Age-associated neurodegenerative diseases are not only a great scientific challenge but a medical, economic, and social burden of enormous dimensions. This family of diseases includes Alzheimer’s (AD), Parkinson disease, Huntington disease, and others, among which AD is the most common. AD alone affects more than 35 million people worldwide. Although these disorders are products of different etiological factors, they have a common pathological feature, accumulation and aggregation of one or more proteins in the brain, and hence are referred to as proteopathies. The abnormal protein accumulation/aggregation causes proteotoxicity, which is believed to underlie neurodegeneration (1). How different proteins accumulate and aggregate in the brain in various neurodegenerative diseases and by what mechanisms these proteins lead to neurodegeneration are currently hot research subjects. In PNAS, Wang et al. (2) identify O-GlcNAc cycling enzymes as unique regulators of proteotoxicity in four Caenorhabditis elegans models of neurodegenerative diseases. Their findings open a new avenue to investigate neurodegenerative diseases.
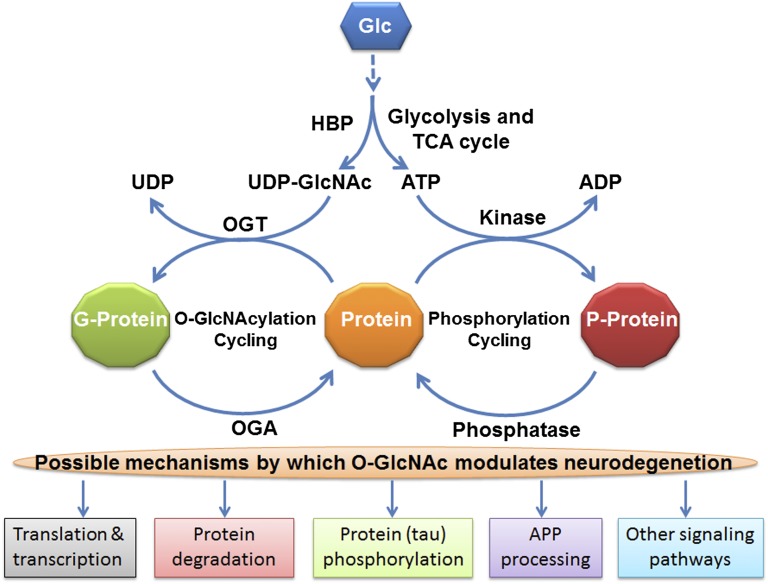
O-GlcNAcylation is a dynamic posttranslational modification of proteins with a sugar moiety, in which β-GlcNAc is transferred enzymatically from a UDP-GlcNAc donor, which is generated from glucose through the hexosamine biosynthetic pathway, to the hydroxyl group of serine or threonine residues of proteins (Fig. 1). In contrast to classic protein glycosylation, which occurs in the endoplasmic reticulum and Golgi apparatus, and modifies mainly secreted and membrane proteins, O-GlcNAcylation modifies nucleocytoplasmic proteins and resembles protein phosphorylation (3). The enzymes catalyzing the O-GlcNAc cycling, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), are expressed in the nucleocytoplasmic compartments (3, 4). O-GlcNAcylation regulates a variety of vital cellular processes and is itself regulated developmentally in the brain (5). OGT knockout, which eliminates O-GlcNAcylation, results in embryonic lethality in vertebrates (6, 7), indicating an essential role of O-GlcNAcylation during early development. Deregulation of O-GlcNAc modification has been linked to several human diseases, including neurodegenerative diseases (8–10). Both OGT and OGA are highly expressed in the brain (11). A recent proteomic study identified 274 O-GlcNAcylated proteins and mapped 458 O-GlcNAc sites in the adult mouse cerebrocortical brain tissue (12).
Fig. 1.
