Abstract
In regions where endemic measles virus has been eliminated, diagnostic assays are needed to assist in correctly classifying measles cases irrespective of vaccination status. A measles IgG avidity assay was configured using a commercially available measles-specific IgG enzyme immunoassay by modifying the protocol to include three 5-min washes with diethylamine (60 mM; pH 10.25) following serum incubation; serum was serially diluted, and the results were expressed as the end titer avidity index. Receiver operating characteristic analysis was used for evaluation and validation and to establish low (≤30%) and high (≥70%) end titer avidity thresholds. Analysis of 319 serum specimens expected to contain either high- or low-avidity antibodies according to clinical and epidemiological data indicated that the assay is highly accurate, with an area under the curve of 0.998 (95% confidence interval [CI], 0.978 to 1.000), sensitivity of 91.9% (95% CI, 83.2% to 97.0%), and specificity of 98.4% (95% CI, 91.6% to 100%). The assay is rapid (<2 h) and precise (standard deviation [SD], 4% to 7%). In 18 samples from an elimination setting outbreak, the assay identified 2 acute measles cases with low-avidity results; both were IgM-positive samples. Additionally, 11 patients (15 samples) with modified measles who were found to have high-avidity IgG results were classified as secondary vaccine failures; one sample with an intermediate-avidity result was not interpretable. In elimination settings, measles IgG avidity assays can complement existing diagnostic tools in confirming unvaccinated acute cases and, in conjunction with adequate clinical and epidemiologic investigation, aid in the classification of vaccine failure cases.
INTRODUCTION
Although measles was declared eliminated from the United States in 2000, sporadic outbreaks have continued to occur due to importations from areas of endemicity (30). Most cases have occurred in unvaccinated individuals presenting with the classic clinical picture of descending macular papular rash, fever, and either cough, coryza, or conjunctivitis; consequently, they can be readily diagnosed. Suspected cases can be easily laboratory confirmed following timely collection of specimens, usually a serum specimen assayed for the presence of measles-specific IgM and concordant epidemiological information (7–9, 24, 30).
A limited number of cases, however, have occurred within the vaccinated population. In contrast to measles in unvaccinated individuals, measles in vaccinated persons may present as a spectrum of symptoms ranging from classic to modified measles, the latter being a less severe disease with milder rash and/or fever and none, some, or all of the other typical measles symptoms (12). Furthermore, IgM test results in these suspect cases may be falsely negative because vaccinated persons may not make measurable measles IgM antibody in response to infection and specimens may be collected at suboptimal times due to poor symptom recognition in mild cases of modified measles (19, 21, 34). New measles assays or modified applications of currently available assays that confirm and classify active cases irrespective of vaccination status will be required to certify and maintain measles elimination.
Avidity enzyme immunoassays differentiate early (primary) from distant (secondary) antibody responses. Avidity describes the net force by which multivalent antibodies bind to multivalent antigens (18, 23). In the naive host, low-avidity IgG antibodies are elicited at the first immunological challenge. Somatic hypermutation of antibody binding sites in the presence of limiting antigen leads to the selection of high-affinity antibodies (affinity maturation), and over time, antibody matures from low to high avidity (13, 35). In measles, IgG avidity maturation is a dynamic process that begins with the first encounter with antigens from wild-type or vaccine virus (14, 27, 37).
Traditionally, measles IgG avidity assays have been used to classify primary (PVF) and secondary (SVF) vaccine failures (2, 16, 31, 33). PVF are vaccinated individuals who never responded to the vaccine and present with classic measles upon measles virus infection. In serum collected within 4 weeks of rash onset, measles IgM is present and measles IgG is of low avidity. Low-avidity results classify PVF and help confirm suspect cases even with a record of prior vaccination. In contrast, SVF are individuals with documented IgG antibody responses to vaccination whose antibody has waned over time, making them susceptible to measles virus infection. SVF produce high-avidity antibodies upon challenge with measles virus (31, 32). High-avidity results classify SVF and can be used to verify secondary immune responses in modified-measles cases with elevated plaque reduction neutralization (PRN) titers (> 30,000 mIU/ml), a newly proposed biomarker to confirm SVF that otherwise would not be confirmed by routine serology or molecular-based tests (19, 34).
