis, thrombocytopenia, and leukocytosis (2, 3). Approximately 10% of DS newborns exhibit a clonal preleukemia, termed transient myeloproliferative disease (TMD), which progresses to acute megakaryoblastic leukemia (AMKL) in ∼30% of cases. Both TMD and AMKL are accompanied by somatic mutations in the GATA1 gene, causing production of an amino-truncated form of the transcription factor GATA-1 (reviewed in refs. 4 and 5). Importantly, somatic GATA1 mutations do not predispose to leukemia without T21 (6), and the most common DS blood abnormalities occur without GATA1 mutations. Thus, T21 influences blood formation independently, particularly during embryogenesis (7, 8). These derangements likely predispose to TMD/AMKL and could also contribute to the early fetal demise that occurs throughout gestation in approximately one-third of T21 pregnancies (1). For these reasons, it is of biological and medical importance to fully define the effects of T21 on blood formation at all stages of human ontogeny.
During mammalian development, hematopoiesis occurs in multiple waves that differ with respect to timing, origin, and the types of blood cells produced (reviewed in refs. 9 and 10). At about week 3 of human gestation, “primitive” blood cells produced by the embryonic yolk sac are released into circulation. By weeks 4–5, “definitive” progenitors emerge from the yolk sac and begin to seed the fetal liver. After wk 5, hematopoietic stem cells emerge in the aorta-gonad-mesonephros (AGM) region and colonize the fetal liver, which becomes the major site of blood production until birth, when hematopoiesis shifts to bone marrow (11–13).
T21 alters hematopoiesis during embryonic development. Human T21 fetal livers with normal GATA1 alleles contain expanded megakaryocyte-erythroid progenitors, the progeny of which exhibit enhanced proliferation (7, 8). How T21 impacts yolk sac hematopoiesis is unknown and difficult to examine in human tissues at such early stages of embryogenesis. Moreover, murine models for DS only partially recapitulate the hematopoietic abnormalities observed in humans (14–16). As an alternative approach, we examined the effects of T21 on embryonic hematopoiesis by studying human induced pluripotent stem cells (iPSCs) with germ-line T21.
iPSCs generated by reprogramming somatic cells resemble ES cells in their ability to self-renew in culture and generate numerous mature cell types (17). In vitro differentiation of ES/iPSCs recapitulates the ontogeny of hematopoiesis (18, 19). Human ES/iPSCs can be differentiated into erythroid cells expressing mainly ε- or γ/β-globins, likely reflecting primitive and definitive hematopoietic lineages, respectively (20–24). However, the culture conditions and mechanisms that specify these distinct developmental outcomes are not well defined or understood. We analyzed iPSC lines from seven individuals (four T21, three euploid) using an in vitro differentiation protocol optimized for primitive hematopoiesis (20). Our results further define the scope of T21 abnormalities during early human embryogenesis and illustrate that the associated hematopoietic defects are species- and developmental stage-specific. More generally, our findings illustrate the power of iPSCs for studying the consequences of genetic disorders on human ontogeny, particularly the earliest stages that are least accessible via primary tissue samples.
Results
Generation of iPSCs.
iPSCs were derived from four T21 and three euploid control subjects (Table S1) by reprogramming somatic tissues using four retroviruses, encoding OCT4, SOX2, KLF4, or MYC individually (17), or by a single polycistronic lentivirus encoding all four genes regulated by a doxycycline-inducible promoter (25). Consistent experimental results were obtained using iPSC clones derived from different cell types and reprogramming vectors. All clones used in this study exhibited typical morphology, cell-surface expression of pluripotency markers (Tra1-60, Tra1-81, SSEA3, SSEA4, KIT, KDR), expression of endogenous pluripotency genes (ABCG2, DNMT3B, NANOG, OCT4, REX1), silencing of viral encoded reprogramming genes, and production of three embryonic germ layers in teratomas (Fig. S1). Karyotype analysis of iPSCs (passages 11–21) showed no acquired abnormalities and confirmed T21 in the appropriate lines. Cells were passaged at least 20 times to avoid “memory effects” that could exert lineage bias to iPSC lines derived from different tissues (26, 27).
Trisomy 21 iPSCs Produce Primitive Hematopoietic Progenitors at Normal Frequency.
