Abstract
Trans-isomers of cytokinins (CK) are thought to predominate and have greater biological activity than corresponding cis-isomers in higher plants. However, this study demonstrates a system within which the predominant CK are cis-isomers. CK were measured at four developmental stages in developing chickpea (Cicer arietinum L. cultivar Kaniva) seeds by gas chromatography-mass spectrometry. Concentrations were highest at an early endospermic fluid stage and fell considerably when the cotyledons expanded. The cis-isomers of zeatin nucleotide ([9R-MP]Z), zeatin riboside ([9R]Z), and zeatin (Z) were present in greater concentrations than those of corresponding trans-isomers: (trans)[9R-MP]Z, (trans)[9R]Z, (trans)Z, or dihydrozeatin riboside. Dihydrozeatin, dihydrozeatin nucleotide, and the isopentenyl-type CK concentrations were either low or not detectable. Root xylem exudates also contained predominantly cis-isomers of [9R-MP]Z and [9R]Z. Identities of (cis)[9R]Z and (cis)Z were confirmed by comparison of ion ratios and retention indices, and a full spectrum was obtained for (cis)[9R]Z. Tissues were extracted under conditions that minimized the possibility of RNase hydrolysis of tRNA following tissue disruption, being a significant source of the cis-CK. Since no isomerization of (trans)[2H]CK internal standards occurred, it is unlikely that the cis-CK resulted from enzymic or nonenzymic isomerization during extraction. Although quantities of total CK varied, similar CK profiles were found among three different chickpea cultivars and between adequately watered and water-stressed plants. Developing chickpea seeds will be a useful system for investigating the activity of cis-CK or determining the origin and metabolism of free CK.
Seed tissues were the source for isolation of the first naturally occurring CK, trans-Z (Miller, 1961; Letham, 1963). Seeds have turned out to be a rich source of CK, and in the past 30 years investigators have described a range of different CK from seed tissues (van Staden et al., 1982). This may reflect their relatively high levels in seeds (van Staden et al., 1982), a status that is believed to indicate a role for CK in establishing developing seeds as strong assimilate sinks (Brenner and Cheikh, 1995). Despite a vast literature concerning the occurrence, form, and significance of CK in plant development, the nature and site(s) of their synthesis is yet to be established. In fact, Holland (1997) recently proposed that CK are not formed by plants at all but rather by bacterial symbionts that colonize plant tissues. Although there is good evidence for the transfer of CK synthesized by Rhizobium in legume nodules (Upadhyaya et al., 1991), a role for bacteria in providing CK to roots or shoot organs needs to be investigated more thoroughly. Because unequivocal evidence for a plant isopentenyl transferase is lacking, a persistent hypothesis, which has recently been reviewed (Prinsen et al., 1997), is that the free CK in plants are not synthesized de novo but are released during tRNA turnover.
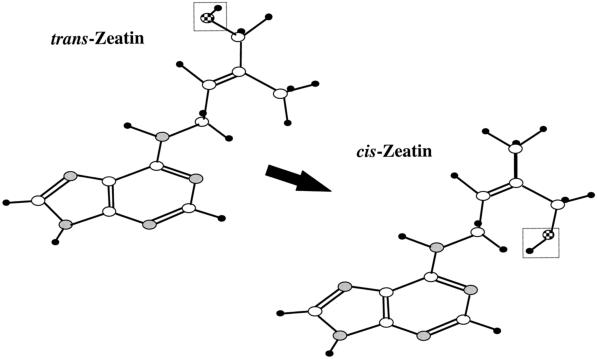
Z, [9R-MP]Z, and [9R]Z have an unsaturated isopentenyl side chain that can exist in the cis or trans conformation. The cis-isomer occurs when the hydroxyl group of the isopentenyl side chain is oriented toward the N-1 position of the purine ring, whereas in the trans-isomer the hydroxyl group is oriented away from the purine ring (Korszun et al., 1989; Fig. 1). The trans-isomers of [9R]Z and Z are by far the more commonly reported forms and are considered the predominant isomers in higher plants (McGaw and Burch, 1995; Prinsen et al., 1997). Systems in which the existence of cis-CK can be demonstrated unequivocally would be significant for two reasons. First, because cis-CK show much lower activity than trans-CK in bioassays (Kaminek, 1982) and their interconversion may constitute a mechanism for reducing CK bioactivity in vivo. Second, cis-CK provide evidence for the hypothesis that the free CK pool in higher plants may be at least partially derived from the breakdown of tRNA. The major criticism of this hypothesis has been the structural distinctness between tRNA-bound CK and free-pool CK (Letham and Palni, 1983; McGaw and Burch, 1995; Prinsen et al., 1997). tRNA-bound CK are predominantly cis-isomers, whereas the large majority of studies to date have reported that free CK are predominantly or exclusively trans-isomers. It has even been suggested by Tay et al. (1986) that where cis-isomers have been detected (Mauk and Langille, 1978; Watanabe et al., 1982; Takagi et al., 1985) they are artifacts formed in extractions that did not rigorously exclude the possibility of tRNA breakdown following cell disruption.
Figure 1.
The stereochemical conformation of (trans)Z and (cis)Z (adapted from Korszun et al., 1989). Open oval, C; gray oval, N; checkered oval, O; black oval, H.
Recent evidence indicates that the occurrence and significance of cis-CK need to be reexamined. Using an analytical procedure requiring no extraction step, Parker et al. (1989) showed in wheat and oat that cis-CK are minor components of xylem. An enzyme that converts cis- to trans-isomers, Z cis-trans-isomerase, has been partially purified and assayed in extracts of developing bean seeds (Bassil et al., 1993). Furthermore, in potato tuber sprouts, Nicander et al. (1995) identified cis-Z-9-glucoside, a compound that could not have been derived directly from tRNA breakdown. Clearly, there is a need to identify the source of cis-isomers and to establish their significance, both as precursors for “active” CK and in general for regulatory roles proposed for this group of compounds. Preliminary studies of the CK composition of pulses indicated that cis-isomers of Z and [9R]Z were minor components of species of lupin but major components of chickpea (Cicer arietinum L.; Emery et al., 1997). In the present study we used GC-MS to identify and quantify CK profiles in developing chickpea seeds, and we determined a system within which the predominant CK are unambiguously identified as cis-isomers.
