Abstract
Influenza A virus (IAV) is widely circulating in the swine population and causes significant economic losses. To combat IAV infection, the swine industry utilizes adjuvanted whole inactivated virus (WIV) vaccines, using a prime-boost strategy. These vaccines can provide sterilizing immunity toward homologous virus but often have limited efficacy against a heterologous infection. There is a need for vaccine platforms that induce mucosal and cell-mediated immunity that is cross-reactive to heterologous viruses and can be produced in a short time frame. Nonreplicating adenovirus 5 vector (Ad5) vaccines are one option, as they can be produced rapidly and given intranasally to induce local immunity. Thus, we compared the immunogenicity and efficacy of a single intranasal dose of an Ad5-vectored hemagglutinin (Ad5-HA) vaccine to those of a traditional intramuscular administration of WIV vaccine. Ad5-HA vaccination induced a mucosal IgA response toward homologous IAV and primed an antigen-specific gamma interferon (IFN-γ) response against both challenge viruses. The Ad5-HA vaccine provided protective immunity to homologous challenge and partial protection against heterologous challenge, unlike the WIV vaccine. Nasal shedding was significantly reduced and virus was cleared from the lung by day 5 postinfection following heterologous challenge of Ad5-HA-vaccinated pigs. However, the WIV-vaccinated pigs displayed vaccine-associated enhanced respiratory disease (VAERD) following heterologous challenge, characterized by enhanced macroscopic lung lesions. This study demonstrates that a single intranasal vaccination with an Ad5-HA construct can provide complete protection from homologous challenge and partial protection from heterologous challenge, as opposed to VAERD, which can occur with adjuvanted WIV vaccines.
INTRODUCTION
Influenza A virus (IAV) infection in swine can lead to significant economic losses through decreased weight gain and increased time to market. IAV also increases the susceptibility to secondary bacterial infection, leading to pneumonia, and in severe cases, to death (8, 16, 18). Due to the high rates of antigenic drift and antigenic shift, there are multiple antigenically diverse strains of IAV currently circulating throughout the swine population (32, 33). Furthermore, introductions of human and avian IAV into the swine population continue to increase the number of distinct circulating IAV strains (2, 11, 20, 33). The ever-changing diversity in circulating IAV strains is problematic for vaccine-mediated protection because the vaccine has to be updated repeatedly to provide sufficient protection against circulating strains.
Vaccines currently used in the swine industry for the control of IAV are whole inactivated virus (WIV) preparations. WIV vaccines are typically multivalent mixtures prepared with an adjuvant and administered intramuscularly using a prime-boost vaccination strategy. Adjuvanted WIV vaccines can elicit sterilizing immunity against homologous virus (14, 30, 31). However, WIV vaccines are often ineffective at protecting against heterologous strains beyond a reduction in clinical presentation of disease (1, 6, 17, 24, 31). Moreover, recent evidence indicates that WIV vaccines may, in some circumstances, result in the development of vaccine-associated enhanced respiratory disease (VAERD) when a vaccinated pig is infected with an antigenically divergent virus (6, 14, 31). VAERD is characterized by the presence of cross-reactive, nonneutralizing antibodies to heterologous virus and by enhanced lung pathology in WIV-vaccinated pigs following heterologous infection compared to that in nonvaccinated pigs (6, 14, 31). Thus, there is a need for alternative vaccine platforms that protect against heterologous infection without resulting in VAERD. Aside from the possible enhancement of disease, WIV vaccines can also be plagued by relatively long production times (38).
The large amount of time needed to license, approve, and produce a WIV vaccine for swine severely hinders its use during a novel IAV outbreak. An alternative platform to WIV that has quick production potential is a replication-defective human adenovirus 5 vector (Ad5) carrying IAV genes. Ad5 is a complete virion that was made replication defective by the removal of two segments of the Ad5 genome (10). Deletion of two Ad5 genomic sequences permits the insertion of an IAV antigen sequence for recombinant expression (reviewed in reference 29). A recent report indicates that a novel Ad5 construct can be created in less than 21 days once an antigen sequence is identified (25). The Ad5 construct can be replicated rapidly using a small bioreactor system, with viral titers of ∼1010 to 1011 PFU per ml in as few as 3 days (see the supplemental material). Considering that traditional WIV vaccine production for humans has been reported to take 5 to 6 months and takes at least as long for fully licensed commercial veterinary vaccines, the Ad5 construct is considerably faster (38). In addition to fast production potential, Ad5 makes an excellent intranasal vaccine platform due to its natural predisposition for respiratory tract infection (28). The Ad5 platform allows for the delivery and presentation of IAV antigen to the site of natural infection, and because Ad5 is an infectious particle, it initiates local immune activation in the absence of an adjuvant (28). Subcutaneous and intramuscular vaccinations with Ad5 constructs containing the hemagglutinin (HA) of IAV (Ad5-HA) have been validated as effective means of eliciting protection against IAV in mice, poultry, and swine (4, 25, 35–37). The advantages of a short production time and the option of intranasal administration make the Ad5-HA platform an attractive alternative to the vaccines currently used in swine.
Ad5-HA as a vaccine for IAV was recently improved by Steitz et al. (25) via incorporation of codon-optimized IAV HA into the Ad5 vector to improve protein expression, a change that increased immunogenicity. Thus, we sought to evaluate the efficacy of a single intranasal vaccination with an Ad5 vector encoding codon-optimized HA against homologous and heterologous challenges in swine. We report that vaccination primes a cross-reactive antigen-specific immune response, provides complete protection from homologous challenge, and limits the duration of viral shedding and the viral load following heterologous challenge.
MATERIALS AND METHODS
Animals and vaccines.
