Abstract
The 40-fold increase in childhood megakaryocyte-erythroid and B-cell leukemia in Down syndrome implicates trisomy 21 (T21) in perturbing fetal hematopoiesis. Here, we show that compared with primary disomic controls, primary T21 fetal liver (FL) hematopoietic stem cells (HSC) and megakaryocyte-erythroid progenitors are markedly increased, whereas granulocyte-macrophage progenitors are reduced. Commensurately, HSC and megakaryocyte-erythroid progenitors show higher clonogenicity, with increased megakaryocyte, megakaryocyte-erythroid, and replatable blast colonies. Biased megakaryocyte-erythroid–primed gene expression was detected as early as the HSC compartment. In lymphopoiesis, T21 FL lymphoid-primed multipotential progenitors and early lymphoid progenitor numbers are maintained, but there was a 10-fold reduction in committed PreproB-lymphoid progenitors and the functional B-cell potential of HSC and early lymphoid progenitor is severely impaired, in tandem with reduced early lymphoid gene expression. The same pattern was seen in all T21 FL samples and no samples had GATA1 mutations. Therefore, T21 itself causes multiple distinct defects in FL myelo- and lymphopoiesis.
Keywords: transient myeloproliferative disorder, aneuploidy, human fetus
Constitutional trisomy 21 (T21) causes Down syndrome (DS), the most common syndrome-associated chromosomal anomaly in humans (1). As well as with neurodevelopmental, cardiac, and gut anomalies (2), there is a striking increase in childhood acute leukemia in DS, even though the risk of solid tumors is much lower than with the general population (3). Intriguingly, this susceptibility to hematopoietic tumors manifests as an increased risk both of acute megakaryocyte (MK)-erythroid leukemia (known as ML-DS) by 150-fold and of acute B-lymphoblastic leukemia (B-ALL) by 33-fold (3, 4).
DS leukemias display distinct characteristics that support a crucial role for T21 in their pathogenesis. Hallmarks of ML-DS are the megakaryoblastic phenotype, clinical presentation confined to the first 5 y of childhood (5, 6), an antecedent clonally linked preleukemic condition (termed transient myeloproliferative disorder, TMD) in most cases, and acquired N-terminal truncating mutations in the erythroid-MK transcription factor GATA1 (7–9). Such mutations in GATA1 are present in both ML-DS and TMD (9) but are not found in patients without DS who develop megakaryoblastic leukemia (7) and are not leukemogenic in the absence of T21 (10).
Molecular, biologic, and clinical data indicate that TMD is initiated before birth (9, 11–14). We previously reported that by the second trimester, the T21 fetal liver (FL) myeloid progenitor compartment is abnormal and that this occurs in the absence of GATA1 mutation (11, 12). Specifically, the MK-erythroid progenitor (MEP) population is expanded with increased cell-intrinsic MK and erythroid lineage proliferation from CD34+ cells. These data suggest that T21-mediated developmental alterations to FL myeloid progenitor development provide a cell-specific substrate for selection and expansion of mutant GATA1 clones. This finding is consistent with results in mice genetically engineered to express N-terminal truncated GATA1 protein, which develop an altered MK lineage proliferation/differentiation phenotype, restricted to FL progenitors and not seen in adult mice (13).
The abnormalities in the myeloid progenitor compartment of T21 FL would be consistent with one of at least two contrasting scenarios. The first scenario is that effects of T21 are confined to the myeloid progenitor compartment, which through the combination of T21 and mutant GATA1 acquires increased self-renewal and a selective growth advantage; these cells would be predicted to have a relatively short lifespan, which could account for frequent spontaneous resolution of TMD (∼80%) within a few months of birth (14). An alternative model, given the increased risk of childhood B-ALL and B-cell immune deficiency (4, 15), is that T21 might perturb hematopoiesis at the hematopoietic stem cell (HSC) or multipotential progenitor level. To distinguish between these two possibilities, we performed detailed immunophenotypic and functional analysis of the HSC/multipotential progenitor compartment and committed myeloid and B-lymphoid compartments of T21 FL without GATA1 mutations and compared these with normal FL. Here, we are unique in reporting that in human FL, T21 itself increases immunophenotypic HSC frequency, clonogenicity and MK-erythroid output with associated MEP expansion, and severe impairment of B-lymphoid development. Perturbation of FL HSC/progenitor proliferation and lineage commitment may underlie the striking susceptibility of T21 hematopoietic cells to both myeloid and lymphoid leukemic transformation and, together with human T21 ES and induced pluripotent stem cells (iPSCs), T21 FL provides a tractable model for dissecting T21-mediated leukemia.
