Abstract
Mutations that cause defects in levels of the signaling lipid phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] lead to profound neurodegeneration in mice. Moreover, mutations in human FIG4 predicted to lower PI(3,5)P2 levels underlie Charcot–Marie–Tooth type 4J neuropathy and are present in selected cases of amyotrophic lateral sclerosis. In yeast and mammals, PI(3,5)P2 is generated by a protein complex that includes the lipid kinase Fab1/Pikfyve, the scaffolding protein Vac14, and the lipid phosphatase Fig4. Fibroblasts cultured from Vac14−/− and Fig4−/− mouse mutants have a 50% reduction in the levels of PI(3,5)P2, suggesting that there may be PIKfyve-independent pathways that generate this lipid. Here, we characterize a Pikfyve gene-trap mouse (Pikfyveβ-geo/β-geo), a hypomorph with ∼10% of the normal level of Pikfyve protein. shRNA silencing of the residual Pikfyve transcript in fibroblasts demonstrated that Pikfyve is required to generate all of the PI(3,5)P2 pool. Surprisingly, Pikfyve also is responsible for nearly all of the phosphatidylinositol-5-phosphate (PI5P) pool. We show that PI5P is generated directly from PI(3,5)P2, likely via 3′-phosphatase activity. Analysis of tissues from the Pikfyveβ-geo/β-geo mouse mutants reveals that Pikfyve is critical in neural tissues, heart, lung, kidney, thymus, and spleen. Thus, PI(3,5)P2 and PI5P have major roles in multiple organs. Understanding the regulation of these lipids may provide insights into therapies for multiple diseases.
Keywords: phosphoinositide, spongiform
Phosphorylated phosphatidylinositol lipids (PPI) function as signaling molecules. Mammalian cells phosphorylate PI on three available hydroxyl groups, in all combinations, giving rise to seven lipids. Each PPI regulates multiple pathways through specific downstream effectors. The localization of lipid kinases and phosphatases dictate the organelles where each lipid species is generated.
In the yeast Saccharomyces cerevisiae, only four of the seven PPIs are made, and the initial elucidation of some of the pathways required to synthesize and catabolize these lipids was determined in yeast. The yeast PI3P 5-kinase, Fab1, converts PI3P to phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] (1, 2). Generation of PI(3,5)P2 requires a protein complex composed of Fab1, the scaffolding protein Vac14 (3–5), the PI(3,5)P2 5-phosphatase Fig4 (6, 7), a Fab1 activator, Vac7 (8, 9), and a negative regulator, Atg18 (10, 11). Although Fig4 is a lipid phosphatase, its presence also is required for Fab1 activity (6, 12). Thus, the absence of Vac14 or Fig4 results in a similar defect, reduced PI(3,5)P2.
All homologs of Fab1 [Pikfyve in mammals (13)], contain a FYVE domain predicted to bind PI3P (14). Mammalian Pikfyve, Vac14, and Fig4 form a complex (5, 15).
Yeast Fab1 and its regulators are localized on the vacuole (lysosome) and on late endosomes (1–6, 8, 9, 12), localizations that are consistent with the phenotypes associated with the loss of Fab1, including defects in retrograde traffic from vacuoles (10), vacuole membrane fission (6), and vacuole acidification (3). Similarly, in mammalian cells, PIKfyve colocalizes with late endosomes and lysosomes (16, 17) and with early endosomes (17–19). Defects observed when Pikfyve function is compromised include the formation of large vacuoles that contain LAMP1 and LAMP2 (19–25), defects in retrograde traffic from early endosomes to the trans-Golgi network (19), defects in EGF receptor degradation (22), defects in autophagy (22, 26, 27), and dysregulation of TRPML1 (28).
Mouse models with mutations in Vac14 and Fig4 genes exhibit profound neurodegeneration (5, 21, 29). Moreover, Pikfyve plays critical roles in early development. A whole-body knockout of Pikfyve causes preimplantation lethality (30).
Here we characterize Pikfyveβ-geo/β-geo, a gene-trap mouse mutant. Pikfyveβ-geo/β-geo, a hypomorph, generates about 10% of the wild-type levels of Pikfyve protein. In fibroblasts, shRNA silencing of the residual Pikfyve transcript revealed that the entire PI(3,5)P2 pool is generated by Pikfyve. Moreover, most of the PI5P pool depends on Pikfyve. Furthermore, characterization of the Pikfyveβ-geo/β-geo mouse revealed additional phenotypes caused by defects in Pikfyve: In addition to neurodegeneration, the Pikfyveβ-geo/β-geo mice exhibit profound defects in the heart, lung, kidney, spleen, and thymus.
Results
Characterization of a Pikfyveβ-geo/β-geo Hypomorphic Mouse.
Mice were generated from embryonic stem cells with a gene-trap insertion in Pikfyve. The insertion site in intron 17 is upstream of the kinase domain and within the CCT domain (Fig. 1A). The predicted chimeric protein contains the first 765 amino acids of Pikfyve fused to β-galactosidase-neomycin phosphotransferase II (β-Geo) and is the same size as wild-type Pikfyve.
Fig. 1.
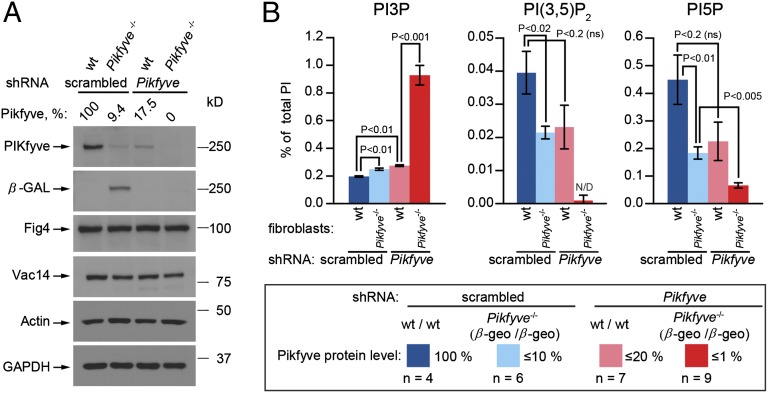
The Pikfyveβ-geo/β-geo mutant has low levels of Pikfyve protein. (A) Schematic of the Pikfyve coding region indicating position of the β-Geo insert, the 5′ and 3′ regions with primers spanning exons 2 and 3 and exons 41 and 42 used for quantitative RT-PCR (green arrows), the shRNA target site (red arrow), and peptide (asterisk) used to produce the mouse monoclonal antibody. (B) Western blots show that Pikfyveβ-geo/β-geo fibroblasts have reduced Pikfyve protein but no difference in Vac14 and Fig4 protein levels.