Less traditionally, a measles avidity assay would be valuable in specific situations encountered in the elimination setting. Many laboratories are uneasy about reporting IgM-positive results from single serum specimens obtained from sporadic cases of rash illnesses, particularly in elimination settings, where tests are rarely used. Additionally, regarding quality of detection, declining levels of IgM antibodies in samples collected more than 4 weeks after rash onset may lead to false-negative results (4). In this context, detection of low-avidity IgG antibodies could be used to rule in these measles cases and provide assurance that a costly investigation is not initiated based on a false IgM-positive result (36, 38). Many public health laboratories do not have the resources to maintain stocks of reagents for sporadic measles serology, and often samples are tested in commercial laboratories. Furthermore, because of the low prevalence of measles, there are fewer companies in the United States selling measles-specific IgM assays. In addition, the availability of well-documented measles IgM-containing serum specimens needed to validate both commercial and “home brew” IgM assays has declined. In these situations, measles IgG avidity testing may be a less expensive and a more reliable choice to confirm suspected measles cases (36).
A well-validated measles avidity assay is needed for use as a complement to IgM testing in the serological confirmation of clinically ambiguous cases and to aid in the classification of measles vaccine failures. This study describes the development, evaluation, and applicability of a measles avidity assay. A commercially available measles IgG enzyme immunoassay was adapted for wider use.
MATERIALS AND METHODS
Samples. (i) Assay development.
Serum samples and low- to high-avidity controls used to develop the assay contained measles-specific IgG antibodies, as determined by the Captia Measles IgG enzyme immunoassay (Trinity Biotech, Jamestown, NY) (Table 1). Samples from healthy adults exposed to measles virus infection or vaccination more than a year before collection were assumed to have high-avidity antibodies. Samples collected within 3 months after measles rash onset or first-time vaccination from IgM-positive children aged 9 months and older (to avoid maternal antibody interference) were expected to have results showing low- and intermediate-avidity antibodies, depending on the timing of sample collection (14, 37). IgM was detected using the CDC measles capture IgM enzyme immunoassay (20).
Table 1.
Characteristics and number of sera used in the validation of a measles avidity assay
| Characteristic | No. of wk after vaccination or rash onset (IgM result) |
|||
|---|---|---|---|---|
| 0 to 3 (+) | 4 to 12 (+ or −) | 13 to 18 (not tested) | >1 year (+ or −) | |
| Group Aa | ||||
| Confirmed measles cases | 45 | 14 | 0 | 33 |
| MMR1 recipients | 31 | 50 | 0 | 31 |
| Total | 76 | 64 | 0 | 64 |
| Group Bb | ||||
| MMR1 recipientsc | 0 | 0 | 8 | 0 |
| Unvaccinated case, age younger than 9 monthsc | 3 | 2 | 0 | 0 |
| Outbreak immunization response recipientc | 2 | 1 | 0 | 0 |
| Collected during outbreaks in endemic settingsc | ||||
| Unknown vaccination status | 21 | 13 | 0 | 0 |
| Unknown date of rash onsetd | 0 | 4 | 0 | 0 |
| Unvaccinated, unreliable information | 6 | 0 | 0 | 0 |
| Vaccinated with symptomse,f | 0 | 0 | 0 | 20 |
| Collected during outbreaks in elimination settingse | ||||
| Unknown vaccination statusg | 0 | 0 | 0 | 29 |
| Unknown date of rash onseth | 0 | 0 | 0 | 4 |
| Unknown date of collection (both vaccinated) | 0 | 0 | 0 | 2 |
| Total | 32 | 20 | 8 | 55 |
Samples for ROC analysis were distributed into two groups: (i) a recent-exposure group with previously unvaccinated measles IgM-positive persons aged ≥9 months collected 0 to 12 weeks after their first dose of measles, mumps, and rubella vaccine (MMR1) or after rash onset (samples collected at 0 to 3 weeks were used to select the low-avidity threshold, and samples collected at 0 to 12 weeks were used to select the high-avidity threshold) and (ii) a distant-exposure group with samples collected from IgM-negative adults more than a year after natural measles or vaccination and used to select both avidity thresholds.