Results
Perturbation of HSC/Progenitor Frequency in T21 FL.
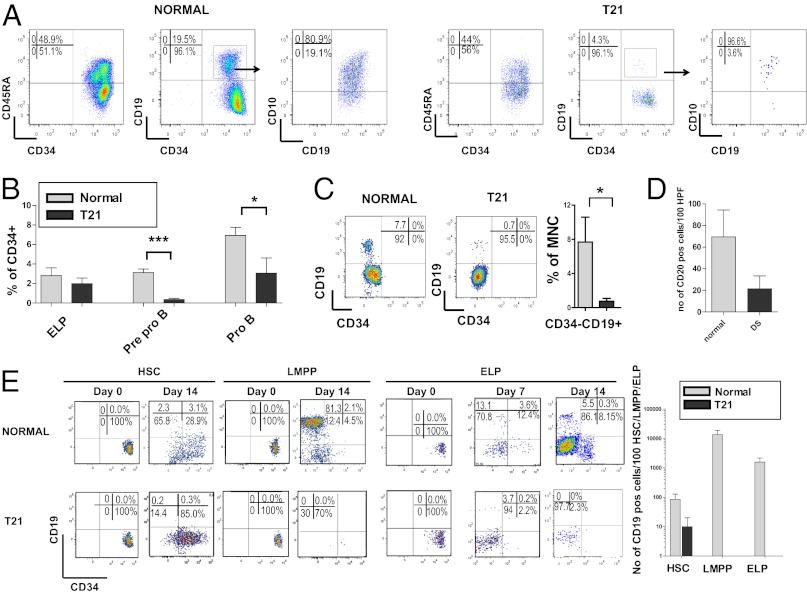
We previously found marked MEP expansion, reduced granulocyte-macrophage progenitors (GMP), and qualitative common myeloid progenitor (CMP) abnormalities in T21 FL in the absence of GATA1 mutation (11, 12). To investigate whether T21 causes wider perturbation of T21 FL hematopoiesis, we used the same scheme to define the HSC/progenitor hierarchy as established for cord blood and adult marrow (16, 17) and compared the HSC/multipotential progenitors (MPP)/lymphoid-primed multipotential progenitors (LMPP) compartment in T21 FL (n = 8; median gestation 16 wk) with normal FL (n = 13; median gestation 15 wk) by flow cytometry. There was a 3.5-fold increase in immunophenotypic HSC frequency in T21 compared with normal FL (7.9 ± 0.9% vs. 2.3 ± 0.4%; P = 0.0025) but MPP and LMPP frequency were preserved and we confirmed the changes in MEP and GMP frequency we found previously (11, 12) (Fig. 1). The same pattern was seen in all T21 FL samples and no samples had GATA1 mutations. There was a marked reduction in CD34+CD19+ committed B progenitors (CBP) in T21 compared with normal FL (3.1 ± 1.3% vs. 10.7 ± 1.3%; P = 0.0026), suggesting a block to B-lymphoid differentiation because early lymphoid progenitor (ELP) (CD34+CD127+CD10−CD19−) frequency was normal (Fig. 1). These data show multiple defects in T21 FL hematopoiesis, characterized by expansion of immunophenotypic HSC and MEP with commensurate reductions in GMP and B-progenitors. Because the frequency of CD34+ cells was the same in T21 FL as normal FL, both by flow cytometry (3.1 ± 0.8% vs. 4.9 ± 0.9% of mononuclear cells; T21 vs. normal; P = 0.185) and immunohistochemistry of FL sections (15.1 ± 2 vs. 17.7 ± 4.7/100 high-power fields; P = 0.624) (Fig. S1), these differences in HSC/progenitor frequency also reflect changes in absolute numbers of these populations in T21 FL.
Fig. 1.
Perturbation of T21 FL HSC/progenitor frequency. (A) Representative plots from normal FL (n = 13) and T21 FL (n = 8) CD34+ cells gated on CD34+CD38+CD19− cells for CMP, MEP, and GMP, and on CD34+CD38lo/− cells for HSC, MPP, and LMPP. (B) Mean + SEM HSC and progenitor frequencies in normal (n = 13; light bars) and T21 (n = 8) FL (dark bars) showing increased HSC and MEP frequency and reduced CBP and GMP. **P < 0.01; *P < 0.05.
Increased Clonogenicity and MK-Erythroid Potential of T21 FL HSC/Progenitors.