Offspring from intercrosses of heterozygous mice produced pups at the expected Mendelian ratio of 1:2:1 (Fig. S1A). At birth, homozygous mutants were about 50% smaller than wild-type and heterozygote littermates (Fig. S1 B and C). Most died within a few days of birth. A few Pikfyveβ-geo/β-geo mutants survived to 2 wk of age (Fig. S1A).
To test the efficacy of the gene trap, Pikfyve mRNA levels were determined in fibroblasts from postnatal day 0 (P0) pups (Fig. 1A and Fig. S1D). Quantitative real-time PCR of exons 2 and 3 did not detect significant differences in transcript levels of Pikfyve in wild-type, heterozygous, and homozygous fibroblasts, indicating that the chimeric and wild-type transcripts are equally stable. Differences were observed for exons 41 and 42, which are present in the wild-type allele only. In heterozygous mice, the levels of transcript containing exons 41 and 42 was ∼50% of wild-type. Notably, ∼15% of full-length Pikfyve transcripts were still present in homozygous Pikfyveβ-geo/β-geo fibroblasts.
To distinguish the gene-trap chimeric protein from wild-type Pikfyve, we generated a monoclonal antibody to residues 1793–1995 within the kinase domain (Fig. 1A, asterisk). The level of Pikfyve in Pikfyveβ-geo/βgeo fibroblasts was reduced, but Vac14 and Fig4 were present at normal levels (Fig. 1B). This result contrasts with that in Vac14−/− fibroblasts, which have no Vac14 and have reduced Fig4 (Fig. 1B and ref. 31). Western blots of a dilution series of cell lysates indicated that the Pikfyveβ-geo/β-geo and Pikfyveβ-geo/+ mutant fibroblasts expressed about 10% (Fig. S1E), and 50% (Fig. S1F), of the wild-type levels of Pikfyve, respectively.
Heterozygous Pikfyveβ-geo/+ mice have no apparent defects, suggesting that the Pikfyveβ-geo allele does not have a dominant negative effect in vivo. Moreover, this allele does not interact with Vac14. In wild-type lysates, immunoprecipitation of Pikfyve, using an amino-terminal Pikfyve antibody, coprecipitates Vac14 and Fig4. Conversely, immunoprecipitation of Vac14 coprecipitates Pikfyve and Fig4 (Fig. S1G). In lysates from Pikfyveβ-geo/β-geo fibroblasts, immunoprecipitation with the amino terminal Pikfyve antibody immunoprecipitated both the Pikfyveβ-geo/β-geo chimera and residual full-length Pikfyve; however, little Vac14 or Fig4 coprecipitated. This small amount is likely caused by a pull-down of residual full-length Pikfyve. Consistent with this postulate, immunoprecipitation of Vac14 from Pikfyveβ-geo/β-geo fibroblasts coprecipitated Fig4 and full-length Pikfyve but not the chimeric protein (Fig. S1G). Thus, the Pikfyveβ-geo/β-geo chimera does not integrate into the Vac14 complex. Furthermore, depletion of Pikfyve does not interfere with the ability of Vac14 and Fig4 to form a complex.
Pikfyve Protein Is Not Depleted Uniformly in Tissues of the Pikfyveβ-geo/β-geo Mouse.
We determined relative levels of Pikfyve, Vac14, and Fig4 in multiple tissues from wild-type P0 pups. The levels of Pikfyve, Vac14, and Fig4 were highest in the brain, twofold higher than in other tissues (Fig. S2A). In Pikfyveβ-geo/β-geo P0 pups, Pikfyve was reduced dramatically in all tissues, by 65–90%, although the extent of Pikfyve reduction was not uniform (Fig. S2 B and C). There was much less change in the levels of Vac14 or Fig4 in the tissues of Pikfyveβ-geo/β-geo mice.
Neonatal Pikfyveβ-geo/β-geo Mice Display Modest Defects in the Nervous System.
Pikfyveβ-geo/β-geo P0 mice died perinatally; however the general cytoarchitecture of the brains was unexpectedly normal. There was no obvious vacuolation in most brain regions. One exception was the caudal cortical subventricular neuroepithelium, a region of rapidly dividing cells, where there was modest vacuolation (Fig. S3A). This spongiform appearance was present in all four Pikfyveβ-geo/β-geo brains examined but in none of the four Pikfyve+/+ brains. There were vacuoles in the dorsal root ganglia of the four Pikfyveβ-geo/β-geo pups; the surrounding tissue appeared normal (Fig. S3B).
To test the potential of neurons from the brain to form vacuoles, we generated primary neuronal cultures from the hippocampus of Pikfyveβ-geo/β-geo and wild-type P0 mice. Many of the neurons cultured from Pikfyveβ-geo/β-geo mice formed vacuoles, but neurons from wild-type mice did not (Fig. S3C). These results demonstrate that neuronal tissues from the Pikfyveβ-geo/β-geo mouse have the potential to become vacuolated when cultured ex vivo.
Pikfyveβ-geo/β-geo Mutants that Survive Beyond 2 Wk Develop Extensive Neurodegeneration.
A small number of Pikfyveβ-geo/β-geo mutant mice survived for 16–19 d (Fig. S1A). In studies of the survivors, Pikfyve+/β-geo heterozygous littermates served as controls; there are no differences in appearance or behavior of wild-type and Pikfyve+/β-geo heterozygous mice.