MATERIALS AND METHODS
Plant Material
Plants of chickpea (Cicer arietinum L.) were grown in 7.5-L free-draining polyvinylchloride pots (42.5 cm long; 15 cm in diameter) sealed at the base, in a greenhouse at day/night temperatures of 25.5°C ± 2.7°C/16.5°C ± 1.7°C and maximum/minimum RH of 78.7% ± 20.9%/70.3% ± 12.9% at Floreat Park (Perth, Western Australia). The pots were filled with ground, dry soil from the surface of a native red-brown earth (U.S. Department of Agriculture, Calcic Haploxeralf) with neutral to alkaline pH, collected at Merredin, Western Australia (31o30′ S, 118o12′ E). The soil was mixed with 10% (w/w) yellow sand to avoid compaction and treated with fertilizer corresponding to 2.1 g of N, 0.8 g of P, and 1.2 g of K per pot (1 g of commercial microelement preparation [Richgro, Canning Vale WA, Australia], 7.51 g of KNO3, 7.13 g of NH4NO3, 10.67 g of Ca(NO3)2, and 7.61 g of triple superphosphate per 50 kg of soil). All seeds were inoculated with commercial rhizobia inocula immediately before sowing using group N Bradyrhizobium.
Seeds of large-seeded kabuli-type (cv Kaniva) or small-seeded desi-type (cv Tyson or ICCV88201) chickpeas were sown to a depth of 5 cm. All pots were irrigated every 2nd d to maintain the soil near field capacity (17%, w/w) until the end of the flowering period. Plants of selected pots were water stressed. Water deficit was imposed at 90 DAS by watering once every 9 d with 200 mL per pot until 115 DAS and then by stopping watering until terminal harvest at 157 DAS. Pods were tagged between 69 and 87 DAS when they were 3 mm long (pod set), and were later collected at several stages of development (1, 14, and 40 DAPS) in irrigated plants of cv Kaniva, at 30 DAPS in irrigated and water-stressed plants of cvs Tyson and Kaniva, and at 50 DAPS in irrigated plants of cv ICCV88201. Pods harvested at 14 DAPS were partitioned into pod wall, seed coat, embryo with cotyledons, and endospermic fluid. Pods harvested at 30, 40, and 50 DAPS were partitioned into pod wall, seed coat, cotyledons, and embryonic axis. CK were extracted from whole pods (fertilized ovaries, 1 DAPS, 30–50 mg dry weight), embryo with cotyledons, endospermic fluid and seed coat (14 DAPS; 4–84 mg dry weight), and cotyledons (30, 40, and 50 DAPS; 16–85 mg dry weight for GC-MS-SIM and 500–900 mg dry weight for full-scan GC-MS). All samples were freeze-dried before extraction of CK. Harvests were chosen to correspond to previously determined critical phases of seed development in cvs Kaniva and Tyson: start of cell division (1 DAPS), end of cell division and commencement of seed filling (14 DAPS), maximum rate of seed filling (30 DAPS), and end of seed filling (40 DAPS).
Collection of Xylem Exudate
Selected plants at 105 DAS were decapitated, and the root system was enclosed in a pressure apparatus to assist in the collection of root-bleeding sap from the cut surface of the stem. The pressure used did not exceed 15 p.s.i. and collections were stopped after 10 min. This resulted in collection volumes of 1 to 2 mL from each plant. Samples were frozen and stored at −80°C before their analysis for CK content.
Tissue Extraction
Samples were kept as cold as possible during the initial extraction. Solvents were kept at −20°C and grinding was done on ice. Freeze-dried samples were powdered in liquid nitrogen and ground into a slurry with cold, modified Bieleski extraction buffer 1 (Bieleski, 1964; CH3OH:CHCl3:HCOOH:H2O [60:15:5:20, v/v]) together with 20 ng each of [2H6]iP, [2H6][9R]iP, (trans)[2H5] Z, [2H3]DHZ, (trans)[2H5][9R]Z, and [2H3][9R]DHZ, and 50 ng each of [2H6][9R-MP]iP, (trans)[2H5][9R-MP]Z, and [2H3][9R-MP]DHZ (Apex Organics, Devon, UK) added as internal standards. Additional extraction buffer was added to bring the buffer volume:sample weight ratio to 10:1; the sample was vortexed, sonicated for 1 min, and centrifuged for 5 min to sediment debris; and the supernatant was removed and filtered (0.45 μm). The residue was re-extracted twice more, each time in cold, modified Bieleski extraction buffer 2 (CH3OH:HCOOH:H2O [60:5:35, v/v]), vortexed, sonicated, and centrifuged. The supernatants were pooled and freeze-dried. No extraction step was necessary for samples of xylem exudate, which were directly freeze-dried and purified in the same manner as tissue extracts.
For analyses involving the assessment of RNase activity on CK recovery and tRNA degradation using Bieleski reagents, all glassware was baked at 180°C for 6 h before use and gloves were worn at all times. Extraction solvents and cation-exchange column buffers were prepared using diethyl pyrocarbonate-treated, autoclaved water. Extractions (cotyledons 40 DAPS, 1–4 g dry weight) were carried out as described above, except that the modified Bieleski extraction buffers contained 10 mm RVC (Sigma-Aldrich). Degradation of tRNA was assessed using yeast tRNA (20 μg; Sigma-Aldrich) incubated for 30 min at 37°C with 0.001 unit RNase A (Amresco, Solon, OH) in 50 mm Tris-HCl (pH 8.0) or Bieleski extraction buffer 2, with and without RVC. Reactions were snap frozen in liquid nitrogen and freeze-dried. tRNA breakdown was assessed quantitatively by measuring tRNA recovery following electrophoresis on a 3% agarose gel containing 3 μg mL−1 ethidium bromide in TBE buffer (8.9 mm Tris-borate. 8.9 mm boric acid, and 0.2 mm EDTA). Gels were photographed with a digital camera (model DC40, Kodak) and tRNA was quantified with 1D image analysis software (Kodak).