Next, we performed in vitro clonogenic assays of these immunophenotypic HSC and progenitor populations (sorting strategy shown in Fig. S2). This process showed increased clonogenicity of HSC (fivefold P < 0.01), CMP (2.7-fold P < 0.05), and MEP (2.5-fold P < 0.05) (Fig. 2A). Lineage output from HSC, MPP, CMP, and MEP showed marked skewing toward the MK lineage with increased MK and MK-erythroid colonies (Fig. 2B and Table S1). Furthermore immunohistochemistry of FL sections confirmed the marked absolute increase in MKs (Fig. S1). T21 FL HSC also generated increased absolute numbers of erythroid burst-forming units (BFU-E), given that overall clonogenicity was higher in T21 HSC (Table S1), although the relative proportion was similar to normal FL (Fig. 2B).
Fig. 2.
Increased clonogenicity and megakaryocyte/erythroid potential of normal and T21 FL HSC and progenitors. (A) Clonogenicity of flow-sorted HSC and progenitors from normal (n = 8) and T21 (n = 5) FL (mean + SEM). Cells (100 cells/mL) were plated in Methocult H4230 with IL-3, IL-6, IL-11, SCF, FLT3, GM-CSF, TPO, and EPO. Clonogenicity of T21 HSC, CMP, and MEP was increased compared to normal FL. **P < 0.01; *P < 0.05. (B) Lineage read out of clonogenic data in A showing increased MK and MK-erythroid (MkE) colonies from T21 FL HSC, MPP, CMP, and MEP, and Blast-My colonies from T21 FL LMPP compared to normal FL. T21 HSC generated increased absolute numbers of CFU-MK, MkE, BFU-E, Blast-E, and Blast-My compared to normal FL HSC and T21 CMP and MEP increased CFU-MK, MkE, and Blast-E (for quantitation see Table S1). (C) Representative colonies (Scale bars, 100 μm.) and (D) colony cytospins (Scale bars, 10 μm.) from normal (Left) and T21 (Right) FL clonogenic assays. (E) Only Blast-My and Blast-E had secondary replating activity. No tertiary replating was seen. There was no difference between replating activity of normal and T21 FL Blast-My or Blast-E.
Two types of blast-cell colony (Blast-My and Blast-E) were identified morphologically in normal and T21 FL HSC, MPP, and CMP. Blast-E were also generated by FL MEP (Fig. 2 B–F). Blast-E, small compact hemoglobinized colonies (Fig. 2C) appearing after day 14 of culture (in contrast to BFU-E, which were easily seen by day 10), contained late and early normoblasts, blast-like cells, and macrophages (Fig. 2D), coexpressed CD34 and GlyA in contrast to BFU-E (Fig. 2E) and, unlike BFU-E, had high replating efficiency, generating solely erythroid colonies (BFU-E) and no myeloid colonies. Neither Blast-E–derived BFU-E nor BFU-E arising directly from plated FL HSC/MPP/CMP or MEP were replatable (Fig. 2F), suggesting they lie immediately upstream of BFU-E. Absolute numbers of Blast-E colonies were increased in T21 FL HSC, CMP, and MEP because overall clonogenicity was higher (Table S1), although the relative proportion was similar to normal FL (Fig. 2B). Blast-My, which appeared on day 10–12 of culture and did not hemoglobinize even after 4 wk (Fig. 2C), contained almost exclusively myeloid blast cells (Fig. 2D), coexpressed CD34 and mature myeloid markers (Fig. 2E), and had high replating efficiency, generating CFU-GM, BFU-E, and BFU-MK, which had no further replating ability (Fig. 2F), suggesting that they lie immediately upstream of CFU-GEMM. Blast-My were increased in T21 FL HSC and CMP compared with normal FL (Fig. 2B). Although Blast-My are detectable in normal cord blood and adult bone-marrow HSC and myeloid progenitors, Blast-E appear to be fetal in origin because we do not detect them in adult bone marrow (Table S2).
Impaired B-Lymphoid Differentiation of T21 HSC, LMPP, and ELP.