At P16 mutant Pikfyveβ-geo/β-geo pups had half the body weight (Fig. S1A) and 30% the brain weight of heterozygous littermates (Fig. S4 B and C). The three homozygous Pikfyveβ-geo/β-geo mice that survived for 18–19 d also displayed severely impaired mobility (Movie S1). At 16 d, the Pikfyveβ-geo/β-geo pups had prominent vacuolation in the deep cerebellar nuclei and in the brainstem, particularly the midbrain and pons (Fig. S4D). There also was vacuolation in the spinal cord (Fig. S4E). Brain and spinal cord from heterozygous littermates showed no evidence of degeneration. Most Pikfyveβ-geo/β-geo mice died around P0, but in the surviving mutants, defects in the nervous system become more prominent later, raising the likelihood of serious defects in other organs.
Neonatal Pikfyveβ-geo/β-geo Mice Exhibit Defects in Several Organs.
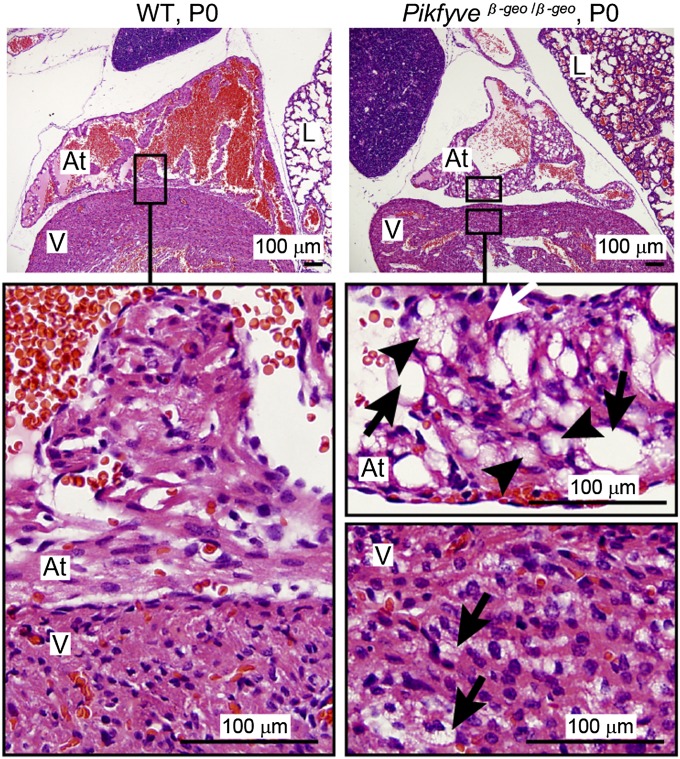
The hearts of Pikfyveβ-geo/β-geo mice were severely affected. Compared with wild-type littermates, the Pikfyveβ-geo/β-geo hearts had a reduced density of atrial myocytes, consistent with reduced myofibrillar content. In addition, large translucent vacuoles were present (Fig. 2). The lungs of the Pikfyveβ-geo/β-geo mutants also were abnormal, with an increase in the ratio of cellularity in the alveolar region and decreased airspace in the lung (Fig. 2 and Fig. S5A).
Fig. 2.
Spongiform degeneration of the neonatal Pikfyveβ-geo/β-geo mouse heart. Sagittal sections of neonatal wild-type (WT) and Pikfyveβ-geo/β-geo mice stained with H&E. The atria (At), ventricles (V), and lung (L) of wild-type littermates exhibit normal morphology. At higher magnification, atrial and ventricular myocytes strongly stain with H&E. There is no apparent vacuolation. Pikfyveβ-geo/β-geo mice have reduced H&E staining of atrial myocytes consistent with reduced myofibrillar content. In the Pikfyveβ-geo/β-geo mutants, both atria and ventricles have vacuoles (arrows), with a high level of vacuolation in the atria. Smaller vacuoles (arrowheads) give the myocytes [identified by heavily stained myofibrils (white arrow)] a foamy appearance.
We analyzed four P0 Vac14−/− (Fig. S5B) and three E18.5 Fig4−/− mice (Fig. S5C). The hearts from the Vac14−/− and Fig4−/− mutants had significant degeneration of the atria marked by the presence of large, transparent vacuoles. These data strongly suggest that defects in the levels of PI(3,5)P2 and PI5P lead to degeneration of the heart, particularly the atria.
The hearts from 2-wk-old Pikfyveβ-geo/β-geo mutants were smaller than those of their heterozygous littermates (Fig. S5D). The right atrium and superior vena cava were enlarged, but cardiac structure was normal otherwise. There was extensive vacuolation of the atrial myocytes. Smaller vacuoles also were noted in ventricular myocytes (Fig. S5E).
To determine if these histologic abnormalities would result in myocardial dysfunction, echocardiograms were performed on four pups that survived beyond 2 wk. The hearts were significantly smaller, resulting in a decreased calculated left ventricular (LV) mass (Fig. S5F). Because of the marked difference between the size of the Pikfyveβ-geo/β-geo mutants and their Pikfyve+/β-geo littermates, the calculated LV mass was corrected for calculated body surface area. After correction, no detectable difference in LV size or LV mass was noted (Fig. S5F). However, there were differences in right ventricular (RV) size and function. When corrected for differences in body surface area, the RV end diastolic dimension was significantly larger in the Pikfyveβ-geo/β-geo mutants than in their Pikfyve+/β-geo littermates (Fig. S5F), as is consistent with RV dilatation.
These defects suggested a primary pulmonary pathology. Indeed, the lungs had marked abnormalities that might contribute to the early demise of the Pikfyveβ-geo/β-geo mutants (Fig. S5G). There was increased cellularity with reduced alveolar cross-sectional area and apparent pulmonary vascular congestion. In addition, there were areas lacking alveolar development. These findings suggest a primary role for Pikfyve in lung development.