Samples for ROC analysis were distributed into two groups: (i) a recent-exposure group with samples described in note c (samples collected at 0 to 3 weeks were used to select the low-avidity threshold, and samples collected at 0 to 18 weeks were used to select the high-avidity threshold) and (ii) a distant-exposure group with samples described in note f was used to select both avidity thresholds. Assumptions were made to use samples with uncertain epidemiological information. Groups A and B together make up group C.
Recent-exposure group for group B.
Vaccination status was unknown for three individuals, and one was unvaccinated.
Distant-exposure group for group B.
Samples were collected 0 to 50 days after onset of rash.
Samples were collected −1 to 38 days after onset of rash.
Vaccination status was unknown for three individuals, and one was vaccinated.
(ii) Assay validation.
A total of 319 serum samples were retrospectively gathered from specimen collections acquired from diverse locations in the Americas and Africa in the years 1988 to 2004 and archived at the Centers for Disease Control and Prevention in Atlanta, GA. Institutional review board approval was obtained for outbreak and vaccine study samples. Specimens were also obtained from blood donated by healthy individuals under a CDC Institutional Review Board-approved protocol and from individuals involved in outbreaks who previously had natural measles or had received at least one dose of measles vaccine. The samples were distributed into group A and group B (Table 1).
Avidity assay for measles virus-specific IgG antibodies.
The protocol of the Captia Measles IgG enzyme immunoassay (Trinity Biotech, Jamestown, NY) was modified for use with the denaturant agent diethylamine (DEA) to develop a measles avidity assay. The antigen of the Captia assay is whole measles virus extracts. Briefly, serum avidity controls (15 μl) and test samples (15 μl) were diluted in Captia serum diluent (135 μl) and were 10-fold serially diluted in two single-well dilution series by combining diluted serum (30 μl) with serum diluent (270 μl). One dilution series started at 1:100 (or at 1:10 with serum specimens with known low levels of measles-specific IgG) and was washed with the manufacturer's suggested wash buffer (WB). The other dilution series started at 1:10 and was washed with 60 mM DEA in WB and adjusted to pH 10.25 (±0.1) with 1.0 M hydrochloric acid. Three washes were performed for 5 min each at room temperature. Next, the plates were washed three times with WB without soaking. All other steps of the assay were performed as described by the manufacturer. Incubation times were 20 min for the serum and conjugate and 10 min for the chromogen tetramethylbenzidene. A run was accepted according to the kit's quality control criteria and the avidity control criteria as defined by the precision estimates (see “Assay diagnostic performance and precision evaluation” below) (Table 2). End titer avidity index percentages (etAI%) were obtained using the formula graphically described by Jenum et al. (22) and modified as shown in Fig. S1 in the supplemental material. The result classification was low avidity if the etAI% was ≤30% and high avidity if the etAI% was ≥70%. An etAI% between 30% and 70% was considered an equivocal result, and the sample was retested. If the result after retest was still between these values, the sample was considered to be of intermediate avidity and not interpretable. Samples at 1:10 dilution with undetectable IgG after DEA treatment were classified as low avidity.
Table 2.
Evaluation of the precision of a diethylamine-based measles IgG avidity assay
| Control serum | Avg etAI% | No. of daysa | No. of lotsb | Src (%) | CV%d | STe (%) | CV%f |
|---|---|---|---|---|---|---|---|
| High | 88 | 20 | 5 | 5.76 | 6.55 | 6.24 | 7 |
| Intermediate 1 | 47 | 8 | 3 | 5.41 | 11.51 | 6.51 | 14 |
| Intermediate 2 | 46 | 8 | 2 | 3.48 | 7.56 | 6.98 | 15 |
| Low-intermediate | 28 | 13 | 3 | 3.32 | 11.85 | 4.62 | 17 |
| Low | 17 | 20 | 4 | 1.97 | 11.62 | 4.23 | 25 |
Number of days that the control was tested, in duplicates; two runs were performed per day.