To characterize the B-progenitor defect we performed more detailed immunophenotypic analysis of CBP (CD34+CD19+). Both immature CBP, CD34+CD19+CD10− (PreproB), and mature CBP, CD34+CD19+CD10+ (ProB), were seen in normal FL (Fig. 3 A and B), as in cord blood (18), and CD19+/CD20+ B cells were easily seen in normal FL (Fig. 3 C and D, and Fig. S1). Normal FL HSC, LMPP, and ELP generated CD34−CD19+ B cells in MS5 cocultures consistent with FL as a site of normal prenatal B-cell development (Fig. 3E). In T21 FL, both PreproB (0.33 ± 0.1 vs. 3.5 ± 0.5%; T21 vs. normal) and ProB progenitors (3.1 ± 0.5 vs. 7.1 ± 1.0) were reduced (Fig. 3 A and B), as were mature (CD19+/CD20+) B cells (Fig. 3 C and D, and Fig. S1). Moreover, T21 FL HSC demonstrated marked impairment in B-cell differentiation with very few CD34−CD19+ cells after 14 or 21 d in MS5 cocultures (Fig. 3E). T21 FL LMPP and ELP MS-5 cocultures also failed to generate CD19+ cells apart from transient occasional CD19+ cells from ELP after 7 d but not thereafter (Fig. 3E). In contrast, there was no difference in T21 FL T progenitor frequency (CD34+CD4+ cells: 5.5 ± 1.9 vs. 3.7 ± 0.9% of CD34+ cells; normal vs. T21; P = 0.70). These data suggest specific impairment of B-cell development in T21 FL which is evident at all stages of B-cell differentiation.
Fig. 3.
Impaired lymphoid differentiation of T21 FL HSC, LMPP, and ELP. (A) Representative plots from normal (Left; n = 11) and T21 (Right; n = 6) FL showing reduced Pre-proB (CD34+CD19+CD10−) and ProB progenitors (CD34+CD19+CD10+). (B) Mean B-lymphoid progenitor frequencies in normal (light bars; n = 11) and T21 (n = 6) FL (dark bars) showing reduced PreProB and ProB progenitors in T21 FL. ***P < 0.001; *P < 0.02. (C) Representative plots showing reduced CD34−CD19+ cells in T21 compared to normal FL and summary data in normal (light bars, n = 5) and T21 FL (dark bars, n = 8). *P < 0.05. (D) Mean number of CD20+ cells/high-power fields from normal (n = 4; light bars) and T21 FL (n = 4; dark bars). (E) Differentiation of flow-sorted 100 HSC, LMPP, and ELP on MS5 stroma showing representative results on day 0 and day 14 (day 7 also shown for ELP) from normal (n = 3) and T21 FL (n = 4) and (Right) mean absolute number of CD19+ cells on day 14 from normal FL HSC, LMPP, and ELP (light bars) or T21 HSC, LMPP or ELP (dark bars).
Expression of MK-Erythroid and B-Lineage Genes in T21 FL HSC and Progenitors.
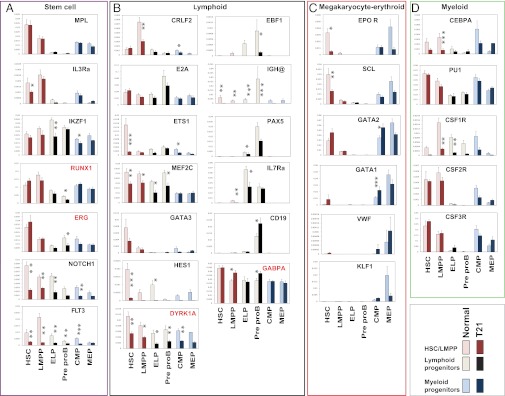
To determine whether changes in HSC/progenitors in T21 FL reflected alterations in lineage-affiliated gene-expression signatures, we flow-sorted normal and T21 HSC, CMP, MEP, LMPP, ELP, and PreproB progenitors, as shown (Fig. S2), and measured expression of key lineage-associated genes by quantitative RT-PCR. Both normal and T21 FL HSC-expressed genes highly expressed in human adult bone-marrow HSC, including MPL, IL3RA, IKZF1, RUNX1, ERG (17), and interestingly also expressed high levels of NOTCH1 compared with differentiated progenitors, consistent with murine fetal HSC (19) (Fig. 4A). Intriguingly, high-level expression of EPOR was seen in normal FL HSC, equivalent to FL MEP (Fig. 4C), in contrast to low EPOR expression in adult marrow HSC (17), suggesting FL HSC may be erythroid-primed to meet the fetal demand for red cells. FL HSC also expressed genes important in early lymphoid specification, including FLT3, CRLF2, E2A, ETS1, MEF2C, GATA3, and HES1 (20–22) (Fig. 4B), as well as early stem MK-erythroid genes, including SCL, GATA2, low levels of GATA1 and VWF (Fig. 4C), and early myeloid lineage-affiliated genes, such as CEBPA, PU1, CSF1R, CSF2R, and CSF3R (Fig. 4D). These data are consistent with multilineage priming of human FL HSC, analagous to murine and adult HSC (17, 22, 23).