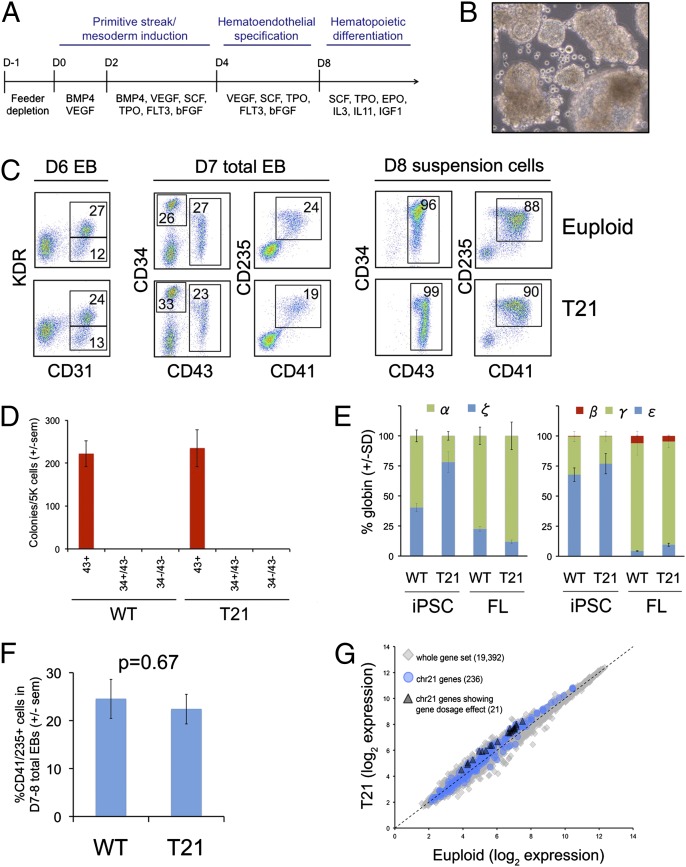
We compared the blood-forming capacities of T21 and euploid iPSC lines by generating embryoid bodies (EBs) in defined media containing sequential combinations of cytokines that support primitive streak/mesoderm formation, transition to hematoendothelial progenitors, and terminal hematopoietic differentiation (Fig. 1A), using modifications of a published protocol by Kennedy et al. (20). The kinetics for emergence of hematopoiesis varied slightly among different iPSC lines. We controlled for these differences by examining progenitors that were stage-matched by surface-marker expression.
Fig. 1.
T21 iPSCs produce normal levels of early hematopoietic progenitors. (A) Schematic of hematopoietic differentiation protocol via EB formation with the following cytokines: BMP4, VEGF, SCF, TPO, FLT3, bFGF, EPO, IL-3, IL-11, and IGF1. (B) Photograph of iPSC-derived EB culture with hematopoietic cells released into the medium. Original magnification, 10×. (C) Flow cytometry analysis showing CD31+KDR+ hematoendothelial precursor cells (Left) and CD34+/−43+235+41+ progenitors within EBs (Center), and released into the medium (Right). (D) Methylcellulose colony assays of various purified populations. Cytokines include SCF, IL-3, EPO, and GMCSF. Results show mean values ± SEM, n = 3 per group. (E) Globin gene expression in erythroid colonies from iPSCs or fetal liver (FL) determined by quantitative real-time PCR. Charts show fraction of α- (Left) or β-like (Right) genes. Ten to 20 colonies were pooled per sample, n = 3 per group. (F) Frequency of CD43+41+235+ progenitor cells in EB cultures on day 7–8 of hematopoietic differentiation (n = 14 and 11 independent experiments for euploid and T21 iPSCs, respectively. (G) Scatter plots of microarray data showing average mRNA expression values in purified CD43+41+235+ progenitors from between-group comparison of three euploid and three T21 biological replicate samples.
Beginning at days 4–5 of differentiation, a prehematopoietic cell population expressing KDR (VEGF-R) and CD31 (platelet endothelial cell adhesion molecule-1) was detected in EBs (Fig. 1C, Left). By days 7–9, the initial wave of hematopoietic progenitors, which coexpress CD43 (leukosialin), CD41 (integrin αIIb), and CD235 (glycophorin A) (28, 29), were present in EBs comprising ∼25% of the total culture (Fig. 1C, Center). At this same time point, EBs released a relatively homogenous population of CD43+41+235+ blood progenitors into the medium (Fig. 1 B and C, Right). These progenitors differentiated into committed erythroid (CD235+41–), myeloid (CD45+18+), and megakaryocytic (CD41+42+235–) lineages by day 12 of differentiation (Fig. S2). Colony-forming assays of flow cytometry-sorted cells from whole EBs performed on days 7–8 of differentiation showed that all hematopoietic progenitors reside in the CD43+ population (Fig. 1D). Erythroid colonies produced from iPSC progenitors express mainly ζ- and ε-globin genes, indicative of primitive hematopoiesis. In contrast, fetal liver-derived colonies express mostly α- and γ-globin genes, reflecting definitive hematopoiesis (Fig. 1E). The CD43+41+235+ progenitors were present at similar proportions within day 7–8 EB cultures generated from T21 and euploid iPSCs (Fig. 1 C and F). Transcriptome analysis of flow cytometry-sorted CD43+41+235+ progenitors showed remarkable similarity between T21 and euploid cells. Only 21 of 236 HSA21 genes were up-regulated ≥1.5-fold in T21 progenitors (Fig. 1G), consistent with complex lineage-specific patterns of gene-dosage imbalance in human and murine T21 tissues (30). Taken together, our results suggest that T21 and euploid iPSCs produce similar frequencies of progenitors in the first wave of hematopoiesis to emerge within EB cultures, and that T21 progenitors exhibit only subtle alterations in gene expression.