Analysis of other organs revealed that the thymus and spleens from the P16 Pikfyveβ-geo/β-geo mutants were smaller and had less cellular density than the organs from wild-type littermates and contained numerous small vacuoles (Fig. S5 D and H and Fig. S6A). Kidneys from Pikfyveβ-geo/β-geo mutants exhibited defects at P0, and the defects were worse in older animals. At P0 the kidneys contained multiple small vacuoles in renal tubules, but the overall structure of the glomeruli was unaffected (Fig. S6B). At P19 there was a progression in the kidney degeneration in Pikfyveβ-geo/β-geo homozygotes (Fig. S6C), with some tubular cells filled with vacuoles and some apparent loss of tubules.
Pikfyve Generates All of the Cellular PI(3,5)P2.
The dramatic reduction of Pikfyve protein in the Pikfyveβ-geo/β-geo mutant mice (Figs. S1E and S2 B and C) suggested that the mutants would be largely depleted of PI(3,5)P2. We examined steady-state levels of PI(3,5)P2 in heterozygous and homozygous Pikfyveβ-geo/β-geo fibroblasts, where the levels of Pikfyve are about 50% and 10% of wild-type levels, respectively (Fig. S1 F and E). Heterozygous Pikfyve+/β-geo fibroblasts had wild-type levels of PI(3,5)P2 (Fig. S7A). Pikfyveβ-geo/β-geo fibroblasts had a 50% reduction in PI(3,5)P2, with a corresponding 40% rise in the precursor, PI3P. These findings indicate that Pikfyve has a role in the generation of PI(3,5)P2 from PI3P. Note that these data provide information about PI(3,5)P2 levels only in fibroblasts. To measure the effects of these mutations on PPI levels in specific tissues, new techniques will need to be developed.
Notably, although PI(3,5)P2 levels were normal in heterozygous Pikfyve+/β-geo fibroblasts, there was a 30% reduction of PI5P (Fig. S7A). Moreover, in homozygous Pikfyveβ-geo/β-geo fibroblasts there was an additional reduction of PI5P to 50% of wild-type levels, which is comparable to the reduction of PI(3,5)P2 in the same cells. These observations suggest that Pikfyve also plays a major role in the generation of PI5P. There were no detectable differences in the levels of PI4P or PI(4,5)P2 (Fig. S7A).
To test further the requirement for Pikfyve in the generation of PI(3,5)P2, we depleted the remaining Pikfyve protein in wild-type and Pikfyveβ-geo/β-geo fibroblasts by culture for 4 d in the presence of shRNA (Fig. S7B). This depletion resulted in the formation of vacuoles (Fig. S7C). In the Pikfyveβ-geo/β-geo fibroblasts, vacuoles were present before shRNA silencing. After silencing, the number and size of the vacuoles increased. shRNA knockdown in wild-type fibroblasts resulted in a 85% reduction in Pikfyve protein, whereas shRNA knockdown in Pikfyveβ-geo/β-geo fibroblasts caused a complete loss of detectable protein (Fig. 3A). In Pikfyveβ-geo/β-geo fibroblasts, there also was a loss of the β-gal gene-trap chimeric protein. There was no detectable reduction in Vac14 or Fig4.
Fig. 3.
Generation of PI(3,5)P2 and most of the PI5P requires Pikfyve. Lentiviral expression of Pikfyve shRNA for 4 d. Scrambled sequence was used as a negative control. (A) After shRNA, Pikfyve protein levels in the wild-type and Pikfyveβ-geo/β-geo mouse fibroblasts were decreased or were below detectable levels, respectively. (B) Depletion of Pikfyve in Pikfyveβ-geo/β-geo fibroblasts resulted in a fivefold elevation of PI3P, a loss of 85% of PI5P, and nearly complete loss of PI(3,5)P2.
Depletion of Pikfyve by shRNA in Pikfyveβ-geo/β-geo cells resulted in a fivefold elevation of PI3P and total loss of PI(3,5)P2 (Fig. 3B); no peak was detected at the position of PI(3,5)P2 (Fig. S8A). Summation of the 2-min elution region that includes these products demonstrated that PI(3,5)P2 in wild-type cells comprised 0.040 ± 0.006% of total phosphoinositide lipids, whereas in the mutant cells PI(3,5)P2 was not detectable (0.001 ± 0.002%). These findings show that the total cellular pool of PI(3,5)P2 is generated via the Pikfyve pathway and that Pikfyve is the source of PI(3,5)P2 in mammalian cells.
Most of the PI5P Pool Is Generated from PI(3,5)P2.
shRNA treatment of Pikfyveβ-geo/β-geo fibroblasts resulted in a loss of 85% of PI5P (Fig. 3B and Fig. S8B). These findings provide strong support for the hypothesis that Pikfyve is required to generate most of the PI5P pool. Moreover, Vac14- and Fig4-deficient cells also have a 50% (21) and 55% (Fig. S8C) reduction in PI5P, respectively. The small amount of PI5P detectable after shRNA treatment leaves open the possibility that a minor portion of the PI5P pool is generated via Pikfyve-independent pathways.
In addition to effects on PI5P, there was an ∼30% reduction in PI(4,5)P2 after shRNA treatment of Pikfyveβ-geo/β-geo fibroblasts (Fig. S7D). This finding raises the possibility that the Pikfyve-dependent PI5P pool contributes to the generation of PI(4,5)P2.
Note that nearly complete reduction of Pikfyve protein is necessary for substantial changes in PI(3,5)P2 levels (Fig. S7E). Because Pikfyve is an enzyme, only a catalytic amount of Pikfyve is required to act on a large pool of substrate. For example, shRNA knockdown of Pikfyve to 17% of its normal levels in wild-type cells reduces the level of PI(3,5)P2 by only 40%. Thus, unless Pikfyve is lowered to nondetectable levels, significant amounts of PI(3,5)P2 and PI5P will remain.