Fig. 4.
(A–D) Altered gene expression in T21 FL HSC/progenitors Mean gene expression levels by quantitative RT-PCR from flow-sorted HSC/progenitors (50 cells in triplicate for each population) from normal (n = 5; light bars) and T21 FL (n = 3; dark bars) shown relative to GAPDH. Significant differences between T21 and normal FL are shown as *P < 0.05; **P < 0.01, and ***P < 0.001, using Bayesian analysis of differences in mean (see SI Experimental Procedures).
T21 FL HSC also expressed a multilineage program but with marked reductions in expression of early lymphoid genes compared with normal FL HSC: NOTCH1, FLT3, ETS1, MEF2C, HES1, and DYRK1A (Fig. 4B). Increased MK-erythroid commitment by T21 FL HSC did not appear to be a result of of increased MPL or EPOR or to the key MK-erythroid regulators SCL or KLF1, because their levels of expression were normal or reduced in T21 FL HSC (Fig. 4C). Expression of several early stem cell MK-erythroid genes, including GATA2, GATA1, VWF (Fig. 4C), RUNX1, and ERG (Fig. 4A), was higher in T21 FL HSC and, although the increase for each gene individually was not statistically significant, collectively these changes may contribute to the increased MK-erythroid commitment. In line with this finding, GATA2 and GATA1 expression were increased in T21 FL CMP (Fig. 4C). Taken together, differences in T21 FL HSC gene expression are consistent with their increased MK-erythroid and impaired B-lymphoid potential.
T21 FL LMPP also failed to up-regulate the early lymphoid gene program (IKZF1, RUNX1, NOTCH1, and FLT3) seen in normal FL LMPP and several key B-lymphoid genes, including EBF, IGH@, CRLF2, and IL7RA (Fig. 4 A and B). In contrast, E2A and PU1 expression in T21 FL was the same as normal FL, consistent with their wider roles in hematopoiesis (21). Similar patterns of reduced expression of early lymphoid and B-lymphoid genes, including EBF1, IGH@, and PAX5, but not CD19, were seen in T21 ELP and PreproB progenitors (Fig. 4B).
Finally, to investigate chromosome 21 (HSA21) genes important in hematopoiesis, we compared levels of RUNX1, ERG, DYRK1A, and GABPA (Fig. 4 A and B) in T21 and normal FL HSC/progenitors. Importantly, none of these genes showed the predicted 1.5-fold increase in expression across all HSC/progenitor populations expected for cells trisomic for HSA21. Instead, a significant increase in expression, compared with normal FL, was found only for GABPA (1.3- to 1.4-fold) and this was specific to the LMPP and PreproB populations, because GABPA expression in HSC and other progenitors was the same in normal and T21 samples. RUNX1 and ERG expression were comparable in T21 and normal FL across all populations apart from significantly reduced expression in PreproB, although modest, potentially relevant, increases in RUNX1 (1.4-fold) and ERG (1.2-fold) were seen specifically in HSC (Fig. 4A). Interestingly, DYRK1A expression was lower in all T21 HSC/progenitors compared with normal FL (Fig. 4A).
Discussion
The striking association of T21 with myeloid and B-cell malignancies in young children (3) led us to investigate whether T21 itself alters HSC/progenitor biology using detailed immunophenotypic and functional analysis of GATA1 mutation-negative, second trimester T21 FL compared with normal FL. Here, we are unique in reporting evidence in primary human FL cells that T21 itself causes multiple distinct defects in FL myelo- and lymphopoiesis, altering not only the myeloid progenitor compartment (MEP/CMP/GMP), but also causing perturbation of immunophenotypically defined HSC, MPP, LMPP, and of early and committed B-lymphoid progenitors. Gene-expression studies demonstrate the molecular complexity underlying these changes and argue that effects of T21 are cell context-dependent and influenced not only by cell lineage, but also by maturational stage.
Expansion of HSC was accompanied by functional and molecular evidence of MK-erythroid bias, although confirmation that there is a true increase in MK-erythroid–biased HSC would require experiments at a single-cell level and appropriate xenograft models (24). Increased MEP frequency in T21 FL may therefore be a downstream consequence of this, because more profound differences in functional assays and gene expression were seen in HSC rather than MEP in T21 FL. Indeed, both previously (11, 12) and here, we have been unable to demonstrate self-renewal of T21 MEP (replating activity instead resided further up the differentiation hierarchy), although T21 MEP clonogenicity is increased, indicating a proliferative advantage in MEP, as well as HSC and MKs, were increased in vivo in T21 FL (on FL sections). Because all samples were screened (and were negative) for the presence of the disease-causing exon 2/3 mutations in GATA1 characteristic of TMD (7–9), this argues strongly that T21 is responsible for these effects. This theory is supported by data from human iPSCs reported by MacLean et al. (25), which recapitulated many of the abnormalities in fetal myelopoiesis we observed in primary fetal cells.