Altered Myelo-Erythroid Differentiation in T21 iPSCs.
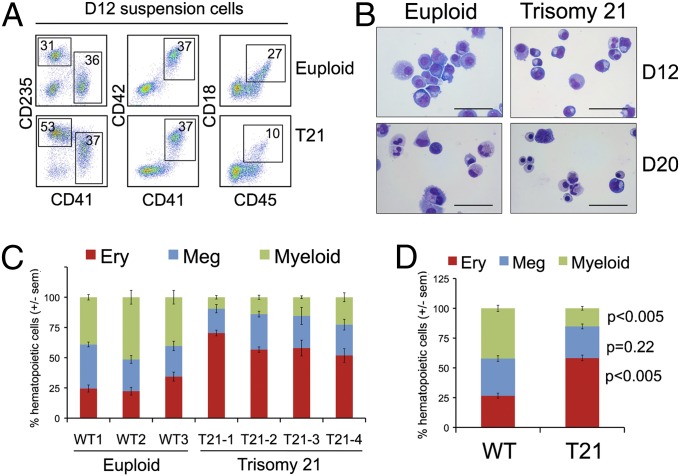
Between days 7 and 14 of EB differentiation, most hematopoietic progenitors were released into the medium and underwent erythroid, myeloid, and megakaryocytic differentiation, as evidenced by flow cytometry and cell morphology (Fig. 2 A and B, and Fig. S2). By day 12, EB cultures from T21 iPSC lines produced about twofold increased proportion of erythroid (CD235+41–) cells with a concomitant decrease in the fraction of myeloid (CD45+18+) cells, compared with controls (Fig. 2 A, C, and D). Morphologies of cells released from EBs at days 12 and 20 are shown in Fig. 2B. By day 20, the T21 samples contained a greater proportion of basophilic erythroblasts and normoblasts but neutrophils were more apparent in the euploid cultures. Of note, T21 fetal liver hematopoietic cultures also exhibit enhanced erythroid differentiation (7, 8). The percentage of megakaryocytic (CD41+42+235−) cells was similar in T21 and euploid cultures (Fig. 2 A, C, and D), which contrasts with findings in T21 fetal liver where megakaryocytic progenitors are expanded, suggesting developmental stage-specific hematopoietic patterns (7, 8).
Fig. 2.
Propensity for erythroid differentiation by T21 iPSCs. (A) Flow cytometry analysis of suspension cells in day 12 differentiation cultures showing mature hematopoietic lineages: erythroid (Ery, CD41−235+), megakaryocytic (Meg, CD41+42+), and myeloid (CD45+18+). (B) May–Grunwald Giemsa-stained cells from EB suspension cultures at days 12 and 20. (Scale bars, 50 μm.) (C) Distribution of lineage-committed cells in EB suspension cultures at days 12–14 of differentiation. n = 3–5 independent experiments per iPSC line. (D) Summary of data with all iPSC lines combined according to genotype (n = 15 per group).
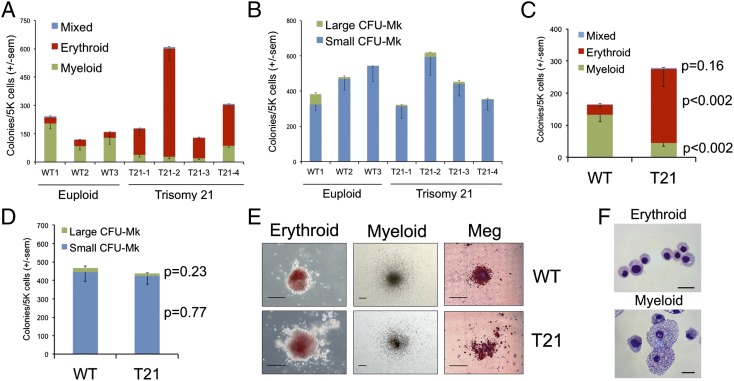
We assessed the colony-forming potential of day 7–9 EB-derived CD43+41+235+ progenitors in methylcellulose assays and in collagen-based megakaryocyte assays. CD43+41+235+ progenitors were purified by flow cytometry from the entire culture (disaggregated EBs plus the cells released into the medium) or obtained from the cells released by the EBs (90% CD43+41+235+ on days 7–9) (Fig. 1C, Right). The clonogenic potential varied among progenitors derived from different iPSC lines (31), but no consistent differences were noted in the overall efficiency of colony formation between T21 and control cultures (Fig. 3 A and B). However, T21 iPSC-derived progenitors produced a significantly increased proportion of erythroid colonies compared with controls (Fig. 3 A and C). In contrast, the majority of colonies formed from euploid iPSC progenitors were myeloid (Fig. 3 A and C), in agreement with the suspension culture differentiation assays. We observed no significant differences in the absolute numbers of megakaryocyte colonies generated by T21 compared with euploid iPSCs (Fig. 3 B and C). Additionally, there was no consistent difference in the size of erythroid or megakaryocyte colonies produced by T21 progenitors compared with controls (Fig. 3E). The cell morphology of iPSC-derived erythroid and myeloid colonies is shown in Fig. 3F. Similar results were obtained by analyzing progenitor populations from whole EBs that were disaggregated into single cells, although the clonogenicity was lower by ∼fivefold (Fig. S3). Importantly, the total number of colonies generated from T21 and euploid total EB cultures was equivalent, suggesting that the primitive hematopoietic progenitor compartment is not expanded with T21 (Fig. S3). Semiquantitative RT-PCR (Fig. 1E) of globin chains from iPSC-generated erythrocytes confirmed primarily embryonic globin gene expression. Overall, our findings demonstrate that T21 iPSC-derived hematopoietic progenitors exhibit increased propensity for erythroid differentiation, similar to DS fetal liver hematopoiesis (7, 8). However, in contrast to T21 fetal liver, T21 iPSCs did not generate increased megakaryocyte progenitors or larger erythroid or megakaryocytic colonies compared with controls. Thus, T21 fetal liver and the iPSCs examined under the in vitro differentiation conditions specified here exhibit common abnormalities in blood formation and also notable differences. Most likely, the differences reflect distinct effects of T21 on primitive vs. definitive hematopoiesis (see Discussion).