Number of lots used.
Estimate of repeatability standard deviation or within-run precision.
Coefficient of variation of Sr (CV% = Sr/mean × 100).
ST is the global precision estimate.
Coefficient of variation of ST (CV% = ST/mean × 100).
Assay development.
The effect of DEA on the antigen was studied by washing the plates with WB or DEA solution at 30 mM, 60 mM, and 80 mM each at pH values of 10.00, 10.25, 10.50, and 10.75 (±0.1). Then, a serum containing high-avidity IgG antibodies was 10-fold serially diluted and incubated on the plate. The assay was completed according to the insert instructions. The results were expressed as the end titer of each curve calculated as explained in Fig. S1 in the supplemental material. The effect of DEA on bound serum antibody was examined using sera expected to contain either high- or low-avidity antibodies (see “Samples” above). Then, washes were performed with WB or with the DEA solutions described above. The results were expressed as the etAI% (see Fig. S1 in the supplemental material).
Assay diagnostic performance and precision evaluation.
Standard EP05-A2 was followed to evaluate the precision of the assay and to calculate the within-device precision standard deviation estimate (ST). ST is a global precision estimate that considers estimates of repeatability and between-day and between-run standard deviations of the assay (10). All 319 validation samples were randomized and tested in 28 separate runs using preliminary thresholds established during a pilot study (data not shown). For the precision experiment, five controls ranging from low to high avidity values were tested by two operators.
Statistical analysis.
Receiver operating characteristic curve (ROC) analysis was used to evaluate assay accuracy, establish avidity thresholds, and estimate sensitivity and specificity (15). The null hypothesis for sample size calculation was an area under the curve (AUC) of ≤0.75 (fair diagnostic accuracy) and a standard error of the AUC of ≤5% (17). A gold standard for measles avidity does not exist. Instead, measles clinical and epidemiological information was used. Accuracy was evaluated in a two-step analysis: (i) using well-defined samples (group A) and (ii) using outbreak samples with uncertain epidemiological information (group B) (Table 1) (42). Three assumptions were made to use some of the samples in group B: (i) sera from cases with vaccination records contained high-avidity IgG antibodies, (ii) sera collected from confirmed measles cases in highly vaccinated elimination settings contained high-avidity IgG antibodies from past vaccination, and (iii) sera collected in settings of endemicity up to 12 weeks after rash onset contained low-avidity IgG antibodies typical of acute measles. In the absence of date records, samples were assumed to have been collected late (4 to 18 weeks). Paired samples were accounted for during ROC analysis; SUDAAN (SAS-callable SUDAAN version 9.2) was used to estimate the variance of sensitivity and specificity, while the Taylor series linearization method (41a) was used to estimate the additional covariance due to repeated measures. ROC analysis and graphing were performed with MedCalc for Windows, version 8.1.1.0 (MedCalc Software, Belgium).
RESULTS
Avidity assay development.
The pH of DEA increases with the concentration, and both the DEA concentration and final pH influenced the stability of antigen on the microtiter plate matrix and the stability of antibody-antigen complexes. A solution of 60 mM DEA adjusted to pH 10.25 (±0.1) was effective in eluting low-avidity antibodies with minimal effect on binding of high-avidity antibodies and coating antigen (see Fig. S2 in the supplemental material). The original pH of 60 mM DEA was greater than 11.5 and resulted in loss of the optical density signal, indicating damage to either bound antibody or bound antigen. A similar effect was observed when 6 M to 8 M urea in WB was used (data not shown).
Assay diagnostic performance and precision evaluation.