Forty-eight 3-week-old crossbred pigs were procured from a high-health-status herd known to be free of IAV and porcine reproductive and respiratory syndrome virus (PRRSV). The pigs were randomly distributed into 6 treatment groups of 8 pigs each (Table 1). Pigs were housed in biosafety level 2 (BSL2) containment, and animal care was performed in compliance with the Institutional Animal Care and Use Committee (IACUC) of the National Animal Disease Center (NADC). Replication-defective adenovirus 5 constructs containing the codon-optimized HA from A/CA/04/09 pH1N1 (CA09) and empty vector (referred to as Ad5-HA and Ad5-empty, respectively) were generated as previously described (25). The E1 and E3 gene segments of the adenovirus genome had been removed, rendering the virus replication defective. Sixteen pigs were vaccinated with 2-ml preparations containing 1010 PFU of Ad5-HA and 16 pigs received Ad5-empty at the same concentration in phosphate-buffered saline (PBS) via the intranasal route at 5 weeks of age (Table 1). One group of 8 pigs was vaccinated intramuscularly at 5 weeks of age with 128 hemagglutination units (HAU) of UV-inactivated CA09 (human isolate) mixed with an oil-in-water adjuvant (Emulsigen-D; MVP Technologies, Omaha, NE) at a virus-to-adjuvant ratio of 4:1 (vol/vol) (this preparation is referred to as kaCA) as previously described (6). The same 8 pigs were boosted 21 days later with the same preparation. Sera and nasal washes (NW) were collected every 7 days from all pigs, beginning on the day of vaccination (day 0), for the measurement of antigen-specific antibody using a previously described method (16). Blood was collected and peripheral blood mononuclear cells (PBMC) were isolated for gamma interferon-specific enzyme-linked immunosorbent spot (IFN-γ ELISpot) assay at 21 and 42 days postvaccination (dpv). Prior to challenge, one pig in the Ad5-HA group to be challenged with CA09 died from causes unrelated to the experiment (Table 1). At 42 dpv, pigs were challenged by intranasal inoculation with Madin-Darby canine kidney (MDCK) cell-propagated CA09 or A/swine/MN/02011/08 (H1N2) (MN08) at a final volume of 2 ml per pig. Results of back titrations of CA09 and MN08 challenge viruses were 104.5 and 105.5 50% tissue culture infective doses (TCID50) per ml, respectively. Nasal swabs (NS) were collected to evaluate viral shedding at 0, 1, 3, and 5 days postinfection (dpi), as previously described (6). At 5 dpi, all pigs were humanely euthanized with a lethal dose of pentobarbital (Fort Dodge Animal Health, Fort Dodge, IA). Postmortem sample collection included serum, nasal swab, nasal wash, bronchoalveolar lavage fluid (BALF), lung, and trachea collection. Collection of BALF consisted of lavage with 50 ml of minimal essential medium (MEM) as previously described (31).
Table 1.
Description of experimental treatment groupsa
| Group | Vaccine | Challenge virus | No. of mice in group |
|---|---|---|---|
| Ad5-empty/NC | Ad5-empty | Sham | 8 |
| Ad5-empty/CA09 | Ad5-empty | CA09 | 8 |
| Ad5-empty/MN08 | Ad5-empty | MN08 | 8 |
| Ad5-HA/CA09 | Ad5-HA | CA09 | 7 |
| Ad5-HA/MN08 | Ad5-HA | MN08 | 8 |
| kaCA/MN08 | kaCA | MN08 | 8 |
NC, nonchallenged; CA09, A/CA/04/09; MN08, A/swine/MN/2011/08; HA, codon-optimized HA from A/CA/04/09; kaCA, killed, adjuvanted CA09.
Microbiology.
Prior to the start of the study, all pigs were screened for antibody against IAV nucleoprotein (NP) to verify a lack of previous exposure and immunity (Influenza A Ab test; Idexx, Westbrook, MA). BALF samples collected at 5 dpi were screened for aerobic bacteria by plating 100 μl of lavage fluid on blood agar and Casmin (NAD enriched) agar plates and incubating them at 37°C for 48 h.
Antibody detection and characterization assays.
For use in hemagglutination inhibition (HI) assays, sera were heat inactivated at 56°C for 30 min, treated with a 20% kaolin (Sigma-Aldrich, St. Louis, MO)-PBS suspension, and absorbed with 0.5% turkey red blood cells (RBCs) to remove nonspecific hemagglutination inhibitors and natural serum agglutinins. The MN08 and CA09 viruses were used as antigens in the HI assays, following standard techniques with turkey RBCs (39). Reciprocal titers from HI assays were divided by 10, log2 transformed, analyzed, and reported as geometric means. Total IgG and IgA antibodies against MN08 and CA09 were detected by enzyme-linked immunosorbent assays (ELISAs) using whole-virus preparations diluted in carbonate-bicarbonate buffer to an HA concentration of 100 HAU per 50 μl; these assays are referred to as isotype ELISAs. Isotype ELISAs were performed on serum, nasal wash, and BALF samples as previously described (15, 31), with some modifications. Briefly, 100 μl of virus was used to coat Nunc Immuno 96-well plates (Nunc, Rochester, NY), which were incubated at room temperature overnight. Sera were heat inactivated at 56°C, while nasal wash and BALF samples were diluted in a 10 mM dithiothreitol-PBS buffer at a 1:1 ratio for mucus dissociation and incubated at 37°C for 1 h. All samples were assayed in triplicate. The mean optical density (OD) for triplicate wells was calculated, and antibody titers are reported as the average OD for all pigs in each respective group.
IFN-γ ELISpot assay.
On days 21 and 42 postvaccination, whole blood was collected in sodium citrate CPT tubes (BD Vacutainer, Franklin Lakes, NJ), and PBMC were separated according to the manufacturer's recommendations. Total PBMC were processed as previously described (7), enumerated, and adjusted to 5 × 105 cells per 0.1 ml. The IFN-γ ELISpot assay was performed according to the manufacturer's recommendations (porcine IFN-γ ELISpot assay; R&D Systems, Minneapolis, MN). Wells were seeded with 0.1 ml of PBMC suspension and stimulated with 50 μl containing 5 × 106 TCID50/ml live CA09 or MN08 virus, 5 μg/ml of concanavalin A, or MDCK cell sham medium. The final volume was brought to 0.25 ml. Following an 18-h incubation in a 37°C humidified 5% CO2 incubator, the assay was completed according to the manufacturer's recommendations. Plates were scanned and analyzed with UV-5 CTL-ImmunoSpot instrumentation and software (Cellular Technology Ltd., Shaker Heights, OH). The mean count for triplicate wells for each treatment for each pig was determined and used to calculate the mean for each vaccine group.
Pathology.