Fig. 3.
Increased erythroid progenitors in T21 iPSC differentiation cultures. CD43+41+235+ hematopoietic progenitors derived from three euploid and four T21 iPSC lines were analyzed. (A) Methylcellulose colony assays of CD43+41+235+ progenitors containing SCF, IL-3, EPO, GM-CSF, and (B) Colony-forming megakaryocyte (CFU-Mk) assays that include TPO, IL-3, and IL-6. Results show mean values ± SEM for three independent experiments per iPSC line. (C) Summary of data with all iPSC lines combined according to genotype for methylcellulose colony assays and (D) CFU-Mk assays. Results show mean values ± SEM, n = 9 for WT, n = 12 for T21. (E) Representative hematopoietic colonies from iPSC-derived progenitors. (Scale bars, 200 μm.) Meg, megakaryocyte. (F) Morphology of cells from iPSC-derived erythroid and myeloid colonies. May–Grunwald Giemsa stain. (Scale bars, 20 μm.)
mRNA Expression in DS and Control iPSC-Derived Hematopoietic Progenitors.
As noted, transcriptome profiling showed remarkably few differences in gene expression between iPSC-derived T21 and euploid CD41+235+ progenitors (Fig. 1G). Applying a parametric two-sample Student t test within the entire set of 19,392 genes interrogated failed to identify differentially expressed genes beyond the expected number of false positives. Focusing on HSA21, which produces a relatively small proportion of transcripts within the total dataset (1.2%), we found 71 differentially expressed genes that passed a false-discovery rate (FDR) threshold of <0.2 (Table S2) (32), 13 of which lie within the “Down Syndrome Critical Region,” defined by studies of children with partial trisomy 21 (33, 34) (Table 1, top section). Of these genes, DYRK1a, a regulator of calcineurin-nuclear factor of activated T cells signaling that has been implicated in DS pathology (35, 36), was up-regulated 1.3-fold with high statistical significance. Among HSA21 genes implicated in pathological DS hematopoiesis (reviewed in refs. 4 and 5), BACH1, GABPA, and SON, in addition to DYRK1a, were significantly up-regulated in T21 progenitors (range 1.25–1.50, P ≤ 0.05) but RUNX1, ERG, ETS2, and DCSR1 were not (Table 1, middle section). Similar differences in expression were observed with semiquantitative RT-PCR validation, with the exception that DSCR1 was up-regulated 2.4-fold in T21 iPSC-derived progenitors (P = 0.01) (Fig. S4).
Table 1.