Purification and Assay of CK
The residue from the extraction was dissolved in 3 mL of cold, acidified water (0.1 n acetic acid) and transferred to a 10-mL of polypropylene tube, adjusted to less than pH 3.0 (with acetic acid) and passed through a sterene divinylbenzene (500 mg) SCX column (Alltech Associates, Baulkham Hills NSW, Australia) that had been preconditioned with 10 mL of 0.1 n acetic acid. The sample was loaded and the column washed with 10 mL of 0.1 n acetic acid. The eluates from the load and wash steps were retained for CK-nucleotide analysis.
Nucleoside and free-base CK were eluted from the SCX column in 20 mL of 2 n NH4OH. The eluate was dried in vacuo (38°C), redissolved in neutral, deionized water (pH 5.0–6.0), and further purified using a syringe-tip 300-mg C18 solid-phase extraction cartridge (Alltech Associates). The cartridge was conditioned with 20 mL of methanol and 20 mL of neutral, deionized water before the sample was loaded. The cartridge containing the sample was washed with 20 mL of neutral, deionized water and the CK was eluted with 20 mL of methanol:water (80:20, v/v). The sample was dried in vacuo (38°C) and, except for xylem samples, further purified by HPLC on a C18 Alphabond column (Alltech Associates; length = 300 mm, i.d. = 3.9 mm, 10-μm particle size) at a flow rate of 2 mL min−1. CK eluted from a solvent program of acetonitrile in water adjusted to pH 7.0 with triethylammonium bicarbonate. The gradient was linear from 5% to 30% acetonitrile over 40 min. Two fractions were collected. The first, from about 15 to 20 min, contained (cis)Z, (trans)Z, DHZ, (cis)[9R]Z, (trans)[9R]Z, and [9R]DHZ. The second, from 24 to 26 min, contained iP and [9R]iP. Both were freeze-dried and the residues were transferred in methanol to 1.0-mL tapered-bottom glass vials for derivatization.
CK nucleotides recovered in the acetic acid wash of the SCX column were converted to nucleosides by incubation with alkaline phosphatase for 12 h at 37°C (type III, Sigma, 3.4 units in 1 mL of 0.1 m ethanolamine-HCl, pH 10.4). Resultant CK-nucleosides were purified as described above.
The CK were permethylated as described previously (Emery et al., 1998), and an aliquot in ethyl acetate was analyzed by GC-MS. The Hewlett-Packard 5890 gas chromatograph was equipped with a split/splitless injector operating at 250°C in splitless mode and was linked to a Hewlett-Packard 5970 series Mass Selective Detector. The GC was fitted with a BP5 capillary column (25 m, 0.22-mm i.d.; 0.25-μm film, 5% phenyl-95% dimethyl siloxane; SGE, Ringwood, Victoria, Australia). The helium flow was 60 cm s−1 and the column head pressure was 1.5 p.s.i. The GC temperature program ramped from 60°C to 200°C at 20°C min−1 and then at 5°C min−1 to 300°C, which was held for 5 min. Ions for SIM mode are listed in Table I for permethylated-iP, [9R]iP, (cis)Z, (trans)Z, DHZ, (cis)[9R]Z,(trans)[9R]Z, and [9R]DHZ. Individual endogenous CK levels were calculated using the ratio of unlabeled to labeled ion pairs. Where necessary, corrections were made for the contribution of 2H ions to 1H ions (and vice versa). Ion pairs used for quantification of each CK are indicated in Table I. Full-scan mass spectra were obtained for a range of m/z (40 to 300) at a rate of 0.9 scans s−1. KRI values were determined using the method of Gaskin and Macmillan (1991). The use of KRI values is essential to distinguish between isomeric compounds that have almost identical MS patterns but different GC characteristics. A solution of C21- to C36-n-alkanes was coinjected into the gas chromatograph-mass spectrometer with authentic CK standards and samples, and an m/z 85 mass chromatogram was added to SIM run monitoring. KRI values were calculated according to the method of Gaskin and Macmillan (1991).
Table I.
KRIs and relative intensities of characteristic diagnostic ions as determined by GC-MS-SIM for permethylated authentic CK standards and putative CK purified from tissues of developing chickpea seeds
| Compound | KRI | Constituent Ions | ||
|---|---|---|---|---|
| m/z (relative abundance) | ||||
| [9R]Z | ||||
| [2H5]trans-[9R]Z | 3084 | 221a(100) | 395 (48) | 426 (2) |
| Authentic trans-[9R]Z | 3089 | 216 (100) | 390 (52) | 421 (2) |
| Putative trans-[9R]Z | 3089 | 216a(100) | 390 (50) | 421 (3) |
| Putative trans-[9R]Z from hydrolyzed trans-[9R-MP]Z | 3089 | 216a(100) | 390 (62) | |
| Authentic cis-[9R]Z | 3065 | 216 (100) | 390 (54) | 421 (2) |
| Putative cis-[9R]ZR | 3065 | 216a(100) | 390 (46) | 421 (2) |
| Putative cis-[9R]Z from hydrolyzed cis-[9R-MP]Z | 3065 | 216a(100) | 390 (45) | 421 (3) |
| [9R]DHZ | ||||
| [2H3][9R]DHZ | 2997 | 163a(100) | 177 (64) | 253 (78) |
| Authentic [9R]DHZ | 2999 | 162 (100) | 176 (65) | 250 (74) |
| Putative [9R]DHZ | 2998 | 162a(100) | 176 (47) | 250 (32) |
| Putative [9R]DHZ from hydrolyzed [9R-MP]DHZ | 2998 | 162 (100) | 176 (35) | |
| Z | ||||
| [2H5]trans-Z | 2299 | 235a(100) | 188 (18) | 266 (4) |
| Authentic trans-Z | 2304 | 230 (100) | 188 (24) | 261 (5) |
| Putative trans-Z | 2304 | 230a(100) | ||
| Authentic cis-Z | 2280 | 230 (100) | 188 (25) | 261 (5) |
| Putative cis-Z | 2280 | 230a(100) | 188 (25) | 261 (5) |
| DHZ | ||||
| [2H3]DHZ | 2223 | 177a(100) | 191 (25) | 235 (12) |
| Authentic DHZ | 2225 | 176 (100) | 190 (30) | 232 (22) |
| Putative DHZ | 2224 | 176a(100) | 190 (38) | 232 (28) |
| iP | ||||
| [2H6]iP | 2090 | 188 (100) | 237a(42) | 219 (47) |
| Authentic iP | 2096 | 188 (100) | 231 (36) | 216 (44) |
| Putative iP | 2096 | 188 (100) | 231a(32) | |
| [9R]iP | ||||
| [2H6][9R]iP | 2876 | 174 (88) | 397a(100) | 205 (44) |
| Authentic [9R]iP | 2882 | 174 (86) | 391 (100) | 202 (78) |
| Putative [9R]iP | 2882 | 174 (75) | 391a(100) | |
| Putative [9R]iP from hydrolyzed [9R-MP]iP | No detection made except for internal standard | |||
Ions used in quantification.