At necropsy, lungs were removed and evaluated for the percentage of the lung affected by purple-red consolidation typical of IAV infection in swine. The percentage of the surface area affected with pneumonia was estimated visually for each lung lobe, and the total percentage for the entire lung was calculated based on the weighted proportion of each lobe in the total lung volume (9). Tissue samples from the trachea and right middle lung lobe were fixed in 10% buffered formalin for microscopic examination. Tissues were processed by routine histopathologic procedures, and slides were stained with hematoxylin and eosin. Microscopic lesions were evaluated by a board-certified veterinary pathologist blinded to treatment groups. Scoring of lesions was based on parameters adapted from the work of Gauger et al. (6). Individual scores were assigned for four parameters: bronchial and bronchiolar epithelial changes, bronchitis/bronchiolitis, peribronchiolar lymphocytic cuffing, and edema and interstitial pneumonia. Scores were based on the percentage of airways with lesions that included epithelial changes and inflammation, on the following 5-point scale: 0, no lesions; 1, 0 to 25% of airways affected with airway epithelial damage and inflammation; 2, 26 to 50% of airways affected; 3, 51 to 75% of airways affected; and 4, >75% of airways affected. Peribronchiolar cuffing by lymphocytes was graded on a 4-point scale, as follows: 0, none; 1, mild, loosely formed cuff of lymphocytes; 2, moderate, well-formed cuffs of lymphocytes; and 3, prominent, thick, well-formed cuffs. The degree of edema and fibrin exudation was scored on the following 4-point scale: 0, none; 1, focal small area of edema in section (less than 15% of section); 2, 15 to 49% of section, including interlobular and/or pleural edema and alveolar lumina and septa; and 3, >50% of section, including interlobular and/or pleural edema and most alveolar lumina and septa. Interstitial pneumonia was graded on the following 5-point scale: 0, no lesions; 1, mild, focal to multifocal interstitial pneumonia; 2, moderate, locally extensive to multifocal interstitial pneumonia; 3, moderate, multifocal to coalescing interstitial pneumonia; and 4, severe, coalescing to diffuse interstitial pneumonia. Trachea sections were scored similarly to the bronchi and bronchioles and were based on epithelial changes and the degree of inflammation. Tracheal epithelial changes were graded on the following 5-point scale: 0, no lesions; 1, early epithelial changes characterized by focal to multifocal loss of cilia and epithelial degenerative changes; 2, mild epithelial flattening with loss of cilia and goblet cells; 3, moderate epithelial flattening with decreased thickness of respiratory epithelium and loss of cilia and goblet cells; and 4, flattened epithelium with areas of mucosa covered by a single layer of cuboidal epithelium and epithelial loss (necrosis). The degree of tracheitis was graded on a simple 4-point scale, as follows: 0, none; 1, mild; 2, moderate; and 3, severe. IAV antigen was detected in lung tissues by use of a previously described immunohistochemical (IHC) method (34), with modifications. Tissue sections were deparaffinized and hydrated in distilled water. Slides were quenched in 3% hydrogen peroxide for 10 min, rinsed three times in deionized water, and treated with 0.05% protease for 2 min. Slides were then rinsed three times in deionized water and once in Tris-buffered saline (TBS). The monoclonal antibody (MAb) HB65 (ATCC, Manassas, VA), specific for NP of IAV, was applied at a 1:100 dilution, and slides were incubated at room temperature for 1 h. Bound MAbs were stained with peroxidase-labeled anti-mouse IgG followed by a chromogen, using a Dako LSAB2-HRP detection system (Dako, Carpinteria, CA) according to the manufacturer's instructions. The slides were rinsed in deionized water and counterstained with Gill's hematoxylin. Antigen detection was assessed using two scores: (i) airway epithelial labeling and (ii) alveolar/interstitial labeling. For airway epithelium, the following 5-point scale was used: 0, none; 1, few cells with positive labeling; 2, mild scattered labeling; 3, moderate scattered labeling; and 4, abundant scattered labeling (>50% of the epithelium was positive in affected airways). For the interstitium/alveoli, the following 4-point scale was used: 0, none; 1, minimal focal signals; 2, mild multifocal signals; and 3, abundant signals.
Virus isolation from nasal swabs and BALF.
BALF was collected at 5 dpi and stored at −80°C. Nasal swabs collected at 0, 1, 3, and 5 dpi were stored at −80°C and subsequently thawed and vortexed for 15 s, followed by centrifugation for 10 min at 640 × g. Nasal swab supernatants were passed through 0.45-μm syringe filters to remove bacterial contaminants. Tenfold serial dilutions in serum-free MEM supplemented with tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (1 μg/ml; Sigma, St. Louis, MO) and antibiotics were made for each BALF and nasal swab filtrate sample. One hundred microliters of each dilution was plated in triplicate onto confluent MDCK cells in 96-well plates. After 72 h of incubation, MDCK cell monolayers were fixed with 4% phosphate-buffered formalin for 30 min. Fixed cells were stained using a previously described (13) immunocytochemistry technique that utilizes an anti-IAV nucleoprotein monoclonal antibody (HB65). Positive staining was used for the determination of virus titers. A final titer, presented as TCID50 per milliliter, was calculated for each sample by the method of Reed and Muench (23).
Statistical analyses.
Log2-transformed HI titers and log10-transformed NS viral titers were analyzed using a mixed linear model for repeated measures (Proc Mixed in SAS for Windows, version 9.2; SAS Institute Inc., Cary, NC). Covariance structures within pigs across time were tested and modeled using the REPEATED statement to determine the optimal covariance structure. Linear combinations of the least-squares mean estimates were used in a priori contrasts after testing for a significant (P < 0.05) treatment group effect. Comparisons were made between each group at each time point, using a 5% level of significance (P < 0.05), to assess statistical differences. The endpoint data for microscopic tracheal and lung lesions, macroscopic lung lesions, log10-transformed BALF viral titers, and IHC staining of the lung were analyzed by analysis of variance using a general linear model for unbalanced data. A significance level of 5% was also used for comparisons between treatment groups for the microscopic lesions and IHC results.
RESULTS
Microbiological assays.
All sera collected from pigs prior to the start of the study were negative for IAV antibody as evaluated by NP antibody ELISA. At the completion of the study, Arcanobacterium pyogenes was isolated from the BALF of 1 pig in the Ad5-empty/NC group and 1 pig in the Ad5-empty/MN08 group. Streptococcus was isolated from the BALF of one pig in the Ad5-HA/MN08 group.
IAV-specific antibody in prechallenge nasal washes and sera.
Sera from kaCA-vaccinated pigs contained HI antibodies to CA09 virus; however, HI antibody cross-reactive to MN08 virus was not detected in the sera of kaCA-vaccinated pigs. Sera from Ad5-HA-vaccinated pigs did not contain HI antibody to CA09 or MN08 virus (data not shown).
Immunoglobulin isotype-specific ELISAs were used to evaluate IAV-specific IgA and IgG in sera and nasal washes (NW). The Ad5-empty vaccine did not induce IgA or IgG titers against MN08 or CA09 at any time point prechallenge in the NW or sera. However, CA09-specific IgA was detected in the NW from Ad5-HA-vaccinated pigs, only at 14 dpv. IgG to heterologous MN08 virus was not detected in the NW or sera collected at any time point postvaccination from Ad5-HA-vaccinated pigs. Likewise, IgA antibody to MN08 antigen was not detected in prechallenge sera from the Ad5-HA-vaccinated pigs. Conversely, the kaCA-vaccinated pigs had detectable IgG antibodies to CA09 and MN08 in prechallenge sera, similar to what has been described previously (6). A summary of antibody results is provided in Table 2.
Table 2.
Summary of antibody results
| Vaccine | Sample type | Antibody isotype | Presence of antibody to viral antigen |
|
|---|---|---|---|---|
| CA09 | MN08 | |||
| Ad5-HA | Nasal wash | IgG | No | No |
| Nasal wash | IgA | Yesa | No | |
| Serum | IgG | No | No | |
| Serum | IgA | No | No | |
| kaCA | Serum | IgG | Yesb | Yesb |
| Serum | IgA | No | No | |
At day 14 postvaccination only.