The requirement for Pikfyve for normal steady-state levels of PI5P levels could be a direct effect of Pikfyve catalyzing the generation of PI5P from phosphatidylinositol or could occur indirectly through enzymes with 3′-phosphatase activity acting on PI(3,5)P2, or through a combination of the two. We designed three approaches to distinguish between these models.
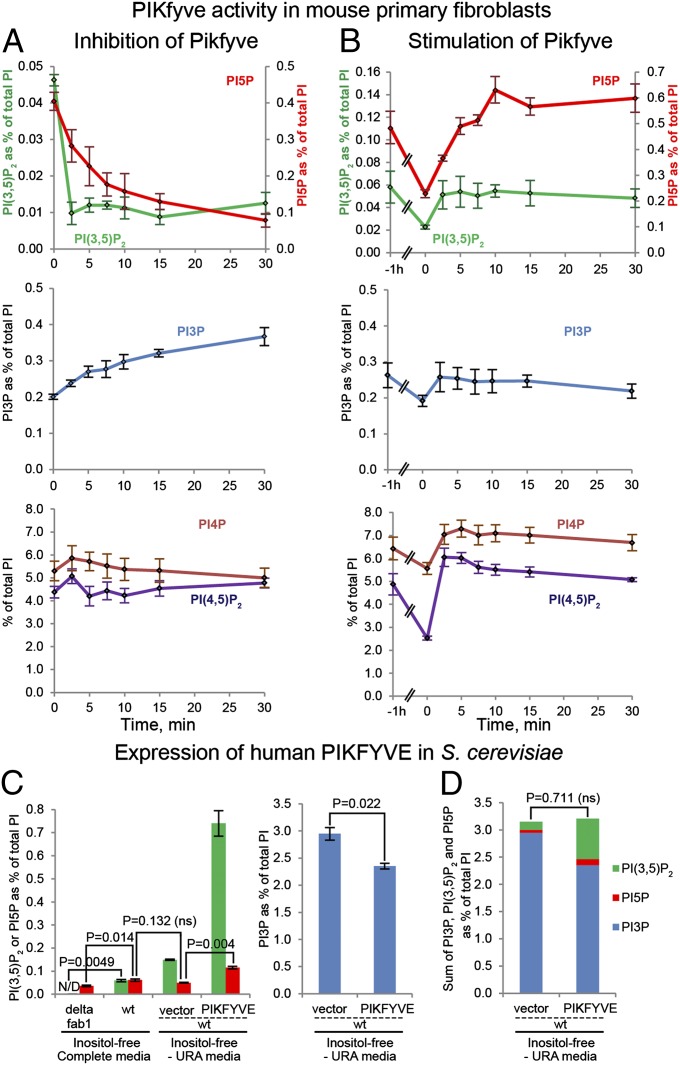
Pikfyve was inhibited acutely in wild-type fibroblasts using the catalytic Pikfyve inhibitor, YM201636 (23). After treatment, PI(3,5)P2 levels were reduced fully at the first time point assessed, 2.5 min. There was a slower, but still rapid, reduction in PI5P, with a half-life of ∼4.5 min (Fig. 4A). This reduction was accompanied by elevation of PI3P during the 30-min treatment. No significant changes were detected for PI4P or PI(4,5)P2 (Fig. 4A).
Fig. 4.
Perturbation of Pikfyve activity suggests that the PI5P derives from PI(3,5)P2. (A) Inhibition of Pikfyve in mouse primary fibroblasts by 1.6 μM YM201636 for the times specified results in a rapid depletion of PI(3,5)P2 and PI5P (n = 4). (B) Stimulation of mouse fibroblasts by refeeding with normal growth medium, insulin, and transferrin following 1-h starvation in HBSS with Mg2+ and Ca2+ reveals that within 2.5 min all lipids, with the exception of PI5P, increased and plateaued at a new steady-state level. PI5P plateaued at its new steady-state level at 10 min (n = 3). (C) Expression of human PIKFYVE in wild-type yeast resulted in a large elevation in PI(3,5)P2, a small increase in PI5P, and a corresponding reduction in PI3P. (D) The sum of PI3P, PI(3,5)P2, and PI5P was the same in wild-type yeast with PIKFYVE overexpression and with vector control (n = 3). This result suggests that PI5P levels are dependent on PI3P via dephosphorylation of PI(3,5)P2.
Starvation, followed by nutrient refeeding, stimulates Pikfyve activity as measured by an increase in PI(3,5)P2 (32). We tested the effects of nutrient refeeding on the pools of PI5P and PI(3,5)P2. Fibroblasts were starved by 1-h incubation in HBSS buffer, causing an acute decrease in all phosphoinositide lipids; PI(3,5)P2 and PI5P were reduced ∼50% (Fig. 4B). Upon readdition of complete medium, PI(3,5)P2 plateaued at prestarvation levels within 2.5 min. In contrast, PI5P returned to prestarvation levels at 5 min and continued to rise for another 5 min. This time lag is consistent with a precursor/product relationship between PI(3,5)P2 and PI5P. PI3P, PI4P, and PI(4,5)P2 returned to prestarvation levels during the first 2.5 min of refeeding (Fig. 4B).
To test further whether most of the PI5P pool in fibroblasts is derived via Pikfyve generation of PI5P from phosphatidylinositol (PI), we tested whether human PIKFYVE can synthesize PI5P directly in S. cerevisiae, which makes little to no PI5P (1, 33). In wild-type yeast there was a small peak in the region where PI5P should elute, which was reduced 1.7-fold in a fab1Δ mutant (Fig. 4C and Fig. S8E). Overexpression human PIKFYVE cDNA in wild-type yeast caused a fivefold elevation in the levels of PI(3,5)P2, demonstrating enzymatic activity of human PIKFYVE (Fig. 4C and Fig. S8D). However, there was only a small increase in PI5P (Fig. 4C and Fig. S8E). The PIKFYVE-dependent pool of PI5P was sixfold less than the PIKFYVE-dependent PI(3,5)P2 pool. There was a corresponding reduction in PI3P levels (Fig. 4C). Moreover, the sum of new PI5P plus PI(3,5)P2 was equal to the total decrease in PI3P (Fig. 4D). There were no alterations in the levels of PI4P or PI(4,5)P2 (Fig. S8F). Together, these studies fit best with the hypothesis that PI5P pools in fibroblasts are regulated indirectly by Pikfyve via the conversion of PI(3,5)P2 to PI5P.