Impaired B-progenitor development in T21 FL manifest both as a marked selective reduction in committed B-progenitor (PreproB and ProB) frequency (T-progenitor frequency was normal) and as reduced ability of HSC, LMPP, and ELP to generate mature B cells. These abnormalities were underpinned by an equally marked reduction in T21 FL HSC of expression of FLT3, one of the earliest regulatory events triggering B-lymphoid development (20). Furthermore, in T21 LMPP, FLT3 expression failed to undergo the up-regulation needed to activate normal B-progenitor development (22). Consistent with this finding, levels of expression of other transcriptional activators that specify B-cell fate, including IL7RA, CRLF2, ETS1, MEF2C, and EBF, were reduced in T21 LMPP and downstream B-progenitors and PAX5 expression was reduced in ELP. These data show extensive dysregulation of B-cell development from HSC to mature B cells. Whether similar dysregulation of B-progenitor development persists beyond birth is unknown, but we hypothesize that “molecular resetting” of the fetal B-lymphoid differentiation program contributes to B-cell immune deficiency (15) and B-ALL in children with DS (26).
It is important to note that the differences between T21 and normal FL hematopoiesis are not only marked, but the pattern of hematologic abnormalities seen in every T21 fetal sample is the same over the gestation range investigated (14–22 wk). This finding supports our earlier observations of consistent FL abnormalities despite the absence of GATA1 mutations, which was independently confirmed in two laboratories (11, 12). The precise mechanisms by which T21 causes the consistent pattern of perturbed FL HSC/progenitor specification and function were not directly addressed in this study. However, by characterizing the effects of T21 on HSC and each progenitor population individually, our data reveal the molecular complexity underlying the effects of T21 and suggest that a single molecular event is unlikely to explain all of these abnormalities and that the impact of changes in expression of HSA21 genes is not just tissue-specific, but also varies according to lineage, differentiation stage, and the regulatory machinery of individual genes, as reported for other T21 tissues in DS (2) and in DS leukemias (27).
The marked phenotypic differences in T21 FL hematopoiesis contrast with the relative modest effects on gene expression. This finding is not surprising given the very modest differences in global gene expression reported in the accompanying article by Chou et al. (28) and in other primary T21 tissues (average 1.5 fold) (29–31), which also show variation in the level of increase both between genes and within different tissues, leading to thresholds for differences in expression between T21 and disomic tissues being set at ∼1.2-fold in some studies (32, 33). In support of this approach, there is good evidence that small changes in gene expression can account for phenotypic/functional differences. In mouse models with different combinations of HSA21, orthologs also show phenotype attenuation when fewer genes are triplicated, demonstrating the importance of multiple small changes of gene expression (34, 35). For example, small changes in mRNA expression (1.5-fold) of both HSA21 genes DYRK1A and DSCR1 caused profound nuclear factor of activated T cells dysregulation and cardiac defects (36). Similarly, our data may provide important clues in primary human hematopoietic cells to possible mechanisms through which T21 may perturb their growth and differentiation and a model with which to investigate these. These data help direct specific questions about how lineage-restricted changes in expression of HSA21 genes (such as GABPA and possibly RUNX1, ERG, and DYRK1A) or the five HSA21 miRs contribute to HSC/progenitor dysregulation, as suggested from ML-DS and TMD (35, 37, 38), or the role of altered HSC/progenitor interactions with microenvironmental regulators, as suggested for insulin-like growth factor signaling (39) and CRLF2/IL7RA (26) in T21 leukemias.
Finally, the impact of T21 on HSC/progenitors is likely to vary with age given the different patterns of hematopoietic abnormalities during fetal and postnatal life (11, 13, 15). Because we found that by 14-wk gestation, FL hematopoiesis is already perturbed, this raises the question of whether T21 also affects HSC/progenitor behavior during early embryonic development and in primitive hematopoietic cells, which is clearly extremely difficult to investigate in detail in human embryos. Indeed, data from human T21 iPSCs recapitulated many, but not all, of the abnormalities we observed in second trimester primary FL cells and show that T21 can also perturb hematopoiesis in the embryonic yolk sac (25, 28). Thus, integration of studies in primary human fetal tissue with the iPSC model systems of fetal and embryonic hematopoiesis characterized by MacLean et al. (25) and Chou et al. (28) will be necessary to properly elucidate the molecular complexity underlying the impact of T21 on human hematopoiesis and leukemogenesis.