At days 14 to 42 postvaccination (weekly bleeds).
Cell-mediated immunity (CMI).
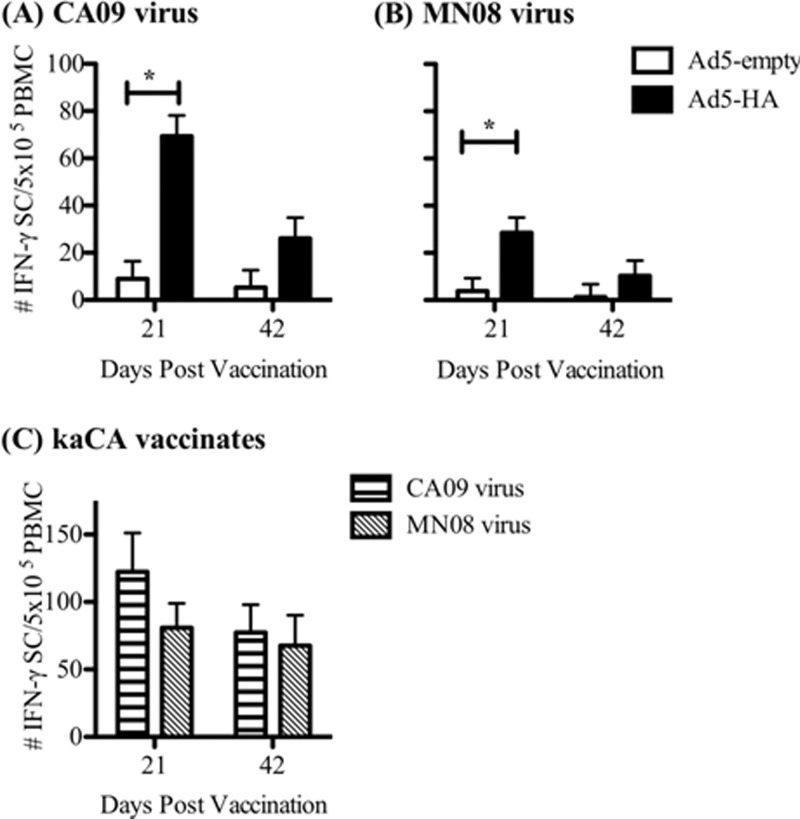
All immunized pigs exhibited antigen-specific IFN-γ recall responses to both homologous CA09 and heterologous MN08 antigens, although responses to the homologous antigen were significantly increased over those to the heterologous antigen (69.8 ± 8.8 and 28.5 ± 6.5 IFN-γ-secreting cells [IFN-γ SC]/5 × 105 PBMC, respectively, at 21 dpv) (Fig. 1A and B). In Ad5-HA-vaccinated pigs, the number of antigen-specific IFN-γ SC decreased over time, as numbers were greater at 21 dpv than at 42 dpv for both viral antigens. The numbers of CA09-specific IFN-γ SC/5 × 105 PBMC for Ad5-HA-vaccinated pigs were 69.8 ± 8.8 at 21 dpv and 26.0 ± 8.8 at 42 dpv. The kaCA vaccine primed antigen-specific IFN-γ responses to both the CA09 and MN08 viruses as well. The average number of antigen-specific IFN-γ SC detected in the kaCA vaccination group was greater than that detected following Ad5-HA vaccination, which was not surprising given that kaCA-vaccinated pigs were exposed not only to HA but also to additional IAV proteins. Although the kaCA group received a boost at 21 dpv, the number of IFN-γ SC detected at 42 dpv was at or below the level detected at 21 dpv, which was prior to the boost (Fig. 1C).
Fig 1.
Ad5-HA vaccination elicits IFN-γ responses to both homologous and heterologous viruses. Pigs were vaccinated intranasally with Ad5-empty or Ad5-HA (CA09) on day 0. PBMC were isolated on day 21 or 42 postvaccination from pigs vaccinated with Ad5-empty or Ad5-HA, and an ELISpot assay was used to determine the number of IFN-γ SC in 5 × 105 PBMC following stimulation in vitro for 18 h with live IAV strain A/CA/04/09 (A) or A/SW/MN/2011/08 (B). (C) PBMC were also collected from pigs in the kaCA group, and the number of IFN-γ SC was determined by ELISpot assay. Results are reported as means ± standard errors of the means (SEM), and statistical differences between nonvaccinated and vaccinated groups challenged with the same virus are indicated with connecting bars and asterisks (P < 0.05).
Macroscopic and microscopic lung lesions.
Macroscopic and microscopic lung lesion scores for the Ad5-HA/CA09 group were indistinguishable from those for the Ad5-HA/NC group and significantly lower than those for the Ad5-empty/CA09 group (Fig. 2 and 3, respectively). The Ad5-HA/MN08 group had macroscopic and microscopic lesion scores that were similar to those of the Ad5-empty/MN08 group, but scores for the Ad5-HA/MN08 group were significantly lower than those for the kaCA/MN08 group (Fig. 2). The kaCA/MN08 group had the highest macroscopic and microscopic lung lesion scores across all vaccination groups (Fig. 2 and 3). Microscopic tracheal lesions were less severe in the Ad5-HA/MN08 group than in either the kaCA/MN08 or Ad5-empty/MN08 group (1.8 ± 0.1 versus 2.9 ± 0.2 and 2.4 ± 0.2, respectively). The Ad5-HA-vaccinated pigs challenged with MN08 or CA09 had lower lung IAV antigen scores than the Ad5-empty or kaCA group, which is suggestive of less viral antigen (Fig. 3C). Furthermore, there was a relationship between decreased virus and lung lesions in Ad5-HA/MN08-vaccinated pigs, in that lung viral loads were reduced at 5 dpi but macroscopic and microscopic lung lesions were not significantly different from those observed in the Ad5-empty/MN08 group. This is in contrast to the case for the Ad5-HA/CA09-vaccinated pigs, which had a reduction in virus and a reduction in lung lesions compared to the Ad5-empty/CA09 group.
Fig 2.