Discussion
These studies revealed that Pikfyve is responsible for all of the intracellular PI(3,5)P2 pool. While this hypothesis was previously assumed, observations that Vac14−/− and Fig4−/− fibroblasts have half the normal levels of PI(3,5)P2 raised the possibility that other pathways contribute to PI(3,5)P2 pools. However, after knockdown of residual Pikfyve protein in Pikfyveβ-geo/β-geo fibroblasts, no PI(3,5)P2 was detected.
Pikfyve is indirectly responsible for most of the PI5P pool. In Vac14−/−, Pikfyveβ-geo/β-geo, or Fig4−/− fibroblasts, PI5P levels are reduced ∼50% (Figs. S7A and S8C) (21). When Pikfyve is further reduced by shRNA knockdown in Pikfyveβ-geo/β-geo cells, only 15% of wild-type PI5P levels remain (Fig. 3B and Fig. S8B). Furthermore, acute treatment of wild-type fibroblasts with the Pikfyve inhibitor YM201636 resulted in a rapid loss of PI5P (Fig. 4A and ref. 34). Thus, loss of Vac14, Fig4, or Pikfyve activity results in significant loss of PI5P.
We used three tests to determine whether Pikfyve has a direct role in the generation of PI5P from phosphatidylinositol or, alternatively, whether PI5P is generated from PI(3,5)P2 via 3′-phosphatases. That PI5P levels are 10-fold higher than PI(3,5)P2 levels initially raised doubt that PI(3,5)P2 would be a major precursor for the generation of PI5P. However, our data suggest that most of the PI5P is derived from PI(3,5)P2.
The strongest support for this hypothesis is that human PIKFYVE expressed in yeast is highly active and produced a fivefold elevation in PI(3,5)P2 (Fig. 4C and Fig. S8D). However, the small additional amount of PI5P produced was sixfold lower than PI(3,5)P2. Notably, if all of the PI5P generated by PIKFYVE was derived indirectly from PIKFYVE via PI(3,5)P2, then the sum total of PI5P plus PI(3,5)P2 plus PI3P, should be equal in wild-type yeast and yeast expressing PIKFYVE—as, indeed, was observed (Fig. 4D). These observations strongly support the hypothesis that the PIKFYVE protein in vivo directly catalyzes the conversion of PI3P to PI(3,5)P2 but does not generate PI5P from PI.
The experiments performed in fibroblasts also fit this hypothesis. Upon acute inhibition of Pikfyve, PI(3,5)P2 was fully depleted at the first measurable time point, 2.5 min. In contrast, PI5P declined more slowly; at 7.5 min, about half of the PI5P remained. Similarly, using 1-h starvation followed by nutrient refeeding to stimulate Pikfyve, PI(3,5)P2 fully increased to its new steady-state levels at 2.5 min following refeeding, whereas the rate of increase of PI5P levels apparently was slower and was completed at 10 min. The more rapid disappearance of PI(3,5)P2 after inhibition of Pikfyve, compared with the disappearance of PI5P, and the more rapid appearance of PI(3,5)P2, after stimulation of Pikfyve fit the hypothesis that PI(3,5)P2 and PI5P are generated by different enzymes and are consistent with a precursor/product relationship between PI(3,5)P2 and PI5P.
An alternative interpretation for the studies in fibroblasts is that the rate of turnover of PI5P is slower than the rate of turnover of PI(3,5)P2. However, collective interpretation of the fibroblast and yeast studies strongly supports the hypothesis that most of the Pikfyve-dependent PI5P pool in fibroblasts is generated via the action of 3′-lipid phosphatases on PI(3,5)P2 (Fig. 5).
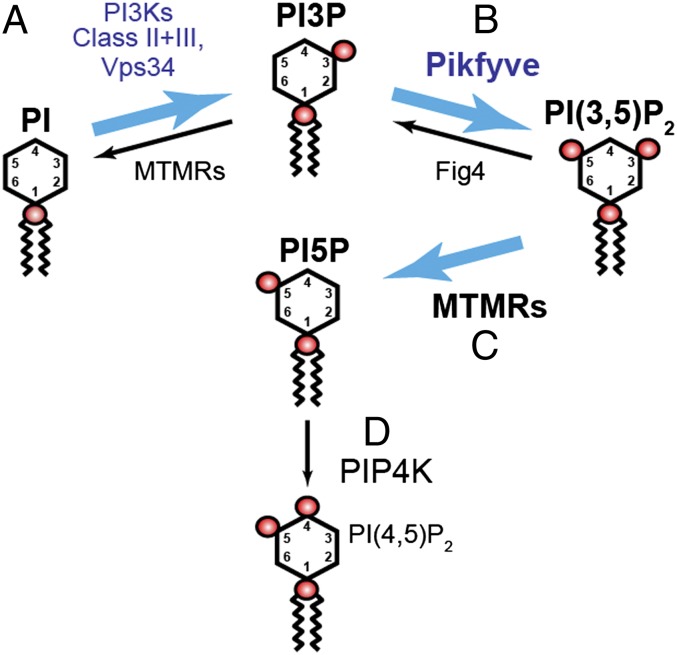
Fig. 5.
Model in which most of the PI5P pool derives from PI(3,5)P2 that is generated via Pikfyve. PI is converted to PI3P by Vps34 and possibly other PI3Ks (A) (48). Some PI3P is converted to PI(3,5)P2 by Pikfyve (Fab1) (B) (1, 2, 13). Fig4 is both a lipid 3′-phosphatase and an activator of Fab1 (6, 7). Pikfyve generates all of the PI(3,5)P2 pool (Fig. 3). Most of the PI5P pool indirectly requires Pikfyve (C). The Pikfyve-dependent pool of PI(3,5)P2 is dephosphorylated to produce PI5P. The most likely 3′-phosphatases are myotubularins (MTMRs) (33, 36–38). Blue arrows denote the indirect generation of PI5P from PI3P through dephosphorylation of PI(3,5)P2. PI5P also may serve as a precursor for some of the PI(4,5)P2 pool (ref. 42 and Fig. S8D) (D).