Experimental Procedures
Samples.
Second trimester FL collected during elective surgical termination of pregnancy were processed immediately. GATA1 analysis, CD34+ separation, and immunohistochemistry were performed as previously described (11) (SI Experimental Procedures). FISH was used to confirm T21 in T21 FL and the lack of chromosomal abnormalities in normal FL. The study was approved by the Hammersmith and Queen Charlotte’s Hospitals Research Ethics Committee (ref 04/Q0406/145).
Flow Cytometric Analysis and Sorting.
Cells were stained with ≤8 fluorophore-conjugated monoclonal antibodies (see SI Experimental Procedures) and analyzed using a BD LSR Fortessa or FACSAria II (Becton Dickinson). Gates were set with unstained controls gating on viable cells using DAPI. Data were analyzed on FlowJo software (Tree Star).
Clonogenic Assays.
Clonogenic assays were performed on flow-sorted HSC/progenitors (100 cells/mL) using Methocult H4230 (Stem Cell Technologies) with cytokines [IL-3 20 ng/mL, IL-6 10 ng/mL, IL-11 10 ng/mL, stem cell factor (SCF) 10 ng/mL, FLT3 10 ng/mL, GM-CSF 50 ng/mL, thrombopoietin (TPO) 50 ng/mL (all Peprotech) and erythropoietin (EPO) 4 U/mL (R&D Systems)] (see SI Experimental Procedures).
MS5 Stromal Cocultures for B-Lymphoid Differentiation.
MS5 stromal cells were cocultured with 100 sorted HSC/progenitors in MS5 medium (a-MEM; Invitrogen) with 10% (vol/vol) FCS, Flt-3L (10 ng/mL), SCF (20 ng/mL), IL-2 (10 ng/mL), IL-7 (5 ng/mL), GM-CSF (20 ng/mL), and G-CSF (10 ng/mL) (Peprotech). B-lymphoid differentiation was assessed weekly by FACS from day 7–21 (see SI Experimental Procedures).
Gene Expression Analysis by Dynamic Arrays.
Gene expression was assessed using the BioMark real-time PCR (qPCR) system (Fluidigm). Fifty HSC or progenitors from five normal FL and three T21 FL were sorted into 200-μL PCR tubes with 10 μL RT-STA mix. Each population was tested in triplicate. Sorted populations were analyzed for relative level of expression of ≤48 genes simultaneously, as previously described (17). Gene expression was normalized to GAPDH expression using the 2^-ΔCq method. Data are presented as mean ± SEM. For list of assays (ABI) used for qPCR, see Table S3.
Statistics.
The difference in means for two sample groups was tested for significance using a Bayesian analysis of differences in mean and Wilcoxon test. Data are expressed as mean ± SEM unless otherwise indicated.
Supplementary Material
Acknowledgments
We thank P. May, A. Reid, and V. Melo for FISH analysis. This study was supported in part by Leukemia and Lymphoma Research Specialist Programme Award 08030 (to G.C., I.R., and P.V.); the Imperial College Biomedical Research Centre (I.R.); Oxford Partnership Biomedical Research Centre National Institute for Health Research Biomedical Research Centre scheme (P.V., A.J.M., and S.E.J.); Leukemia and Lymphoma Research (A.R., G.B., A.J.M., D.A., and A.K.); the Medical Research Council (S.F., M.P.H.S., and S.E.J.); and the Kay Kendall Leukaemia Fund (to O.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211405109/-/DCSupplemental.