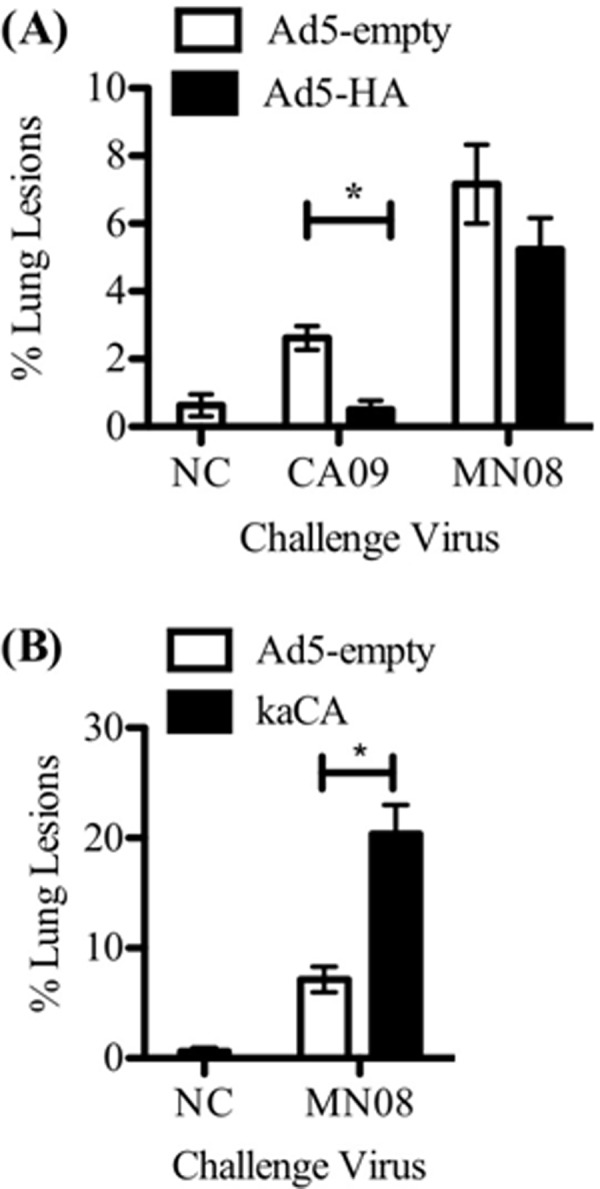
Macroscopic lung lesions on day 5 postinfection were reduced by Ad5-HA vaccination and enhanced in kaCA-vaccinated pigs. Pigs were vaccinated intranasally with Ad5-empty or Ad5-HA 42 days prior to challenge or intramuscularly with kaCA 42 and 21 days prior to challenge. Pigs were challenged intranasally with A/CA/04/09 (CA09), A/SW/MN/2011/08 (MN08), or PBS (NC). The percentage of macroscopic lung lesions in the Ad5-HA- or Ad5-empty-vaccinated pigs (A) and kaCA-vaccinated pigs (B) were evaluated 5 days after infection with the indicated virus. Results are reported as means ± SEM, and statistical differences between nonvaccinated and vaccinated groups challenged with the same virus are indicated with connecting bars and asterisks (P ≤ 0.05).
Fig 3.
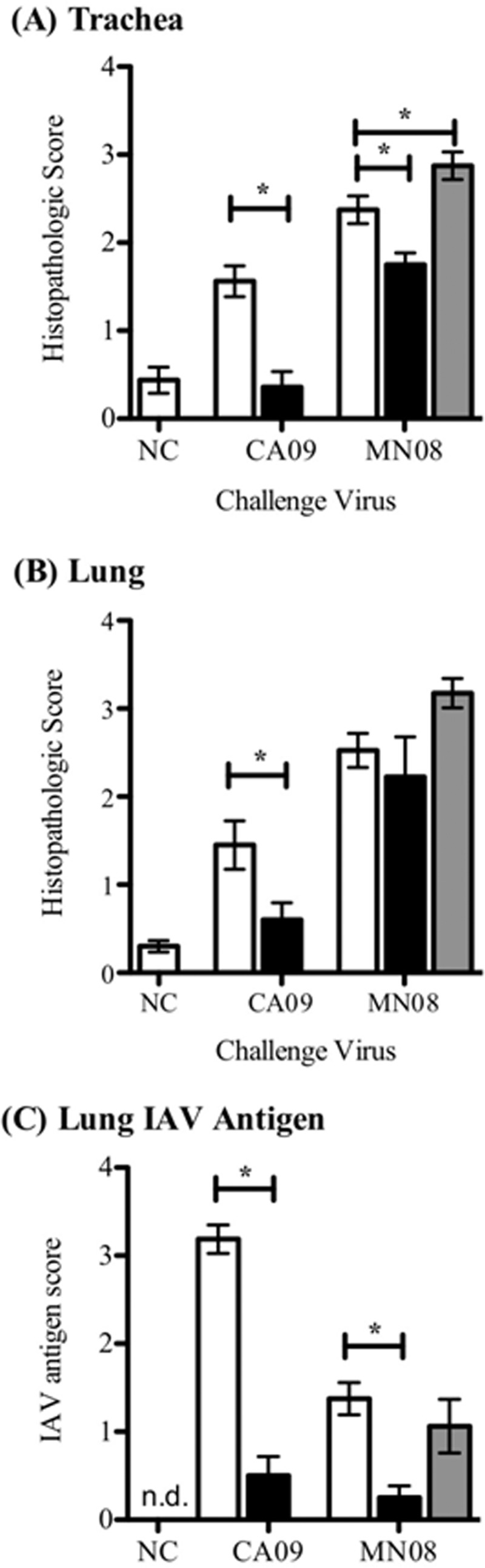
Microscopic pneumonia scores and IAV antigen scores at 5 days postinfection. Tissues were collected from pigs vaccinated intranasally with Ad5-empty (white bars) or Ad5-HA (black bars) 42 days prior to challenge or intramuscularly with kaCA (gray bars) 42 and 21 days prior to challenge. Pigs were challenged intranasally with A/CA/04/09 (CA09), A/SW/MN/2011/08 (MN08), or PBS (NC). Trachea (A) and lung (B) histopathology scores are shown for hematoxylin-and-eosin-stained formalin-fixed tissues collected 5 days following challenge with CA09 or MN08. (C) Lung IAV antigen scores identified using an anti-NP (HB65) antibody on formalin-fixed tissue 5 days after infection with CA09 or MN08 as described in Materials and Methods. Results are reported as means ± SEM, and statistical differences between nonvaccinated and vaccinated groups challenged with the same virus are indicated with connecting bars and asterisks (P ≤ 0.05).
Virus titers in BALF and nasal swabs following challenge.
Virus was not isolated from any of the nasal swab (NS) samples collected from the Ad5-HA/CA09 group but was isolated from NS collected from the Ad5-empty/CA09 group (Table 3), indicating protection from homologous challenge. Conversely, virus was detected in the NS collected at 1 and 3 dpi from pigs in the MN08 challenge group, regardless of vaccination. NS viral titers reached the highest detected level at 3 dpi and remained elevated until 5 dpi for the Ad5-empty group, regardless of the IAV challenge strain. NS samples collected at 5 dpi from the kaCA/MN08 group had lower viral titers than those collected at 3 dpi, and equal to titers observed at 1 dpi, while Ad5-HA/MN08 NS virus titers at 5 dpi were reduced to levels that were lower than the 1-dpi titers. At 5 dpi, virus titers in BALF samples were 4.9 ± 0.2 TCID50 (log10) for the Ad5-empty/CA09 group and 4.7 ± 0.3 TCID50 (log10) for the Ad5-empty/MN08 group. Conversely, virus was not detected in the BALF of Ad5-HA-vaccinated pigs following challenge with homologous or heterologous virus. Virus was not isolated from the NS or BALF collected from the empty/nonchallenged controls at any time in the study. Results are summarized in Table 3.