Transcriptome analysis of T21 vs. euploid iPSC-derived progenitors
| Gene symbol | Chromosome | Fold-change | P value | FDR |
| Differentially expressed genes located in DS critical region | ||||
| SH3BGR | chr21 | 1.66 (+) | 0.0050 | 0.07 |
| BRWD1 | chr21 | 1.64 (+) | 0.0287 | 0.14 |
| DSCR3 | chr21 | 1.60 (+) | 0.0579 | 0.19 |
| MORC3 | chr21 | 1.55 (+) | 0.0024 | 0.06 |
| BACE2 | chr21 | 1.54 (+) | 0.0136 | 0.09 |
| HLCS | chr21 | 1.54 (+) | 0.0141 | 0.09 |
| WRB | chr21 | 1.52 (+) | 0.0041 | 0.07 |
| TTC3 | chr21 | 1.39 (+) | 0.0091 | 0.08 |
| PIGP | chr21 | 1.35 (+) | 0.0288 | 0.14 |
| DYRK1A | chr21 | 1.34 (+) | 0.0027 | 0.06 |
| PSMG1 | chr21 | 1.27 (+) | 0.0066 | 0.07 |
| HMGN1 | chr21 | 1.22 (+) | 0.0223 | 0.12 |
| SETD4 | chr21 | 1.22 (+) | 0.0045 | 0.07 |
| Chromosome 21-encoded hematopoietic regulators | ||||
| BACH1 | chr21 | 1.50 (+) | 0.0313 | 0.15 |
| RUNX1 | chr21 | 1.38 (+) | 0.1074 | 0.30 |
| ETS2 | chr21 | 1.36 (+) | 0.1605 | 0.37 |
| GABPA | chr21 | 1.29 (+) | 0.0023 | 0.06 |
| SON | chr21 | 1.25 (+) | 0.0141 | 0.09 |
| RCAN1 (DSCR1) | chr21 | 1.16 (+) | 0.1455 | 0.35 |
| ERG | chr21 | 1.68 (−) | 0.2175 | 0.42 |
| Genes involved in hematopoietic lineage determination and differentiation | ||||
| GATA2 | chr3 | 1.20 (+) | 0.1230 | 0.78 |
| MYB | chr6 | 1.18 (+) | 0.0003 | 0.01 |
| IKZF1 (Ikaros) | chr7 | 1.07 (+) | 0.6739 | 0.95 |
| SPI1 (PU.1) | chr11 | 1.02 (+) | 0.9308 | 0.95 |
| CEBPA | chr19 | 1.02 (−) | 0.8777 | 0.95 |
| KLF1 | chr19 | 1.04 (−) | 0.7328 | 0.95 |
| GFI1B | chr9 | 1.05 (−) | 0.5540 | 0.95 |
| ZFPM1 (FOG1) | chr16 | 1.06 (−) | 0.3857 | 0.95 |
| GATA1 | chrX | 1.08 (−) | 0.2710 | 0.95 |
| CSF1R (C-FMS) | chr5 | 1.19 (−) | 0.0150 | 0.29 |
| CD34 | chr1 | 1.75 (−) | 0.0443 | 0.56 |
Differential gene expression between T21 and euploid iPSC-derived progenitors is analyzed for two separate comparisons: genes located on chromosome 21 (FDR < 0.2; top two portions of table) and 38 selected genes outside of HSA21 that play key roles in hematopoietic lineage determination and differentiation (bottom portion of table). “Fold-change” is the level of up-regulation (+) or down-regulation (−) of mean gene expression in T21 with respect to euploid cells. P values were obtained from a parametric two-sample Student t test comparing differences in mean expression between T21 and euploid cells (n = 3 distinct iPSC lines per group). FDR values were obtained using the Benjamini–Hochberg-FDR method. Genes encoding regulators of hematopoiesis with significant differences in expression are in boldface.
We separately examined 38 genes outside of HSA21 that play key roles in hematopoietic lineage determination and differentiation (37, 38) (Table 1, bottom section, and Table S2). Thirty-five of these genes, including GATA1, GATA2, FOG1 (ZFPM1), KLF1, PU.1 (SFPI1), CEBPa, IKZF1 (Ikaros), and GFI1B were not differentially expressed between T21 and controls (P > 0.05). Among the cohort of 38 genes queried, the microarrays detected three with statistically significantly different expression: down-regulated CD34 (1.75×) and CSF1R (1.19×), and up-regulated MYB (1.18×). However, semiquantitative RT-PCR demonstrated no significant difference (Fig. S4). Thus, the T21 hematopoietic progenitors exhibit relatively small changes in the expression of numerous hematopoietic and HSA21 genes relative to euploid controls.
Discussion
iPSCs provide new opportunities to model human diseases and investigate early embryonic development. We used iPSCs to assess the effects of T21 at the onset of human hematopoiesis, which is difficult to study in equivalent 4- to 8-wk-gestation embryos. Based on stringent controls and consistent effects in multiple independent iPSC lines, our findings reflect the developmental influence of T21 and are not caused by artifacts of iPSC derivation, culture, or clonal variation. The present findings are unique in indicating that T21 hematopoietic abnormalities begin in the embryonic yolk sac. Specifically, we show that T21 yolk sac-type hematopoiesis is skewed toward the production of erythroid cells, similar to what occurs in T21 fetal liver hematopoiesis (7, 8). Thus, increased erythroid mass may occur in early human T21 embryos, which could potentially impair viability by increasing blood viscosity and contribute to the high rate of early spontaneous abortion observed for this chromosomal aneuploidy (1).