References
- 1.Parker SE, et al. National Birth Defects Prevention Network Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and Down syndrome: From genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 3.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355(9199):165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock JA, et al. Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: A Children's Cancer Group study. Blood. 2005;106(13):4043–4049. doi: 10.1182/blood-2003-10-3446. [DOI] [PubMed] [Google Scholar]
- 5.Lange BJ, et al. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children’s Cancer Group Studies 2861 and 2891. Blood. 1998;91(2):608–615. [PubMed] [Google Scholar]
- 6.Hasle H, et al. Myeloid leukemia in children 4 years or older with Down syndrome often lacks GATA1 mutation and cytogenetics and risk of relapse are more akin to sporadic AML. Leukemia. 2008;22(7):1428–1430. doi: 10.1038/sj.leu.2405060. [DOI] [PubMed] [Google Scholar]
- 7.Wechsler J, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32(1):148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 8.Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003;101(11):4301–4304. doi: 10.1182/blood-2003-01-0013. [DOI] [PubMed] [Google Scholar]
- 9.Alford K, et al. Analysis of GATA1 mutations in Down syndrome TMD and myeloid leukemia. Blood. 2011;118:2222–2238. doi: 10.1182/blood-2011-03-342774. [DOI] [PubMed] [Google Scholar]
- 10.Hollanda LM, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38(7):807–812. doi: 10.1038/ng1825. [DOI] [PubMed] [Google Scholar]
- 11.Tunstall-Pedoe O, et al. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood. 2008;112(12):4507–4511. doi: 10.1182/blood-2008-04-152967. [DOI] [PubMed] [Google Scholar]
- 12.Chou ST, et al. Trisomy 21 enhances human fetal erythro-megakaryocytic development. Blood. 2008;112(12):4503–4506. doi: 10.1182/blood-2008-05-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, et al. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet. 2005;37(6):613–619. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 14.Klusmann JH, et al. Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood. 2008;111(6):2991–2998. doi: 10.1182/blood-2007-10-118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstegen RHJ, Kusters MAA, Gemen EFA, DE Vries E. Down syndrome B-lymphocyte subpopulations, intrinsic defect or decreased T-lymphocyte help. Pediatr Res. 2010;67(5):563–569. doi: 10.1203/PDR.0b013e3181d4ecc1. [DOI] [PubMed] [Google Scholar]
- 16.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goardon N, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19(1):138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Sanz E, et al. Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proc Natl Acad Sci USA. 2010;107(13):5925–5930. doi: 10.1073/pnas.0907942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajcini KV, Speck NA, Pear WS. Notch signaling in mammalian hematopoietic stem cells. Leukemia. 2011;25(10):1525–1532. doi: 10.1038/leu.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Singh H, Medina KL, Pongubala JMR. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci USA. 2005;102(14):4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Månsson R, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26(4):407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30(4):493–507. doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 25.MacLean GA, et al. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci USA. 2012;109:17567–17572. doi: 10.1073/pnas.1215468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shochat C, et al. Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208(5):901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourquin JP, et al. Identification of distinct molecular phenotypes in acute megakaryoblastic leukemia by gene expression profiling. Proc Natl Acad Sci USA. 2006;103(9):3339–3344. doi: 10.1073/pnas.0511150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou ST, et al. Trisomy 21-associated defects in human primitive hematopoiesis revealed through induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:17573–17578. doi: 10.1073/pnas.1211175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birchler JA. Reflections on studies of gene expression in aneuploids. Biochem J. 2010;426(2):119–123. doi: 10.1042/BJ20091617. [DOI] [PubMed] [Google Scholar]
- 30.FitzPatrick DR, et al. Transcriptome analysis of human autosomal trisomy. Hum Mol Genet. 2002;11(26):3249–3256. doi: 10.1093/hmg/11.26.3249. [DOI] [PubMed] [Google Scholar]
- 31.Mao R, Zielke CL, Zielke HR, Pevsner J. Global up-regulation of chromosome 21 gene expression in the developing Down syndrome brain. Genomics. 2003;81(5):457–467. doi: 10.1016/s0888-7543(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 32.Conti A, et al. Altered expression of mitochondrial and extracellular matrix genes in the heart of human fetuses with chromosome 21 trisomy. BMC Genomics. 2007;8:268. doi: 10.1186/1471-2164-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson LE, et al. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum Mol Genet. 2007;16(7):774–782. doi: 10.1093/hmg/ddm022. [DOI] [PubMed] [Google Scholar]
- 34.Roper RJ, Reeves RH. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2006;2(3):e50. doi: 10.1371/journal.pgen.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malinge S, et al. Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J Clin Invest. 2012;122(3):948–962. doi: 10.1172/JCI60455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arron JR, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441(7093):595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 37.Klusmann JH, et al. miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev. 2010b;24(5):478–490. doi: 10.1101/gad.1856210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salek-Ardakani S, et al. ERG is a megakaryocytic oncogene. Cancer Res. 2009;69(11):4665–4673. doi: 10.1158/0008-5472.CAN-09-0075. [DOI] [PubMed] [Google Scholar]
- 39.Klusmann JH, et al. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev. 2010b;24(15):1659–1672. doi: 10.1101/gad.1903410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.