Two observations support the model that Pikfyve makes PI5P directly in vivo. First, in heterozygous PIKfyve+/β-geo fibroblasts (Fig. S7A), PI5P is reduced by 30%, whereas PI(3,5)P2 levels are unaffected. One explanation is that mechanisms that coordinate both synthesis and turnover ensure specific levels of PI(3,5)P2. Perhaps minor perturbations in PI(3,5)P2 synthesis in the heterozygous mutant are compensated by less turnover of PI(3,5)P2 to PI5P. Second, although one study found that Pikfyve cannot generate PI5P from PI in vitro (35), another in vitro study showed that Pikfyve directly generates PI5P (13). However, note that in the latter study Pikfyve was pulled down from cell types that express many lipid 3′-phosphatases, including several myotubularins. A tight association of Pikfyve with myotubularins in vivo and in the immunoprecipitation protocol would explain the differences between in vitro studies of Pikfyve immunoprecipitated from COS cells and of Pikfyve expressed heterologously in yeast.
The most likely candidate 3′-phosphatases for the generation of PI5P from PI(3,5)P2 are myotubularins. Several myotubularin 3′-lipid phosphatases, including Mtm1, Mtmr2, Mtmr3, and Mtmr6, can convert PI(3,5)P2 to PI5P in vitro and, when expressed heterologously, in S. cerevisiae (33, 36–38). Thus, myotubularins may convert PI(3,5)P2 to PI5P in vivo. However, a direct test of this role is not feasible, because there are at least eight active myotubularins (39, 40).
Some PI5P generated via Pikfyve may contribute to PI(4,5)P2 pools (Fig. 5). When PI5P is depleted, PI(4,5)P2 levels are reduced (Fig. S7D). PI5P is converted to PI(4,5)P2 by type II PI-5-P 4-kinases (PIP4K IIs) (41, 42).
The discovery that Pikfyve is critical for the generation of most of the cellular PI5P pool provides a caveat to the interpretation of phenotypes caused by perturbation of Pikfyve activity. Methods will need to be developed that can distinguish between phenotypes caused by loss of PI5P and those caused by loss of PI(3,5)P2.
Loss of PI(3,5)P2 rather than PI5P is likely responsible for the vacuoles observed during inhibition of Pikfyve activity (Fig. S7C and refs. 5, 19–25, and 29). Elevation of PI5P affects early endosomes without affecting late endosomes (43), and large vacuoles seen in metazoans likely are related to the large vacuoles in yeast, an organism that does not make PI5P.
Large vacuoles are a hallmark of partial to full inhibition of Pikfyve activity, but vacuoles may not be the primary cause of organ damage in animals. Neurons become vacuolated ex vivo, suggesting that vacuole formation may not be the most proximal or serious defect caused by loss of Pikfyve activity. PI(3,5)P2 and PI5P likely regulate multiple additional pathways. It is of interest that several aspects of the Pikfyve depletion phenotype closely resemble those observed in mice deficient for Fig4 and Vac14, consistent with the coordinated role of the three proteins in regulation of phosphoinositide levels.
Francois–Neetens mouchetée fleck corneal dystrophy (44) is associated with mutations in the PIKFYVE gene. Mutations in the PIKFYVE regulator FIG4 are responsible for the Charcot–Marie–Tooth disorder CMT4J (29, 31, 45, 46) and for some cases of amyotrophic lateral sclerosis and primary lateral sclerosis (47). It is likely that additional neurological diseases may be linked to PIKFYVE and its regulators. Moreover, additional pathological consequences of mutations in PIKFYVE and its regulators are likely. Most striking in the Pikfyveβ-geo/β-geo mutant are the defects in the heart and lung.
Together, these finding demonstrate that defects in the levels of PI(3,5)P2 and PI5P cause premature lethality and degeneration of multiple organs. Furthermore, in mammalian tissues, Pikfyve is the sole source of PI(3,5)P2, and the resultant PI(3,5)P2 serves as the precursor for most of the cellular pools of PI5P.
Materials and Methods
All experiments with animals were performed in compliance with guidelines of the University Committee on Use and Care of Animals of the University of Michigan and National Institutes of Health. Animals were housed and cared for in accordance with National Institutes of Health guidelines. See SI Materials and Methods for details of experimental procedures.
Supplementary Material
Acknowledgments
We thank Dr. Shenghui He for help with quantitative RT-PCR and Judy Poore and the Microscopy and Image Analysis Laboratory at the University of Michigan for H&E sample preparation. This work used the Vector and Hybridoma Cores of the Michigan Diabetes Research and Training Center, funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Disease, and the University of Michigan Echocardiography and Transgenic Animal Model Cores. This work was supported by National Institutes of Health Research Grants R01 NS064015 (to L.S.W.), R01 GM24872 (to M.H.M.), and R01 DK061618 (to A.R.S.); by a Veterans Administration Merit Review Grant (to R.L.A.), and by a grant from the Fritz Thyssen Foundation (to T.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203106109/-/DCSupplemental.