RESULTS
Identification of cis-CK
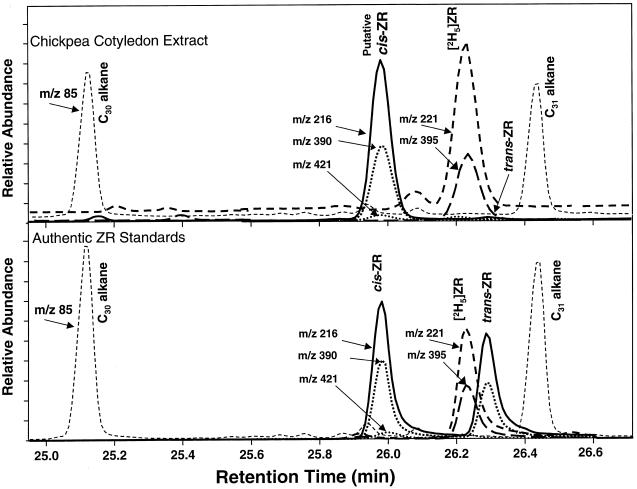
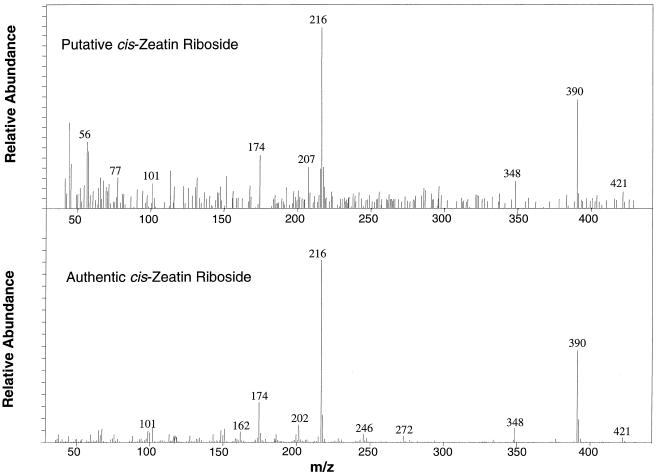
The GC-MS-SIM spectra for authentic standards of (cis)[9R]Z, (trans)[9R]Z, and [2H5][9R]Z and a sample from developing chickpea cotyledons are shown in Figure 2. In the cotyledon sample, a compound was detected that had ion ratios similar to (trans)[9R]Z in GC-MS-SIM runs but eluted from the gas chromatograph with a retention time of approximately 0.3 min before (trans)[9R]Z and had the same retention time as authentic (cis)[9R]Z. Integration of SIM peaks confirmed that the ratios of the major ions for the unknown compound, (cis)[9R]Z and (trans)[9R]Z, were very similar (Table I). A full spectrum of the putative (cis)[9R]Z from chickpea cotyledons was obtained (Fig. 3), which matched closely either (cis)[9R]Z (Fig. 3) or (trans)[9R]Z (not shown). Since permethylated cis- and trans-isomers of [9R]Z had almost identical fragmentation patterns (Table I), the two isomers could not be distinguished by mass spectra alone. KRI values were calculated for each of the compounds in several different samples. All of the [9R]Z standards and the putative chickpea (cis)[9R]Z eluted on GC between the C30- and C31-alkanes (Fig. 2). KRI consistently characterized GC retention time differentials between authentic standards of isomers and confirmed that KRI values were identical for the chickpea putative (cis)[9R]Z and authentic (cis)[9R]Z (Table I). No isomerization of internal standard from (trans)[2H5][9R]Z to (cis)[2H5][9R]Z was observed in any of the samples.
Figure 2.
GC-MS-SIM spectra for putative (cis)[9R]Z purified from cotyledons of immature chickpea seeds (cv Kaniva, 30 DAPS) as compared with authentic standards of (cis)[9R]Z (cis-ZR), (trans)[9R]Z (trans-ZR), and [2H5](trans)[9R]Z ([2H5]ZR).
Figure 3.
Full-scan mass spectrum of putative (cis)[9R]Z purified from cotyledons of immature chickpea seeds (cv Kaniva, 30 DAPS) as compared with that of an authentic standard of (cis)[9R]Z.
A second unknown compound with KRI values corresponding to authentic (cis)Z was detected in extracts from developing chickpea seeds and showed a ratio of major ions from GC-MS-SIM runs similar to those of authentic (cis)Z (Table I). A clear full spectrum for the putative (cis)Z was not obtained, since its concentration in tissue extracts was relatively low, and a direct comparison of spectra could not be done without increasing the scale of extraction considerably.
Treatment of the CK-nucleotide fraction isolated from chickpea seeds by SCX with phosphatase yielded a compound with GC-MS-SIM ion ratios similar to [9R]Z; a GC retention time and KRI value were identical to that of (cis)[9R]Z (Table I). This is consistent with a cis-isomer of [9R-MP]Z being present in the original extraction from chickpea seeds.
CK Profiles during Seed Development
CK profiles were relatively consistent across seed tissues and stages of development (Table II). With the exception of [9R-MP]iP, which was never detected, the CK nucleotides were present in the greatest concentrations, followed by the ribosides and free-base CK, respectively. iP and [9R]iP were present in detectable quantities in only older cotyledon extracts from seeds in which cell division had ceased.