Table 3.
Viral titers in NS and BALF samples collected at the indicated times
| Vaccine | Challenge virus | Mean viral titer ± SEM (log10 TCID50/ml)a |
|||
|---|---|---|---|---|---|
| NS at 1 dpi | NS at 3 dpi | NS at 5 dpi | BALF at 5 dpi | ||
| Ad5-empty | NC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| Ad5-empty | CA09 | 2.1 ± 0.5b | 2.6 ± 0.3b | 2.7 ± 0.2bc | 4.9 ± 0.2b |
| Ad5-HA | CA09 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| Ad5-empty | MN08 | 3.3 ± 0.4b | 3.4 ± 0.2c | 3.3 ± 0.3c | 4.7 ± 0.3b |
| Ad5-HA | MN08 | 3.2 ± 0.4b | 3.2 ± 0.3c | 1.0 ± 0.5a | 0.0 ± 0.0a |
| kaCA | MN08 | 2.8 ± 0.6b | 4.1 ± 0.2d | 1.9 ± 0.5b | 1.1 ± 0.8a |
Different letters indicate a significant difference (P < 0.05) between treatments within the time point for specific samples (NS or BALF).
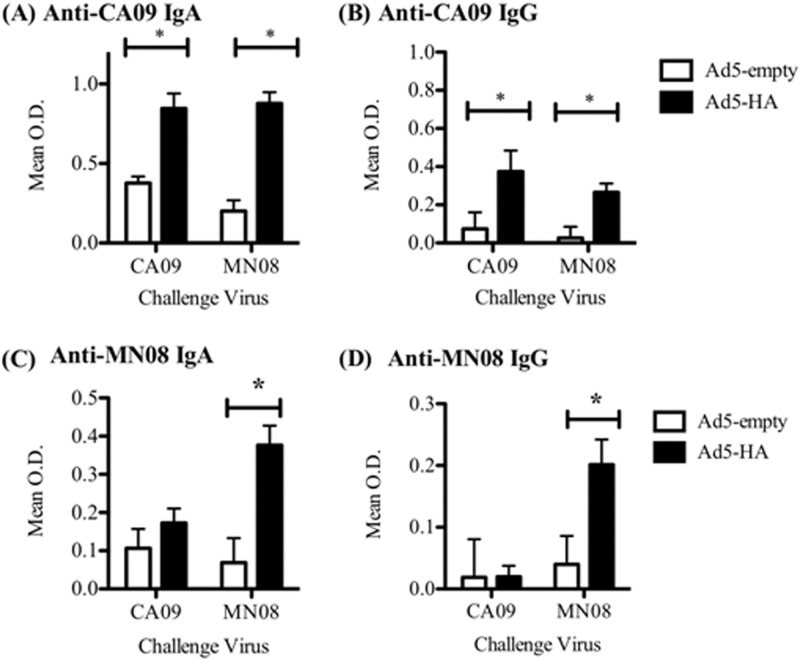
Humoral response to challenge virus in BALF at 5 dpi.
An isotype-specific ELISA using whole virus as antigen was utilized to quantify IAV-specific IgG and IgA antibodies in the BALF 5 days following challenge. The pigs vaccinated with Ad5-HA had detectable BALF IgA antibody specific to CA09, regardless of the challenge virus (Fig. 4A). MN08-specific IgA was also detected in the BALF of the Ad5-HA group challenged with MN08 (Fig. 4A), although the animals in this group were vaccinated with HA from CA09. Anti-CA09 IgG antibodies were present in the BALF of Ad5-HA-vaccinated pigs challenged with either CA09 or MN08 (Fig. 4B). However, MN08-specific IgG was present only in the BALF of the Ad5-HA group challenged with MN08 (Fig. 4B), while anti-MN08 IgG was not present in the Ad5-HA group challenged with CA09. The BALF from the kaCA/MN08-vaccinated pigs had detectable IgG and IgA specific to both the CA09 and MN08 viruses (data not shown).
Fig 4.
Ad5-HA vaccination elicits IAV-specific IgG and IgA in the lung. Pigs were vaccinated with Ad5-empty or Ad5-HA (CA09) intranasally 42 days prior to infection with A/CA/04/09 (CA09) or A/SW/MN/2011/08 (MN08). ELISA plates were coated with CA09 or MN08 as the antigen, and levels of IgA (A and C) and IgG (B and D) antibodies in BALF samples (diluted as described in Materials and Methods) collected 5 days after infection with the indicated challenge virus are shown. Results are reported as the mean OD ± SEM for each group. Statistical differences between nonvaccinated and vaccinated groups challenged with the same virus are indicated with connecting bars and asterisks (P ≤ 0.05).
DISCUSSION
The commercial IAV vaccines currently available for use in swine are based on the WIV platform. Vaccination with WIV can elicit sterilizing immunity against a homologous strain, primarily through production of antibody directed toward the receptor binding domain of the immunodominant surface glycoprotein HA (3). Due to the highly variable nature of HA, the WIV vaccine provides limited protection against heterologous viruses with demonstrated antigenic drift. Furthermore, recent reports suggest that WIV vaccines can result in VAERD when the vaccine strain and infecting virus share some antigenic similarities but vaccination does not elicit neutralizing antibodies to the infecting virus (6, 14, 31). With the high rate of antigenic drift observed in IAV and the diverse IAV strains currently circulating in the U.S. swine population, heterologous mismatches are likely to occur between vaccine and infecting strains in the field. The HA in the MN08 virus belongs to the human-like δ-cluster of HA genes, which was introduced into the swine population from human seasonal IAV, whereas the CA09 HA is a drift variant of the classical swine lineage HA, which is most closely related to the γ-cluster viruses (12, 32, 33). Protein sequence homology between the CA09 HA and the MN08 HA is approximately 77%. Therefore, a vaccine platform that provides protection against a broad range of IAV antigenic types but does not result in VAERD is highly desirable. We report herein that a single intranasal vaccination with Ad5-HA induced full protection against homologous challenge and partial protection against a heterologous challenge, by limiting the duration and amount of viral shedding. In addition, our data indicate that vaccination with Ad5-HA did not result in VAERD upon heterologous challenge for the same vaccine strain-challenge strain combination that induced VAERD with the WIV vaccine. Lastly, Ad5-HA vaccination primed an immune response that resulted in more rapid production of mucosal antibodies that were cross-reactive to heterologous virus, which likely played a role in protection.