Schematic of protein O-GlcNAcylation and possible mechanisms by which it may modulate neurodegeneration. Alterations of O-GlcNAcylation can modulate, directly or indirectly through modulation of protein phosphorylation, multiple pathways involved in neurodegeneration. APP, amyloid β precursor protein; G-protein, O-GlcNAcylated protein; Glc, glucose; HBP, the hexosamine biosynthetic pathway; P-protein, phosphorylated protein; TCA, tricarboxylic acid.
By generating null mutants of OGT and OGA in C. elegans models of neurodegenerative proteotoxicity, Wang et al. (2) find that loss of function of OGT alleviates, whereas loss of OGA enhances, the phenotype of transgenic models of tauopathy, β-amyloid peptide, and polyglutamine expansion. These observations are striking, because previous studies showed that down-regulation of brain O-GlcNAcylation, which is seen in AD brain (9) and was expected in the ogt-1 null mutant animals in the study of Wang et al. (2), facilitates abnormal tau hyperphosphorylation and, consequently, neurofibrillary neurodegeneration (9, 13). On the other hand, the marked increase of brain O-GlcNAcylation after treatment with thiamet-G, an OGA inhibitor, reduces tau phosphorylation and aggregation in rodents and hinders tau-driven neurodegeneration in a mouse model of tauopathy (8, 10). Although the overall O-GlcNAcylation level increases markedly in the brain as well as in other organs, the mice or rats treated with thiamet-G do not show any abnormality or phenotype (10, 14). The observations that Wang et al. (2) report apparently contradict the findings from rodent studies. Furthermore, both the global O-GlcNAcylation level and the tau O-GlcNAcylation level are decreased in AD brain (9), which is consistent with the decreased brain energy/glucose metabolism in AD, because O-GlcNAcylation also serves as a sensor of intracellular nutrients/glucose metabolism (15).
Several possibilities may explain the apparent discrepancy between the observations in C. elegans by Wang et al. (2) and in rodent models by others. First, the role of O-GlcNAcylation, including its developmental regulation, might be very different in C. elegans than in vertebrates. Ogt KO results in embryonic lethality in vertebrates (6, 7), whereas C. elegans animals bearing null mutations of ogt-1 are alive and fertile, and appear to have a normal phenotype (16). The basal level of O-GlcNAcylation might be much higher in vertebrates than in C. elegans, such that this modification is indispensable in vertebrates but not in worms. Thus, it would not be surprising if the role of O-GlcNAcylation in neurodegeneration is different in these different animals. Second, O-GlcNAcylation in the ogt-1 and oga-1 null C. elegans is presumably dysregulated from the beginning of life; thus, the animals have already adapted to the loss of these gene functions. Manipulation of O-GlcNAcylation level in adult animals disturbs the normal O-GlcNAc cycling, and thus likely produces different responses from what is seen in the ogt-1 and oga-1 null C. elegans. Third, different readouts were used in the study of C. elegans and in rodent studies. Wang et al. (2) determine protein aggregates, degenerative neurons, and cell death in the Huntington disease models; animal paralysis in the Aβ1–42 model; and the “thrashing” behavior in the tauopathy model. In rodent studies (8–10), tau phosphorylation and aggregation were the major readouts. Finally, although similar roles of altered O-GlcNAc cycling are seen in the four different C. elegans models of neurodegenerative diseases that Wang et al. (2) study, the ogt-1 null mutation has no effect on a generic proteotoxicity model (GFP-degron). Thus, the implications of the observations in C. elegans on human neurodegenerative diseases must await similar studies in rodents.
Wang et al. (2) further demonstrate that O-GlcNAc cycling may modify proteotoxicity by regulating proteasome and autophagy activity and by splicing isoforms of daf-16, a known regulator of longevity, stress response, and proteotoxicity, in C. elegans. Because the changes in polyubiquitinated proteins and daf-16 isoforms
The work of Wang et al. sheds new light into neurodegenerative proteopathies.
are mild to moderate in the ogt-1 and oga-1 mutants, whether these are indeed the main mechanisms by which O-GlcNAcylation modulates proteotoxicity must be confirmed, especially in vertebrates. However, these studies do provide novel directions for future investigation of the mechanistic role of O-GlcNAc cycling in neurodegeneration.