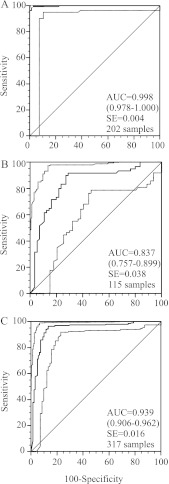
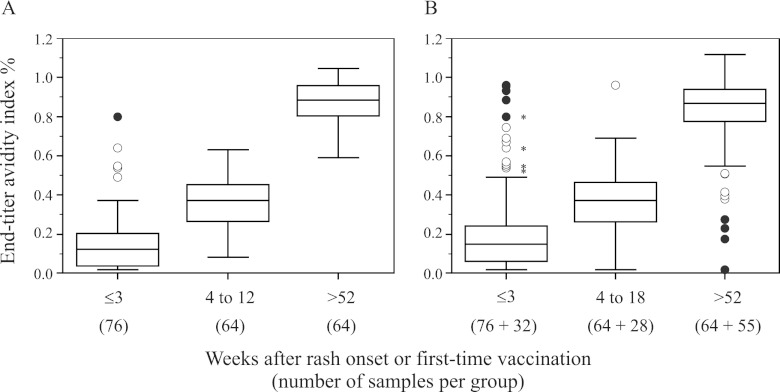
The assay is highly precise, with global precision estimate values (ST) below 7% (Table 2), and highly accurate, with AUC values greater than 0.9, even when samples with uncertain epidemiological information (group B) were included to challenge the assay's accuracy (Fig. 1A and C). When the group B samples were analyzed alone, accuracy was moderate to high (AUC = 0.8) (Fig. 1B) (15, 29, 43). Avidity thresholds were established where sensitivity and specificity were highest, at ≤30% for low avidity and at ≥70% for high avidity (Table 3). As expected, measles avidity increased with time of exposure or immunization (Fig. 2). However, a postulated low-avidity sample among the well-defined samples had a high-avidity result (Fig. 2A). It was an outbreak sample collected 5 days after rash onset from a 9-month-old infant. A follow-up sample collected at day 11 resulted in low avidity. Low-avidity results were not obtained among the distant-exposure group.
Fig 1.
ROC analysis of a diethylamine-based measles avidity assay using samples with good epidemiological records collected from persons aged ≥9 months either 0 to 12 weeks or more than 1 year after vaccination or rash (A), samples with uncertain epidemiological information collected from persons aged ≥3 months (some assumptions were made for 99 samples) (B), and combinations of the samples in panels A and B (C). The AUC is the area under the ROC curve and is plotted as a solid line. The diagonal line is an AUC of 0.5, interpreted as a random guess. The 95% confidence intervals are in parentheses and are plotted as dashed lines. The circle indicates the point with maximum accuracy.
Table 3.
Diagnostic performance indicators of a diethylamine-based measles avidity assay
| Sample group (avidity threshold) | Sensitivitya | Specificityb |
|---|---|---|
| Ac (≤30%) | 91.9 (83.2–97.0) | 100 (94.4–100) |
| Cd (≤30%) | 84.9 (76.6–91.1) | 95.0 (89.3–98.1) |
| A (≥70%) | 99.3 (96.0–100) | 98.4 (91.6–100) |
| C (≥70%) | 96.5 (92.9–98.6) | 86.6 (79.1–92.1) |
Sensitivity is the percentage of samples with low- or intermediate-avidity results among all samples collected 0 to 18 weeks after exposure (95% confidence interval).
Specificity is the percentage of samples with high-avidity results among all samples collected at least 1 year after exposure (95% confidence interval).
Sample group A contained 202 samples with good epidemiological records from persons aged ≥9 months. Sample collection after exposure was 0 to 12 weeks after the first measles vaccine or rash onset or >1 year when collected from healthy donors.
Sample group C included sample group A and 115 samples with uncertain epidemiological information from persons aged ≥3 months. Sample collection ranged from 0 to 18 weeks.
Fig 2.