The heterologous MN08 virus was isolated from the noses of Ad5-HA/MN08-vaccinated pigs at 1 and 3 dpi; thus, vaccination did not completely prevent heterologous infection, but the reduced nasal titers at 5 dpi indicate that prior Ad5-HA vaccination increased the rate of heterologous viral clearance. While the mechanism of heterologous viral clearance is not completely clear, establishment of an infection prior to clearance provides evidence that cell-mediated immune mechanisms likely played an important role. The role of cell-mediated immune responses may be at the level of killing virally infected cells and/or providing more rapid help to naïve B cells. In addition, it is possible that cross-reactive B cell clones already present in the respiratory tract quickly expand following infection and provide some level of protection.
Conserved regions within the CA09 and MN08 HA proteins likely contain T cell epitopes that would be recognized upon heterologous challenge. In the current study, we assessed the quantity of antigen-specific IFN-γ SC as a measure of CMI induced by Ad5-HA intranasal vaccination. Pigs vaccinated with Ad5-HA were exposed only to the CA09 HA antigen, and therefore, although whole virus was used as the recall antigen in the IFN-γ ELISpot assay, responses were likely specific only to the HA of the virus used as the recall antigen. Following Ad5-HA vaccination, PBMC were primed to produce IFN-γ in response to both the CA09 and MN08 viruses (Fig. 1A and B). However, HA-specific antibody was never detected in the blood of Ad5-HA-vaccinated pigs, regardless of the virus used in the assay (CA09 or MN08). Ad5-HA vaccination did provide protection upon heterologous challenge, as evidenced by reduced NS viral titers at 5 dpi and clearance of viable virus from the BALF at 5 dpi. Thus, our data indicate that the priming of CMI toward HA likely contributed to the clearance of heterologous challenge virus. The ELISpot assay did not discern if the IFN-γ SC were CD4+, CD8+, or CD4+ CD8+ T cells (a population of memory T cells in pigs [40]), and therefore it is difficult to pinpoint if more rapid viral clearance was the result of increased activity of cytotoxic T lymphocytes (CTL) or T helper cells. Previous research in mice indicates that CD4+ Th1 cells alone can decrease the severity of IAV infection (26). When primed CD4+ Th1 cells were passively transferred to naïve mice that were subsequently infected with IAV, the infection was quickly cleared (26). Thus, the enhanced clearance of virus in the Ad5-HA/MN08 pigs may have been due to activation of CD4+ Th1 cells that were primed toward a conserved HA epitope.
Further evidence suggesting that a primed CMI response provides protection from heterologous infection is the fact that memory CD4+ T cells have been shown to be more adept at providing B cell help than naïve CD4+ T cells, although the exact mechanism by which this occurs has not been defined clearly (reviewed in reference 27). Antibody levels in the lung lavage fluid samples from pigs in the current study provide additional support for this finding. Antibody detected in the BALF following Ad5-HA vaccination would be expected to react to CA09 HA, which was the case regardless of the challenge strain (Fig. 4). However, MN08-specific antibody was detected in the BALF only following MN08 challenge, not CA09 challenge (Fig. 4C and D). These data suggest that Ad5-HA vaccination alone (CA09 HA) did not induce the production of mucosal antibody that cross-reacted with MN08, because if this had been the case, we would have expected that lung lavage fluid collected from Ad5-HA/CA09-vaccinated pigs would cross-react with MN08 antigen. However, this was not the case. MN08-specific antibody was detected only in the lung lavage fluid of pigs in the Ad5-HA/MN08 group (Fig. 4C and D). The detection of MN08-specific antibody in the BALF of Ad5-HA/MN08-vaccinated pigs was associated with a decrease in virus titers in the BALF at the same time point (5 dpi) (Table 3), as well as a decrease in lung IAV antigen scores compared to those of Ad5-empty/MN08-challenged controls (Fig. 3C). Detection of cross-reactive antibody to MN08 in conjunction with a decrease in IAV in the lungs of Ad5-HA-vaccinated pigs (Table 3) suggests an involvement of antibody in the clearance of virus. We speculate that mucosal antibody participated in the clearance of heterologous virus and that its production was a consequence of MN08 virus challenge and subsequent reactivation of Ad5-HA-primed CMI. This does not exclude the contribution of CTL involvement to clearance of virally infected cells from the respiratory tract, and further work is warranted to investigate the mechanism of more rapid viral clearance. Regardless of the mechanism, the clearance of heterologous virus reduced the duration and amount of viral shedding, a situation that would likely result in a reduction of transmission within and between swine herds. A vaccine that reduces heterologous viral transmission and disease would significantly lessen the economic impact experienced during an outbreak of a novel IAV strain in a herd.
Previous work by Gauger et al. (6) indicates that adjuvanted WIV vaccination can cause VAERD in pigs when a heterologous mismatch between vaccine and challenge viruses occurs (6). Gauger et al. and others reported an association between VAERD and the presence of nonneutralizing antibody to the heterologous virus (6, 14, 31). Similarly, in kaCA/MN08-vaccinated pigs, we detected cross-reactive nonneutralizing antibodies along with an increased percentage of pneumonia at necropsy. Our data and those of others indicate that the involvement of nonneutralizing antibodies in the development of VAERD warrants further investigation (6, 13, 31). The kaCA vaccine did prime an antigen-specific IFN-γ SC response to both CA09 and MN08, and this response was greater in magnitude than that observed following Ad5-HA vaccination. However, the Ad5-HA vaccine encoded only a single IAV antigen, whereas kaCA would have included additional IAV antigens for increased antigen-specific recall responses upon reexposure to live virus. The route of vaccine administration may also have contributed to the differences in the number of peripheral IFN-γ SC observed between vaccine groups. Previous work in mice has shown that intramuscular immunization increases the number of antigen-specific T cells in the periphery, whereas intranasal immunization results in T cells localized in the lung (21, 22). While viral titers were reduced in both the Ad5-HA/MN08- and kaCA/MN08-vaccinated pigs by 5 dpi, the Ad5-HA/MN08-vaccinated pigs had a greater reduction than the kaCA/MN08-vaccinated pigs (Table 3). Conversely, the kaCA/MN08-vaccinated pigs had enhanced lung lesions, while lung lesions in the Ad5-HA/MN08-vaccinated pigs were not significantly different from those in Ad5-empty/MN08-vaccinated pigs (Fig. 2). The reduction in virus in the kaCA group may not have been the result of a protective immune response but, instead, the effect of the severe inflammatory environment that occurs with VAERD (5). Most importantly, our data indicate that Ad5-HA vaccines can partially protect against heterologous virus without the development of VAERD.