The role of O-GlcNAc cycling in AD has been implicated recently. Tau phosphorylation was found to be regulated inversely by O-GlcNAcylation, and the O-GlcNAcylation level of brain proteins, including tau, was found to be decreased in AD brain (9, 13). Inhibition of O-GlcNAcylation or reduction of brain glucose metabolism causes hyperphosphorylation of tau (9, 13, 17), which leads to the formation of neurofibrillary tangles and neurodegeneration (18). Treatment with OGA inhibitor decreases tau hyperphosphorylation in rats and inhibits tau aggregate formation and neuronal loss in a mouse model of tauopathy (8, 10, 13). Amyloid β precursor protein, which gives rise to the amyloid β peptide that forms amyloid plaques, is also modified by O-GlcNAc (19), and this modification appears to modulate the processing of the precursor protein to form amyloid β peptide (20). Genetic studies have linked the oga gene to late-onset AD (21). Furthermore, the majority of neurodegenerative diseases are sporadic and multifactorial, and involve multiple brain signaling pathways. Because O-GlcNAcylation also modulates protein homeostasis and signaling transduction pathways, it can modify the neurodegenerative process by multiple mechanisms (Fig. 1).
The work of Wang et al. (2) sheds new light into neurodegenerative proteopathies. It is clear now that the homeostasis of O-GlcNAc cycling is critical to, and its dysregulation is involved in, neurodegenerative diseases. To date, there is no satisfactory treatment or cure for neurodegenerative diseases, mainly because the disease mechanisms are largely unknown. O-GlcNAc cycling provides a new target for investigation of these disease mechanisms and for drug development for this family of devastating diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 17669.
References
- 1.Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol. 2006;1:151–170. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, et al. O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci USA. 2012;109:17669–17674. doi: 10.1073/pnas.1205748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800(2):96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love DC, Krause MW, Hanover JA. O-GlcNAc cycling: Emerging roles in development and epigenetics. Semin Cell Dev Biol. 2010;21(6):646–654. doi: 10.1016/j.semcdb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, et al. Developmental Regulation of Protein O-GlcNAcylation, O-GlcNAc Transferase, and O-GlcNAcase in Mammalian Brain. PLoS ONE. 2012;7(8):e43724. doi: 10.1371/journal.pone.0043724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafi R, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97(11):5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster DM, et al. O-GlcNAc modifications regulate cell survival and epiboly during zebrafish development. BMC Dev Biol. 2009;9:28. doi: 10.1186/1471-213X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuzwa SA, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4(8):483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132(Pt 7):1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuzwa SA, et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol. 2012;8(4):393–399. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 11.Liu K, et al. Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem. 2004;89(4):1044–1055. doi: 10.1111/j.1471-4159.2004.02389.x. [DOI] [PubMed] [Google Scholar]
- 12.Alfaro JF, et al. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 2012;109(19):7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(29):10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macauley MS, Shan X, Yuzwa SA, Gloster TM, Vocadlo DJ. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 2010;17(9):949–958. doi: 10.1016/j.chembiol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673(1–2):13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Hanover JA, et al. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA. 2005;102(32):11266–11271. doi: 10.1073/pnas.0408771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci. 2006;23(8):2078–2086. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 18.Gong CX, Grundke-Iqbal I, Iqbal K. Targeting tau protein in Alzheimer’s disease. Drugs Aging. 2010;27(5):351–365. doi: 10.2165/11536110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res. 1995;41(2):270–278. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen KT, Iverfeldt K. O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-β precursor protein (APP) Biochem Biophys Res Commun. 2011;404(3):882–886. doi: 10.1016/j.bbrc.2010.12.080. [DOI] [PubMed] [Google Scholar]
- 21.Bertram L, et al. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290(5500):2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]