Table II.
Concentration of CK identified from components of developing chickpea seeds as quantified by isotope dilution assay using GC-MS-SIM
| Compound | CK Concentration
|
|||||
|---|---|---|---|---|---|---|
| Poda1 DAPS | Embryo 14 DAPS | Seed Coat 14 DAPS | Endosperm 14 DAPS | Cotyledon
|
||
| 30 DAPS | 40 DAPS | |||||
| pmol g−1 dry wt | ||||||
| cis-[9R-MP]Z | 3405 ± 732 | 6106 | 2133 ± 1216 | 12,381 ± 2,434 | 193 ± 46 | 190 ± 20 |
| trans-[9R-MP]Z | 256 ± 129 | ndb | 101 ± 52 | 1,993 ± 667 | 15 ± 4 | nd |
| [9R-MP]DHZ | 117 ± 117 | nd | 166 ± 166 | nd | nd | nd |
| cis-[9R]Z | 403 ± 51 | 1572 | 98 ± 21 | 3,149 ± 1,082 | 152 ± 38 | 37 ± 6 |
| trans-[9R]Z | 153 ± 66 | nd | 39 ± 2 | nd | 3 ± 1 | 8 ± 5 |
| [9R]DHZ | 299 ± 50 | 2414 | 81 ± 32 | 534 ± 77 | nd | 8 ± 7 |
| cis-Z | 80 ± 40 | nd | 53 ± 43 | 130 ± 130 | 12 ± 3 | nd |
| trans-Z | 42 ± 42 | 231 | 24 ± 2 | nd | 4 ± 1 | 15 ± 4 |
| DHZ | 1 ± 1 | nd | 24 ± 20 | nd | 6 ± 2 | 6 ± 5 |
| [9R]iP | nd | nd | nd | nd | 6 ± 1 | 7 ± 5 |
| iP | nd | nd | nd | nd | 9 ± 5 | 2 ± 2 |
| [9R-MP]iP | nd | nd | nd | nd | nd | nd |
Data are means ± se (n = 3–6).
Fertilized ovary.
nd, Not detected.
In all tissues tested from developing seeds of cv Kaniva from 1 to 40 DAPS, levels of (cis)[9R-MP]Z, (cis)[9R]Z, and (cis)Z predominated over their corresponding trans-isomers (Table II). Isomer differences were greatest for [9R-MP]Z, for which the concentration of the cis-isomer was 6- to 26-fold that of the trans-isomer. In two cases (14-DAPS embryos and 40-DAPS cotyledons) no (trans)[9R-MP]Z was detected.
The concentration of total CK was highest as cell division ended at 14 DAPS, ranging from 2.7 to 18.2 nmol g−1 dry weight, depending on the tissue. The highest concentrations of CK were measured in endospermic fluid and embryos. These two tissues were from 2 to 67 times more concentrated in CK than any other tissue at all sampling times. During rapid seed filling at 30 DAPS, the concentration of total CK had decreased considerably and continued to decline to a low of 0.3 nmol g−1 dry weight by the end of seed filling at 40 DAPS.
Comparison of CK among Cultivars
Very similar profiles of CK were measured among cotyledons of three different cultivars, with cis-isomers being predominant in cv Kaniva at 30 DAPS (cis-CK = 89% of total CK, trans-CK = 5%), cv Tyson at 30 DAPS (cis-CK = 81%, trans-CK = 1%), and cv ICCV88201 at 50 DAPS (cis-CK = 92%, trans-CK = 5%). The effect of water deficit on CK content was tested in seeds of cvs Kaniva and Tyson at 30 DAPS and the cultivars showed a similar response. The total CK content was markedly reduced by water stress, with water-stressed cotyledons of cvs Kaniva and Tyson containing 23% and 28%, respectively, of the levels observed in well-watered controls. Despite this reduction, the CK composition did not change greatly; the cis-CK concentration decreased slightly (cv Kaniva cis-CK = 63%; cv Tyson cis-CK = 75%), but the trans-CK concentration remained relatively constant (cv Kaniva trans-CK = 5%; cv Tyson trans-CK = 1%).
CK Profiles in Xylem Exudate
The total CK concentration was 114 pmol mL−1. The CK present were (cis)[9R-MP]Z (81%), (cis)[9R]Z (7%), (trans)[9R]Z(6%), and [9R]DHZ (6%). Proportions of CK present were thus similar to the profiles determined from seed tissue extracts.
RNase-Free Extractions
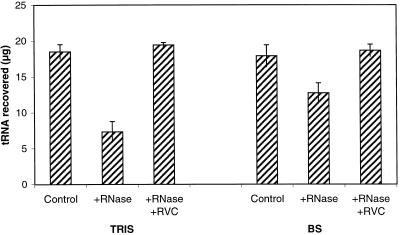
Levels of (cis)[9R]Z or (cis)Z and the ratios of cis-CK to trans-CK were not different between cotyledons extracted in Bieleski solvents and those extracted in Bieleski solvents with RNase inhibitors (20 mm RVC; Table III). Tested in vitro in Tris and Bieleski solvents RVC were effective for preventing the breakdown of tRNA in the presence of RNases (Fig. 4). Bieleski solvents without RVC were also moderately effective for tRNA protection.
Table III.
Concentration of CK in cotyledons of developing chickpea seeds (40 DAPS) extracted in Bieleski solvents alone or with RNase inhibitors
| Buffer Extraction | Bieleski Solvent Alone | Bieleski Solvent with RVC |
|---|---|---|
| pmol g−1 dry wt | ||
| cis-[9R]Z | 37 | 50 |
| trans-[9R]Z | nda | 2 |
| [9R]DHZ | 8 | 8 |
| cis-Z | 6 | 3 |
| trans-Z | 2 | 1 |
| DHZ | 9 | 12 |
| [9R]iP | nd | 6 |
| iP | 19 | 7 |
nd, Not detected.
Figure 4.
In vitro tRNA degradation assays determined in two buffer systems (Tris, Tris-HCl, pH 8.0; BS, Bieleski solvents). tRNA was incubated at 37°C for 30 min alone (Control) or in the presence of RNases with (+RNase) or without (+RVC) RNase inhibitors.