References
- 1.Cooke FT, et al. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol. 1998;8(22):1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- 2.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143(1):65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonangelino CJ, et al. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol. 2002;156(6):1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dove SK, et al. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol. 2002;12(11):885–893. doi: 10.1016/s0960-9822(02)00891-6. [DOI] [PubMed] [Google Scholar]
- 5.Jin N, et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 2008;27(24):3221–3234. doi: 10.1038/emboj.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006;5(4):723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudge SA, Anderson DM, Emr SD. Vacuole size control: Regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell. 2004;15(1):24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17(12):6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gary JD, et al. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell. 2002;13(4):1238–1251. doi: 10.1091/mbc.01-10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dove SK, et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23(9):1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efe JA, Botelho RJ, Emr SD. Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol Biol Cell. 2007;18(11):4232–4244. doi: 10.1091/mbc.E07-04-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol. 2006;172(5):693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sbrissa D, Ikonomov OC, Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J Biol Chem. 1999;274(31):21589–21597. doi: 10.1074/jbc.274.31.21589. [DOI] [PubMed] [Google Scholar]
- 14.Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2(1):157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 15.Sbrissa D, Ikonomov OC, Fenner H, Shisheva A. ArPIKfyve homomeric and heteromeric interactions scaffold PIKfyve and Sac3 in a complex to promote PIKfyve activity and functionality. J Mol Biol. 2008;384(4):766–779. doi: 10.1016/j.jmb.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomov OC, Sbrissa D, Shisheva A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem. 2001;276(28):26141–26147. doi: 10.1074/jbc.M101722200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. Modulation of synaptic function by VAC14, a protein that regulates the phosphoinositides PI(3,5)P(2) and PI(5)P. EMBO J. 2012;31(16):3442–3456. doi: 10.1038/emboj.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabezas A, Pattni K, Stenmark H. Cloning and subcellular localization of a human phosphatidylinositol 3-phosphate 5-kinase, PIKfyve/Fab1. Gene. 2006;371(1):34–41. doi: 10.1016/j.gene.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford AC, et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119(Pt 19):3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sbrissa D, Ikonomov OC, Shisheva A. Phosphatidylinositol 3-phosphate-interacting domains in PIKfyve. Binding specificity and role in PIKfyve. Endomenbrane localization. J Biol Chem. 2002;277(8):6073–6079. doi: 10.1074/jbc.M110194200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lartigue J, et al. PIKfyve regulation of endosome-linked pathways. Traffic. 2009;10(7):883–893. doi: 10.1111/j.1600-0854.2009.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferies HBJ, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9(2):164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicot A-S, et al. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell. 2006;17(7):3062–3074. doi: 10.1091/mbc.E05-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rusten TE, et al. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell. 2006;17(9):3989–4001. doi: 10.1091/mbc.E06-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusten TE, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol. 2007;17(20):1817–1825. doi: 10.1016/j.cub.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson CJ, Lenk GM, Meisler MH. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum Mol Genet. 2009;18(24):4868–4878. doi: 10.1093/hmg/ddp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong X-P, et al. (2010) PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nature Communications 1(4). Available at http://www.nature.com/ncomms/journal/v1/n4/abs/ncomms1037.html. [DOI] [PMC free article] [PubMed]
- 29.Chow CY, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikonomov OC, et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve-/- embryos but normality of PIKfyve+/- mice. J Biol Chem. 2011;286(15):13404–13413. doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenk GM, et al. Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 2011;7(6):e1002104. doi: 10.1371/journal.pgen.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bridges D, et al. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol Biol Cell. 2012;23(15):2955–2962. doi: 10.1091/mbc.E11-12-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaccari I, et al. Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies. PLoS Genet. 2011;7(10):e1002319. doi: 10.1371/journal.pgen.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbrissa D, Ikonomov OC, Filios C, Delvecchio K, Shisheva A. Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636. Am J Physiol Cell Physiol. 2012;303(4):C436–C446. doi: 10.1152/ajpcell.00105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen RK, et al. Complementation analysis in PtdInsP kinase-deficient yeast mutants demonstrates that Schizosaccharomyces pombe and murine Fab1p homologues are phosphatidylinositol 3-phosphate 5-kinases. J Biol Chem. 1999;274(48):33905–33912. doi: 10.1074/jbc.274.48.33905. [DOI] [PubMed] [Google Scholar]
- 36.Berger P, Bonneick S, Willi S, Wymann M, Suter U. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet. 2002;11(13):1569–1579. doi: 10.1093/hmg/11.13.1569. [DOI] [PubMed] [Google Scholar]
- 37.Schaletzky J, et al. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol. 2003;13(6):504–509. doi: 10.1016/s0960-9822(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 38.Walker DM, et al. Characterization of MTMR3. an inositol lipid 3-phosphatase with novel substrate specificity. Curr Biol. 2001;11(20):1600–1605. doi: 10.1016/s0960-9822(01)00501-2. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo O, Urbé S, Clague MJ. Systematic analysis of myotubularins: Heteromeric interactions, subcellular localisation and endosome related functions. J Cell Sci. 2006;119(Pt 14):2953–2959. doi: 10.1242/jcs.03040. [DOI] [PubMed] [Google Scholar]
- 40.Tosch V, et al. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Hum Mol Genet. 2006;15(21):3098–3106. doi: 10.1093/hmg/ddl250. [DOI] [PubMed] [Google Scholar]
- 41.Carricaburu V, et al. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci USA. 2003;100(17):9867–9872. doi: 10.1073/pnas.1734038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390(6656):192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 43.Ramel D, et al. Shigella flexneri infection generates the lipid PI5P to alter endocytosis and prevent termination of EGFR signaling. Sci Signal. 2011;4(191):ra61. doi: 10.1126/scisignal.2001619. [DOI] [PubMed] [Google Scholar]
- 44.Li S, et al. Mutations in PIP5K3 are associated with François-Neetens mouchetée fleck corneal dystrophy. Am J Hum Genet. 2005;77(1):54–63. doi: 10.1086/431346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, et al. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain. 2008;131(Pt 8):1990–2001. doi: 10.1093/brain/awn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholson G, et al. Distinctive genetic and clinical features of CMT4J: A severe neuropathy caused by mutations in the PI(3,5)P2 phosphatase FIG4. Brain J Neurol. 2011;134:1959–1971. doi: 10.1093/brain/awr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow CY, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84(1):85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Y, Backer JM. Regulation of class III (Vps34) PI3Ks. Biochem Soc Trans. 2007;35(Pt 2):239–241. doi: 10.1042/BST0350239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.