In contrast to what was observed in studies of human fetal liver, T21 did not enhance megakaryopoiesis or increase the proliferative capacity of erythro-megakaryocytic precursors in the first wave of hematopoiesis to emerge from iPSCs. Fetal liver contains definitive hematopoietic progenitors, but the iPSC-derived progenitors analyzed in our study likely represent the yolk sac primitive lineage. Of note, the iPSC-derived hematopoietic progenitors produced neutrophils, which are not generated during primitive hematopoiesis in mice (39). This finding may reflect interspecies differences in developmental potential of the primitive lineage or low-level definitive hematopoiesis occurring in our cultures. However, colony and cell morphology, as well as globin analysis, indicates that most of the hematopoietic progenitors examined in our study represent the primitive lineage, a transient population with limited proliferative capacity compared with definitive progenitors (13). Failure of T21 to enhance the expansion of primitive progenitors indicates that this lineage does not likely give rise to DS-associated TMD or AMKL. More likely, DS-associated TMD/AMKL derives from definitive hematopoietic progenitors that originate from either late-stage yolk sac or the AGM region and migrate to the fetal liver, which provides an essential microenvironment for expansion of TMD blasts (40, 41).
Murine models are useful for the study of DS, but do not recapitulate the developmental hematopoietic abnormalities of human T21. Alford et al. (14) and our own data (Fig. S5) show that fetal liver hematopoiesis is normal in the Tc1 and Ts65Dn murine DS models, respectively. Ts65Dn mice, which are trisomic for 104 HSA21 genes and display some postnatal hematopoietic abnormalities that occur in DS (16), also exhibit normal yolk sac-primitive hematopoiesis (Fig. S5), contrasting with our findings using human T21 iPSCs. Taken together, these studies indicate that murine models for DS do not recapitulate human T21 defects in developmental hematopoiesis within the yolk sac or fetal liver, highlighting the need for alternative models to study human T21 hematopoietic disorders.
Controlling developmental shifts in human iPSC hematopoietic differentiation is challenging and highly dependent on culture methods. Our results show that iPSCs can be used to define the effects of T21 on the earliest stage of human hematopoiesis (i.e., yolk sac-derived primitive). In an accompanying article, MacLean et al. (42) generated definitive, but not primitive progenitors from T21 ES and iPSCs (42). This study likely recapitulates late yolk sac or fetal liver-derived definitive hematopoiesis, reflecting a later wave of blood formation in embryos (43). A third accompanying article by Roy et al. analyzes second trimester fetal liver hematopoiesis (44), which represents AGM-derived definitive progenitors. These authors demonstrate that T21 impacts both myeloid and lymphoid lineages. Taken together, these studies better define T21 hematopoiesis throughout ontogeny, illustrating similarities and differences in three distinct, successive stages of embryonic/fetal hematopoiesis.
Notably, our group and Maclean et al. used similar protocols for differentiation of ESC/iPSCs (42), but produced blood lineages representing distinct stages of ontogeny. Specifically, we derived primitive yolk sac-type hematopoietic progenitors and Maclean et al. generated definitive fetal liver-type progenitors. Although our combined findings define T21 hematopoietic abnormalities more comprehensively than either study could alone, the reason for the observed differences in iPSC developmental output is unknown. Numerous properties of iPSCs, including gene-expression patterns, global DNA methylation, developmental capacity, and X chromosome inactivation state can vary, at least in part, from differences in culture methods (45–47). Indeed, prior studies indicate that relatively small differences in ES/iPSC differentiation protocols can affect the developmental stage of erythrocytes generated (23, 24, 48). These findings, along with the present work, suggest that when patient iPSCs are used to model human blood disorders, it is essential to stage the hematopoietic progeny. Long term, it will be important to better define culture conditions that reproducibly drive differentiation of iPSCs into primitive vs. definitive hematopoiesis.
We generated multiple human T21 iPSC lines that all exhibited enhanced erythropoiesis, an abnormality that appears to be a consistent property throughout development in DS. Most likely, multipotent progenitors are directed preferentially toward erythroid or erythro-megakaryocytic fates in T21-associated primitive and definitive hematopoiesis, respectively (7, 8, 42, 44, and the present study). This process must be initiated through altered HSA21-encoded genes that control hematopoietic fate directly and through intermediary factors. In comparing the transcriptomes of CD43+41+235+ progenitors generated from T21 and euploid iPSCs, only a few differentially expressed genes stand out as obvious candidates to explain the lineage-preference phenotypes (Table 1). These candidates include HSA21 genes BACH1, SON, GABPA, and DYRK1A, all of which exhibited relatively small (≤ 1.5-fold) but significant up-regulation in T21 progenitors. The small differences in expression of numerous genes likely produce an aggregate effect. Our finding that only a subset of HSA21 genes are overexpressed in T21 hematopoietic progenitors is consistent with prior studies demonstrating that in DS models, increased dosages of HSA21 are complex and tissue type-specific (30, 49). Although gain- and loss-of-function studies are required to identify the HSA21 genes that contribute to erythroid expansion in T21, standard approaches using siRNA knockdown or cDNA overexpression may create artifacts by altering expression beyond physiologic ranges or at inappropriate times during development. Genetic manipulation of iPSCs using zinc-finger and transcription activator-like effector nucleases should provide more physiological approaches to precisely manipulate copy numbers of candidate HSA21 genes suspected to alter hematopoiesis (50, 51). Similar approaches using directed differentiation of T21 iPSCs into other lineages, including neurons, endothelial, and cardiac cells, should further elucidate how altered HSA21 gene dosages disrupt tissue formation and function in DS.