DISCUSSION
The results establish the presence of cis-CK {(cis)[9R-MP]Z, (cis)[9R]Z, and (cis)Z} in developing chickpea seeds and show that the significant levels of the cis-isomers found were not artifacts of extraction. (cis)[9R-MP]Z, (cis)[9R]Z, and, in some cases, (cis)Z, predominated, whereas the corresponding trans-isomers were detected as minor constituents. Identities of (cis)[9R]Z and (cis)Z were clearly established by comparison of ion levels and retention indices using GC-MS-SIM. In the case of (cis)[9R]Z, a full mass spectrum identical to an authentic standard was obtained. Likewise, (cis)[9R-MP]Z was identified by GC-MS-SIM following its hydrolysis to (cis)[9R]Z.
In previous studies reporting significant levels of cis-CK in potato seedlings (Mauk and Langille, 1978), unfertilized hop cones (Watanabe et al., 1982), rice roots and ears (Takagi et al., 1985), and etiolated squash seedlings (Kuraishi et al., 1987, 1991), the possibility that the cis-CK had resulted from hydrolysis of tRNA during extraction was not rigorously excluded (Tay et al., 1986). Although Kuraishi et al. (1987) used Bieleski solvents to reduce the potential for enzyme hydrolysis, they used prolonged extraction times lasting several days. In the present study cold Bieleski solvents were used and extraction times were minimized by replacing long soaking periods with short applications of sonication, as recommended by Hammerton et al. (1996).
In vitro assays of tRNA integrity in the presence of RNase showed that Bieleski solvents are capable of providing some protection for tRNA over longer periods, and at higher temperatures, than those routinely used in our tissue extractions. In one tissue extraction the tRNA was further protected with the addition of the RNase inhibitor RVC to the extraction buffers. However, the levels of cis-CK did not decrease, as would be expected if there was significant tRNA breakdown during extraction. The in vitro tRNA integrity assays indicated that, even when RNase was added, negligible tRNA breakdown occurred in the presence of RVC in Bieleski solvents. The Bieleski solvents alone provided substantial protection for tRNA in the presence of RNase, and it is unlikely that tRNA hydrolysis during extraction contributes to the pool of free CK detected. These considerations are not likely to apply to the recovery of cis-CK as the major forms in xylem exudate. Parker et al. (1989) suggested that the minor levels of cis-isomers in xylem exudate from cereals precluded extraction artifacts, and this is reinforced by the data for chickpea xylem.
Extraction artifacts other than tRNA breakdown are possible. For example, a cis-trans-isomerase similar to the one isolated from immature bean seeds (Bassil et al., 1993) could have changed the CK isomer ratio following disruption of tissue or cell compartmentation at extraction. In addition, nonenzymatic isomerization of Z or [9R]Z is known to occur in vitro in the presence of light (Bassil et al., 1993). These extraction artifacts are unlikely to have influenced our results. First, all extractions were carried out in cold Bieleski solvents to minimize enzymatic activity. Second, no isomerization of the internal standards (trans)[2H5]Z, (trans)[2H5][9R]Z, or (trans)[2H5][9R-MP]Z was observed in any of the chickpea extracts, even though the standards had been added before the plant tissues were disrupted with buffer. Third, extractions of immature lupin seed tissue under conditions identical to those of the present study yielded predominantly trans-CK, whereas the cis-CK were relatively low or, in some cases, undetectable (R.J.N. Emery, J.E. Barton, and C.A. Atkins, unpublished data).
Four studies have quantified CK in chickpea without reporting the occurrence of cis-isomers. Martin et al. (1987a, 1987b) separated CK from germinating seeds with TLC and used a bioassay that measured chlorophyll synthesis in cucumber cotyledons to identify CK. They would have been unlikely to detect cis-CK given the reported reduction or lack of biological activity of cis-isomers. Saha and Sircar (1996) quantified [9R]Z, DHZ, Z, [9R]iP, and iP in germinating seeds using HPLC and UV absorbance. Although [9R]Z was listed as the major CK, no distinction between the isomers was made. It was unclear whether their HPLC protocol could have resolved the isomers. Turnbull et al. (1997) reported (trans)[9R]Z and (trans)Z as among the major CK in lateral branch buds. However, the antibodies used in their immunoassay would have been unlikely to detect even quite substantial levels of cis-isomers. The polyclonal antibodies used show negligible cross-reactivity with cis-CK, except for those against [9R]DHZ, which cross-react with (cis)[9R]Z at about 5% (C.G.N. Turnbull, personal communication; Turnbull and Hanke, 1985; Parker et al., 1989). Other CK detected included iP, [9R]iP, [9R-MP]iP, [9R-MP]Z, and [9G]Z. In common with the present study, samples had low levels of dihydro-CK (DHZ, [9R-MP]DHZ, and [9R]DHZ).
The present study has shown that levels of total CK may vary markedly over seed development and with water supply, but the profile of individual CK remains relatively constant, with the cis-isomers, especially (cis)[9R]Z and (cis)[9R-MP]Z, predominating. It is interesting to note that, although cis-isomers could be detected in developing lupin seeds, they were minor rather than major constituents of the CK spectrum (R.J.N. Emery, J.E. Barton, and C.A. Atkins, unpublished data). Clearly, a more complete understanding of the role of CK in pod set and seed development in legumes will require an appreciation of the relative levels and bioactivity of both cis- and trans-isomers. It also appears that not all species will show the same relationship between the isomers, and it seems reasonable to suspect that they will also vary in their mechanistic significance.
The unique profile of CK in chickpea seeds raises two issues relating to CK biosynthesis and metabolism. The first is the source of these cis-isomers. The second is the level of bioactivity cis-isomers have in developing chickpea seeds.