In summary, although commercial WIV vaccines in swine can provide sterilizing immunity against homologous viruses, they provide limited protection against heterologous viruses and may lead to VAERD (31). With a single intranasal Ad5-HA vaccination, pigs were protected against homologous challenge and viral shedding, and the length of infection following challenge with heterologous virus was significantly reduced. We clearly demonstrate that intranasal vaccination with an Ad5 vector provides multiple advantages over that with WIV. Some of the benefits of intranasal Ad5-HA vaccines include short production times, stimulation of an immune response similar to that with a natural route of infection, no requirement for added adjuvant, effectiveness with a single dose, and reduced viral shedding without causing VAERD when a viral mismatch occurs (19, 25, 28). The many benefits of intranasal vaccination with Ad5-HA suggest that this platform is a strong candidate as an alternative to the traditional WIV vaccines used in the swine industry.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gwen Nordholm, Michelle Harland, and Zahra Olson for excellent technical assistance and Jason Huegel and Jason Crabtree for assistance with animal work. We also thank Susan Brockmeier for assistance with bacteriology results.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendations or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity employer.
Footnotes
Published ahead of print 29 August 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Bikour MH, Cornaglia E, Elazhary Y. 1996. Evaluation of a protective immunity induced by an inactivated influenza H3N2 vaccine after an intratracheal challenge of pigs. Can. J. Vet. Res. 60:312–314 [PMC free article] [PubMed] [Google Scholar]
- 2. Castrucci MR, et al. 1993. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology 193:503–506 [DOI] [PubMed] [Google Scholar]
- 3. Cox RJ, Brokstad KA. 1999. The postvaccination antibody response to influenza virus proteins. APMIS 107:289–296 [DOI] [PubMed] [Google Scholar]
- 4. Gao W, et al. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80:1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gauger PC, et al. 28 March 2012. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet. Pathol. [Epub ahead of print.] doi:10.1177/0300985812439724 [DOI] [PubMed] [Google Scholar]
- 6. Gauger PC, et al. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–2719 [DOI] [PubMed] [Google Scholar]
- 7. Gorres JP, et al. 2011. DNA vaccination elicits protective immune responses against pandemic and classic swine influenza viruses in pigs. Clin. Vaccine Immunol. 18:1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goulding J, et al. 2011. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J. Infect. Dis. 204:1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halbur PG, et al. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648–660 [DOI] [PubMed] [Google Scholar]
- 10. He TC, et al. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 95:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howden KJ, et al. 2009. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can. Vet. J. 50:1153–1161 [PMC free article] [PubMed] [Google Scholar]
- 12. Karasin AI, Carman S, Olsen CW. 2006. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005). J. Clin. Microbiol. 44:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitikoon P, et al. 2006. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet. Immunol. Immunopathol. 112:117–128 [DOI] [PubMed] [Google Scholar]
- 14. Kitikoon P, et al. 2009. Swine influenza matrix 2 (M2) protein contributes to protection against infection with different H1 swine influenza virus (SIV) isolates. Vaccine 28:523–531 [DOI] [PubMed] [Google Scholar]
- 15. Larsen DL, Karasin A, Zuckermann F, Olsen CW. 2000. Systemic and mucosal immune responses to H1N1 influenza virus infection in pigs. Vet. Microbiol. 74:117–131 [DOI] [PubMed] [Google Scholar]
- 16. Loving CL, et al. 2010. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 49:237–245 [DOI] [PubMed] [Google Scholar]
- 17. Macklin MD, et al. 1998. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J. Virol. 72:1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Invest. 121:3657–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park KS, et al. 2009. Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology 395:182–189 [DOI] [PubMed] [Google Scholar]
- 20. Pereda A, et al. 2010. Pandemic (H1N1) 2009 outbreak on pig farm, Argentina. Emerg. Infect. Dis. 16:304–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price GE, et al. 2009. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine 27:6512–6521 [DOI] [PubMed] [Google Scholar]
- 22. Price GE, et al. 2010. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One 5:e13162 doi:10.1371/journal.pone.0013162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed LJ, Muench HA. 1938. A simple method of estimating fifty percent endpoints. Am. J. Infect. Control 27:493–497 [Google Scholar]
- 24. Reeth KV, Brown I, Essen S, Pensaert M. 2004. Genetic relationships, serological cross-reaction and cross-protection between H1N2 and other influenza A virus subtypes endemic in European pigs. Virus Res. 103:115–124 [DOI] [PubMed] [Google Scholar]
- 25. Steitz J, et al. 2010. A candidate H1N1 pandemic influenza vaccine elicits protective immunity in mice. PLoS One 5:e10492 doi:10.1371/journal.pone.0010492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strutt TM, et al. 2010. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 16:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4 T cells in immunity to viruses. Nat. Rev. Immunol. 12:136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tatsis N, et al. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tutykhina IL, et al. 2011. Development of adenoviral vector-based mucosal vaccine against influenza. J. Mol. Med. (Berl.) 89:331–341 [DOI] [PubMed] [Google Scholar]
- 30. Vincent AL, et al. 2010. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine 28:2782–2787 [DOI] [PubMed] [Google Scholar]
- 31. Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. 2008. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet. Microbiol. 126:310–323 [DOI] [PubMed] [Google Scholar]
- 32. Vincent AL, et al. 2006. Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet. Microbiol. 118:212–222 [DOI] [PubMed] [Google Scholar]
- 33. Vincent AL, et al. 2009. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus Genes 39:176–185 [DOI] [PubMed] [Google Scholar]
- 34. Vincent LL, Janke BH, Paul PS, Halbur PG. 1997. A monoclonal-antibody-based immunohistochemical method for the detection of swine influenza virus in formalin-fixed, paraffin-embedded tissues. J. Vet. Diagn. Invest. 9:191–195 [DOI] [PubMed] [Google Scholar]
- 35. Wesley RD, Lager KM. 2005. Evaluation of a recombinant human adenovirus-5 vaccine administered via needle-free device and intramuscular injection for vaccination of pigs against swine influenza virus. Am. J. Vet. Res. 66:1943–1947 [DOI] [PubMed] [Google Scholar]
- 36. Wesley RD, Lager KM. 2006. Overcoming maternal antibody interference by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of swine influenza virus. Vet. Microbiol. 118:67–75 [DOI] [PubMed] [Google Scholar]
- 37. Wesley RD, Tang M, Lager KM. 2004. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine 22:3427–3434 [DOI] [PubMed] [Google Scholar]
- 38. WHO 2009. Pandemic influenza vaccine manufacturing process and timeline. WHO, Geneva, Switzerland [Google Scholar]
- 39. WHO 2002. WHO manual on animal influenza diagnosis and surveillance, 2nd ed WHO, Geneva, Switzerland [Google Scholar]
- 40. Zuckermann FA. 1999. Extrathymic CD4/CD8 double positive T cells. Vet. Immunol. Immunopathol. 72:55–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.