Materials and Methods
Generation of iPSCs.
Fibroblasts/stromal cells were transduced with pMXs-based retroviral supernatant with human OCT4, SOX2, KLF4, or MYC, as previously described (17). Mononuclear cells were infected with pHage2-CMV-RTTA-W and pHage-Tet-hSTEMMCA-loxP virus, as previously described (25). Reprogramming details and pluripotency characterization methods are provided in SI Materials and Methods.
Hematopoietic Differentiation.
iPSCS were feeder-depleted and EBs generated by standard methods as described in ref. 20. EBs were cultured in StemPro-34 (Invitrogen) media supplemented with 2 mM glutamine, 50 mcg/mL ascorbic acid, 150 mcg/mL transferrin, 0.4 mM monothioglycerol, and with bone morphogenic protein 4 (BMP4) 25 ng/mL, VEGF 50 ng/mL (day 0–2); BMP4 25 ng/mL, VEGF 50 ng/mL, stem cell factor (SCF) 50 ng/mL, thrombopoietin (TPO) 50 ng/mL, FLT3-ligand (FLT3) 50 ng/mL, bFGF 20 ng/mL (day 2–4); VEGF 50 ng/mL, SCF 50 ng/mL, TPO 50 ng/mL, FLT3 50 ng/mL, bFGF 20 ng/mL (day 4–8); SCF 50 ng/mL, TPO 50 ng/mL, IL-3 10 ng/mL, IL-11 5 ng/mL, erythropoietin (EPO) 2 U/mL, and insulin growth factor-1 (IGF1) 25 ng/mL (day 8+). All cytokines except EPO (Amgen) and bFGF (Invitrogen) were purchased from R&D Systems. Cultures were maintained at 37 °C, 5% CO2, 5% O2, and 90% (vol/vol) N2.
Hematopoietic Colony-Forming Assays.
CD43+41+235+ cells were seeded into H4230 methylcellulose (Stem Cell Technologies) with EPO 5 U/mL, IL-3 10 ng/mL, SCF 5 ng/mL, and GMCSF 5 ng/mL, at 2,000–5,000 cells/mL (or 10,000 cells/mL for total EB cells). Colonies were scored at 12 d. 2,000–5,000 cells/mL (or 10,000 cells/mL for total EB cells) were seeded into Megacult-C (Stem Cell Technologies) with TPO 50 ng/mL, IL-6 10 ng/mL, and IL-3 10 ng/mL. After 12 d, cultures were dehydrated, fixed, and stained with anti-GPIIb/IIIa antibody. Large CFU-Mks were defined as having >50 positively stained cells.
Semiquantitative Real-Time PCR.
RNA was isolated with the RNeasy kit (Qiagen), cDNA prepared by the oligo(dT) method (Invitrogen), and PCR-quantified using SYBR green (Roche) on a Light Cycler 480II System (Roche). For all genes except globin, expression was normalized to cyclophilin, and relative quantification determined by comparative CT method. For globin quantification, expression was determined by standard-curve quantification using genomic DNA, and expressed as a fraction of α- or β-like genes. Primers sequences are provided in SI Materials and Methods.
Microarray and Bioinformatic Analysis.
RNA was isolated using an RNeasy kit (Qiagen) and hybridized to Affymetrix HuGene 1.0 ST microarrays. To obtain gene-expression values, raw intensity values from Affymetrix CEL data files were processed using the Robust Multichip Average method (52), implemented by the Bioconductor R “oligo” package (53, 54). Robust Multichip Average processing was applied to the whole dataset, which resulted in 33,297 probesets that were then mapped to 19,392 RefSeq genes. In cases where several probe sets mapped to one RefSeq gene, the expression values were averaged to obtain one value per gene. Additional methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Darrell Kotton and Gustavo Mostoslavsky for the reprogramming vectors; Nancy Spinner for the DS1 fibroblast cell line; Danielle Crawford and Sandra Ryeom for assistance in mouse studies; and the Children’s Hospital of Philadelphia human embryonic and induced pluripotent stem cells core facility, located in the Center for Cellular and Molecular Therapeutics, for technical and scientific advice. This work was supported by National Institutes of Health Grants K08 HL093290 (to S.T.C.), RC2 HL10166 (to M.J.W.), P30 DK090969 (to M.J.W. and S.T.C.), R01 HL091724 (to N.A.S.), and R01 DK065806 (to R.C.H.). S.T.C. is an American Society of Hematology Scholar. J.M.T. is a Special Fellow of the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE35561).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211175109/-/DCSupplemental.