With respect to the source of the cis-isomers, there appear to be a few possibilities. Xylem exudates collected from the root system contained predominantly cis-isoforms; therefore, deposition in pods as a result of transpiration could be one source. However, Zhang and Letham (1990) used estimates for xylem delivery alone to developing lupin seeds and calculated that xylem accounts for only minor delivery (about 1%) of the total CK. Furthermore, developing legume fruits are mainly phloem fed and it is possible that the assimilate stream also carries CK to the seeds. There is no information regarding CK content of phloem in chickpea; therefore, it is difficult to assess the overall significance of translocation. cis-CK could arise from a de novo synthetic pathway or as a result of tRNA turnover in situ (Prinsen et al., 1997), and either could be due to plant metabolism or to the activity of symbiotic bacteria colonizing the shoots (Holland, 1997). Current models of de novo CK biosynthesis (Binns, 1994; Jameson, 1994, figure 9–3) proceed from [9R-MP]iP to either [9R]iP or (trans)[9R-MP]Z, which are either not found or are present only as minor constituents of chickpea seeds. No metabolic pathway has been described that accounts for (cis)[9R]Z or (cis)[9R-MP]Z synthesis de novo. In plant systems that show major levels of cis-isomers (i.e. rice, squash, or chickpea) there may be a CK pathway involving initial production and subsequent modification of cis-CK nucleotides. Otherwise, considering Holland's (1997) hypothesis for bacterial CK synthesis, it is perhaps significant that in bacteria, which are symbionts of, or are otherwise associated with, plants (Morris et al., 1991; Upadhyaya et al., 1991; Holland and Polacco, 1994), cis-CK can be a significant component of the free CK pool. Should transfer to the tissues of the seed take place this might lead to an accumulation of cis-isomers.
The contribution of tRNA to the CK complement of plant tissues also remains to be resolved. Binns (1994) and Hall (1973) argued that tRNA turnover was not sufficiently rapid to account for the observed CK levels. Furthermore, unlike the vast majority of reported plant systems that contain trans-CK, the cis-isomers should predominate following CK release from tRNA (McGaw and Burch, 1995; Prinsen et al., 1997). Clearly, tissues such as those of developing chickpea seeds, which accumulate cis-CK, challenge this idea. The demonstration of an isomerase that would interconvert the isomers (Bassil et al., 1993) also indicates that the cis-configuration may not be an impediment to a tRNA source. In the case of chickpea seeds it might be argued that the level of isomerase is very low.
The second issue relates to the bioactivity of the cis-isomers in tissues such as those of chickpea seeds in which CK have always been regarded as establishing a strong “sink” to attract assimilates. It has been proposed that CK controls seed size by influencing cell number in very young, developing seeds. Increased cell number would enhance storage capacity (Morris et al., 1993; Brenner and Cheikh, 1995). Studies of corn, wheat, and rice (Morris et al., 1993) show that CK content is highest during developmental stages that encompass periods of the most rapid nuclear and cell division of the endosperm. CK levels are greatest at the early endospermic fluid stages of seed growth in lupin (Davey and van Staden, 1978). Our data demonstrate that CK levels in chickpea are also greatest over phases of rapid cell division. However, current thinking presumes that the CK activity is still low, since the increase is predominantly in cis-isomers. Nonetheless, whereas bioassays used in earlier studies found that the cis-isomers were relatively inactive compared with the trans forms of CK, none of them was based on activity measurement with chickpea tissues (Kaminek, 1982). The possibility that cis forms are active in species such as chickpea and trans-isomers are active in other species such as lupins and soybeans, cannot be ignored. Furthermore, manipulation of endogenous CK-isomer ratios in chickpea and other pulses may offer a means to examine the stereochemical-activity relationships of CK and determine what potential may exist to improve the sink strength of growing seeds. Genetic manipulation of enzymes of CK metabolism, especially of the cis-trans-isomerase, offers a means to explore both questions.
Abbreviations:
- CK
cytokinin(s)
- DHZ
dihydrozeatin
- [9R]DHZ
dihydrozeatin riboside
- [9R-MP]DHZ
dihydrozeatin nucleotide
- DAPS
days after pod set
- DAS
days after sowing
- iP
isopentenyl-adenine
- [9R]iP
isopentenyl-adenosine
- [9R-MP]iP
isopentenyl-adenine nucleotide
- KRI
Kovat's retention index
- m/z
mass to charge ratio
- RVC
RNase vanadyl complexes
- SCX
strong cation-exchange solid-phase extraction column
- SIM
selected ion monitoring
- Z
zeatin
- [9R]Z
zeatin riboside
- [9R-MP]Z
zeatin nucleotide
Footnotes
This research was funded by the Australian Cooperative Research Centre for Legumes in Mediterranean Agriculture and the Grains Research and Development Corporation of Australia.