References
- 1.Hassold T, Hunt P. To err (meiotically) is human: The genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 2.Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with Down syndrome: Data from a multihospital healthcare system. Am J Med Genet A. 2007;143(1):42–50. doi: 10.1002/ajmg.a.31442. [DOI] [PubMed] [Google Scholar]
- 3.Roizen NJ, Amarose AP. Hematologic abnormalities in children with Down syndrome. Am J Med Genet. 1993;46(5):510–512. doi: 10.1002/ajmg.1320460509. [DOI] [PubMed] [Google Scholar]
- 4.Malinge S, Izraeli S, Crispino JD. Insights into the manifestations, outcomes, and mechanisms of leukemogenesis in Down syndrome. Blood. 2009;113(12):2619–2628. doi: 10.1182/blood-2008-11-163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy A, Roberts I, Norton A, Vyas P. Acute megakaryoblastic leukaemia (AMKL) and transient myeloproliferative disorder (TMD) in Down syndrome: A multi-step model of myeloid leukaemogenesis. Br J Haematol. 2009;147(1):3–12. doi: 10.1111/j.1365-2141.2009.07789.x. [DOI] [PubMed] [Google Scholar]
- 6.Hollanda LM, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38(7):807–812. doi: 10.1038/ng1825. [DOI] [PubMed] [Google Scholar]
- 7.Chou ST, et al. Trisomy 21 enhances human fetal erythro-megakaryocytic development. Blood. 2008;112(12):4503–4506. doi: 10.1182/blood-2008-05-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunstall-Pedoe O, et al. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood. 2008;112(12):4507–4511. doi: 10.1182/blood-2008-04-152967. [DOI] [PubMed] [Google Scholar]
- 9.Tavian M, Péault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49(2-3):243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 10.Dzierzak E, Speck NA. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9(2):129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschle C, et al. Embryonic–Fetal Hb switch in humans: Studies on erythroid bursts generated by embryonic progenitors from yolk sac and liver. Proc Natl Acad Sci USA. 1984;81(8):2416–2420. doi: 10.1073/pnas.81.8.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliaccio G, et al. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac–liver transition. J Clin Invest. 1986;78(1):51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palis J, Yoder MC. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp Hematol. 2001;29(8):927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 14.Alford KA, et al. Perturbed hematopoiesis in the Tc1 mouse model of Down syndrome. Blood. 2010;115(14):2928–2937. doi: 10.1182/blood-2009-06-227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael CL, et al. Hematopoietic defects in the Ts1Cje mouse model of Down syndrome. Blood. 2009;113(9):1929–1937. doi: 10.1182/blood-2008-06-161422. [DOI] [PubMed] [Google Scholar]
- 16.Kirsammer G, et al. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111(2):767–775. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106(3):860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa A, et al. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS ONE. 2011;6(7):e22261. doi: 10.1371/journal.pone.0022261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma F, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105(35):13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C, Olivier EN, Velho M, Bouhassira EE. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111(4):2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapillonne H, et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica. 2010;95(10):1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommer CA, et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27(3):543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polo JM, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28(8):848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(12):1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimchenko O, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114(8):1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 30.Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: From genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 31.Choi KD, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57:289–300. [Google Scholar]
- 33.Korbel JO, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci USA. 2009;106(29):12031–12036. doi: 10.1073/pnas.0813248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyle R, et al. Genotype-phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur J Hum Genet. 2009;17(4):454–466. doi: 10.1038/ejhg.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arron JR, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441(7093):595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 36.Malinge S, et al. Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J Clin Invest. 2012;122(3):948–962. doi: 10.1172/JCI60455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClanahan T, Dalrymple S, Barkett M, Lee F. Hematopoietic growth factor receptor genes as markers of lineage commitment during in vitro development of hematopoietic cells. Blood. 1993;81(11):2903–2915. [PubMed] [Google Scholar]
- 38.Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002;21(21):3295–3313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
- 39.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 40.Hitzler JK, Zipursky A. Origins of leukaemia in children with Down syndrome. Nat Rev Cancer. 2005;5(1):11–20. doi: 10.1038/nrc1525. [DOI] [PubMed] [Google Scholar]
- 41.Klusmann JH, et al. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev. 2010;24(15):1659–1672. doi: 10.1101/gad.1903410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLean GA, et al. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci USA. 2012;109:17567–17572. doi: 10.1073/pnas.1215468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGrath KE, et al. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood. 2011;117(17):4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy A, et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci USA. 2012;109:17579–17584. doi: 10.1073/pnas.1211405109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tchieu J, et al. Female human iPSCs retain an inactive X chromosome. Cell Stem Cell. 2010;7(3):329–342. doi: 10.1016/j.stem.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bock C, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144(3):439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu SJ, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112(12):4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyle R, Gehrig C, Neergaard-Henrichsen C, Deutsch S, Antonarakis SE. Gene expression from the aneuploid chromosome in a trisomy mouse model of down syndrome. Genome Res. 2004;14(7):1268–1274. doi: 10.1101/gr.2090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27(9):851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 52.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.