LITERATURE CITED
- Bassil NV, Mok DWS, Mok MC. Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiol. 1993;102:867–872. doi: 10.1104/pp.102.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski RL. The problem of halting enzyme action when extracting plant tissues. Anal Biochem. 1964;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- Binns AN. Cytokinin accumulation and action: biochemical, genetic, and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:173–196. [Google Scholar]
- Brenner ML, Cheikh N. The role of hormones in photosynthate partitioning and seed filling. In: Davies PJ, editor. Plant Hormones, Physiology, Biochemistry, and Molecular Biology, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 649–670. [Google Scholar]
- Davey J, van Staden J. Cytokinin activity in Lupinus albus. III. Distribution in fruits. Physiol Plant. 1978;43:87–93. doi: 10.1104/pp.63.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RJN, Leport L, Atkins CA, Turner NC. cis-Isomers of cytokinins predominate in developing seeds of Cicer arietinum (abstract no. 179) Plant Physiol. 1997;114:S-53. [Google Scholar]
- Emery RJN, Longnecker NE, Atkins CA. Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J Exp Bot. 1998;49:555–562. [Google Scholar]
- Gaskin P, Macmillan J (1991) GC-MS of the Gibberellins and Related Compounds: Methodology and a Library of Spectra. University of Bristol (Cantocks Enterprises), Bristol, UK
- Hall RH. Cytokinin as a probe of developmental processes. Annu Rev Plant Physiol. 1973;24:425–444. [Google Scholar]
- Holland MA. Occam's razor applied to hormonology. Are cytokinins produced by plants? Plant Physiol. 1997;115:865–868. doi: 10.1104/pp.115.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland MA, Polacco JC. PPFMs and other covert contaminants: is there more to plant physiology than just plant? Annu Rev Plant Physiol Plant Mol Biol. 1994;45:197–209. [Google Scholar]
- Hammerton RD, Nicander B, Tillberg E. Identification of some major cytokinins in Phaseolus vulgaris and their distribution. Physiol Plant. 1996;96:77–94. [Google Scholar]
- Jameson PE. Cytokinin metabolism and compartmentation. In: Mok DS, Mok MC, editors. Cytokinins: Chemistry, Activity and Function. Boca Raton, FL: CRC Press; 1994. pp. 113–128. [Google Scholar]
- Kaminek M (1982) Mechanisms preventing the interference of tRNA cytokinins in hormonal regulation. In PF Wareing, ed, Plant Growth Substances 1982. Academic Press, New York, pp 215–224
- Korszun ZR, Knight C, Chen C-M. A stereochemical model for cytokinin activity. FEBS Lett. 1989;243:53–56. [Google Scholar]
- Kuraishi S, Ohara T, Sakuri N. Gas chromatography thermionic detector analysis of cytokinins in etiolated squash seedlings using 14C-BA as an internal standard. Plant Cell Physiol. 1987;28:1033–1041. [Google Scholar]
- Kuraishi S, Tasaki K, Sakuri N, Sadatoku K. Changes in levels of cytokinins in etiolated squash seedlings after illumination. Plant Cell Physiol. 1991;32:585–591. [Google Scholar]
- Letham DS. Zeatin, a factor inducing cell division from Zea mays. Life Sci. 1963;8:569–573. doi: 10.1016/0024-3205(63)90108-5. [DOI] [PubMed] [Google Scholar]
- Letham DS, Palni LMS. The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol. 1983;34:163–197. [Google Scholar]
- Martin L, Diez A, Nicolas G, Legaz ME, Villalobos N. Cytokinins in chick-pea seeds. Identification and transformation during germination and seedling growth. J Plant Physiol. 1987a;128:133–140. [Google Scholar]
- Martin L, Diez A, Nicolas G, Villalobos N. Variation of the levels and transport of cytokinins during germination of chick-pea seeds. J Plant Physiol. 1987b;128:141–151. [Google Scholar]
- Mauk CS, Langille AR. Physiology of tuberization in Solanum tuberosum L. Plant Physiol. 1978;62:438–442. doi: 10.1104/pp.62.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw BA, Burch LR. Cytokinin biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones, Physiology, Biochemistry, and Molecular Biology, Ed 2. Dordrecht, The Netherlands: Kluwer Academic; 1995. pp. 98–117. [Google Scholar]
- Miller CO. A kinetin-like compound in maize. Proc Natl Acad Sci USA. 1961;47:170–174. doi: 10.1073/pnas.47.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RO, Blevins DG, Dietrich JT, Durley RC, Gelvin SB, Gray J, Hommes NG, Kaminek M, Mathews LJ, Meilan R and others. Cytokinins in plant pathogenic bacteria and developing cereal grains. Aust J Plant Physiol. 1993;20:621–637. [Google Scholar]
- Morris RO, Jameson PE, Laloue M, Morris JW. Rapid identification of cytokinins by an immunological method. Plant Physiol. 1991;95:1156–1161. doi: 10.1104/pp.95.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicander B, Bjorkman P-O, Tillberg E. Identification of an N-glucoside of cis-zeatin from potato tuber sprouts. Plant Physiol. 1995;109:513–516. doi: 10.1104/pp.109.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CW, Badenoch-Jones J, Letham DS. Radioimmunoassay for quantifying the cytokinins cis-zeatin and cis-zeatin riboside and its application to xylem sap samples. J Plant Growth Regul. 1989;8:93–105. [Google Scholar]
- Prinsen E, Kaminek M, van Onckelen HA. Cytokinin biosynthesis: a black box? J Plant Growth Regul. 1997;23:3–15. [Google Scholar]
- Saha S, Sircar PK. Cytokinin profile during early phase of germination of gram seeds after application of benzylaminopurine. Biol Plant. 1996;38:293–296. [Google Scholar]
- Takagi M, Yokota T, Murofushi N, Ota Y, Takahashi N. Fluctuation of endogenous cytokinin contents in rice during its life cycle-quantification of cytokinins by selected ion monitoring using deuterium-labeled internal standards. Agric Biol Chem. 1985;49:3271–3277. [Google Scholar]
- Tay SAB, MacLeod J, Palni LMS. On the reported occurrence of cis-zeatin riboside as a free cytokinin in tobacco shoots. Plant Sci. 1986;43:131–134. [Google Scholar]
- Turnbull CGN, Hanke DE. The control of bud dormancy in potato tubers. Measurement of the seasonal pattern of changing concentrations of zeatin-cytokinins. Planta. 1985;165:366–376. doi: 10.1007/BF00392234. [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Raymond MAA, Dodd IC, Morris SE. Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta. 1997;202:271–276. [Google Scholar]
- Upadhyaya NM, Parker CW, Letham DS, Scott KF, Dart PJ. Evidence for cytokinin involvement in Rhizobium (IC3342)-induced leaf curl syndrome of pigeonpea (Cajanus cajan Millsp.) Plant Physiol. 1991;95:1019–1025. doi: 10.1104/pp.95.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden J, Davey J, Brown NAC (1982) Endogenous cytokinins in seed development and germination. In AA Khan, ed, The Physiology and Biochemistry of Seed Development, Dormancy and Germination. Elsevier Biomedical Press, Amsterdam, The Netherlands, pp 137–156
- Watanabe N, Yokota T, Takahashi N. Transfer RNA, a possible supplier of free cytokinins, ribosyl-cis-zeatin and ribosyl-2-methylthiozeatin: quantitative comparison between free and transfer cytokinins in various tissues of the hop plant. Plant Cell Physiol. 1982;23:479–488. [Google Scholar]
- Zhang R, Letham DS. Cytokinin translocation and metabolism in lupin species. III. Translocation of xylem cytokinin into the seeds of lateral shoots of Lupinus angustifolius. Plant Sci. 1990;70:65–71. [Google Scholar]