Diethylamine-based measles avidity assay. Shown are box-and-whisker analyses of results over three time intervals from samples with good epidemiological records, collected from persons aged ≥9 months (A), and samples shown in panel A and samples with uncertain epidemiological information, collected from persons aged ≥3 months (B); some assumptions were made for 99 samples. The asterisks indicate outliers within sample group A. The open symbols are values smaller (larger) than the lower (higher) quartile minus (plus) 1.5 times the interquartile range. The solid symbols are values smaller (larger) than the lower (higher) quartile minus (plus) 3 times the interquartile range. The low-avidity threshold is 30%. The high-avidity threshold is 70%.
Intermediate-avidity results within the well-defined group of samples (group A).
Among samples collected within the first 3 weeks of rash onset, 7 samples out of 76 had intermediate-avidity results (Fig. 2A). Of these seven samples, three were from reported unvaccinated confirmed cases at least 15 months old that were reclassified as low-avidity samples after retest. The remaining four samples were collected from 9- to 15-month-old infants during vaccine studies. Samples 1 and 2 (Table 4) were considered to contain residual maternal antibodies because a paired prevaccine sample contained high-avidity IgG; these two samples were excluded from ROC analysis. Samples 3 and 4 were included in the ROC analysis. Within the distant-exposure group, one sample had an intermediate-avidity result (Fig. 2A).
Table 4.
Intermediate-avidity results in sera collected from 9- to 15-month-old infants within 3 weeks of their first dose of a measles virus-containing vaccine
| Sample | No. of days after vaccination | etAI% | Result |
|---|---|---|---|
| 1 | 0a | 71 | High |
| 6 to 22 | 55 | Intermediate | |
| 28 to 45 | 27 | Low | |
| >120 | 64 | Intermediate | |
| 2 | 0 | 78 | High |
| 6 to 22 | 54 | Intermediate | |
| 28 to 45 | 44 | Intermediate | |
| >120 | 74 | High | |
| 3 | 0 | NTb | NT |
| 6 to 22 | 64 | Intermediate | |
| 28 to 45 | 78 | High | |
| 4 | 0 | NT | NT |
| 14 | 51 | Intermediate |
Prevaccine collection.
NT, not tested; negative IgG result.
Assumptions made for samples with uncertain epidemiological information (group B).
Avidity results for 99 group B samples for which assumptions were made classified 32 acute infections, 2 PVF cases, 16 SVF cases, and 29 possible SVF cases, while results from 20 samples could not be interpreted. Ten samples collected in settings of endemicity were from patients with uncertain rash onset dates and/or vaccination status, and avidity testing determined that 5 had results consistent with acute infection, 2 had high-avidity results, and 3 were not interpretable (data not shown). Table 5 summarizes the avidity results for 69 samples with unknown vaccination status. Four samples collected in elimination settings had results of low avidity, two of which derived from persons exposed during two independent measles outbreaks and were easily confirmed by routine methods. However, the other two IgM-positive samples belonged to two isolated cases, which were more difficult to classify because they were not linked to other measles cases and the IgM result could have been falsely positive. One case was a woman born in 1954, in the prevaccine era, when the majority of persons were infected during childhood, acquiring immunity to the disease. The acute sample for this person, collected on the day of rash onset, was IgG negative, and a follow-up sample had to be ordered. This convalescent sample, collected 31 days after rash onset, contained low-avidity IgG antibodies, thus confirming the case by seroconversion. The second case was a young international traveler who declared he had never been vaccinated. His sample was collected 16 days after rash onset and had low-avidity IgG antibodies.
Table 5.
Measles avidity results for outbreak samples collected in settings of endemicity and elimination with unknown vaccination status
| Result | No. of samples |
|||||||
|---|---|---|---|---|---|---|---|---|
| Endemic settings |
Elimination settings |
|||||||
| ≤3a | 4–6 | Unknownb | Total (%) | ≤3 | 4–6 | Unknown | Total (%) | |
| Low | 14 | 9 | 1 | 24 (65) | 3 | 1 | 0 | 4 (12) |
| Intermediate | 4 | 4 | 2 | 10 (27) | 6 | 0 | 1 | 7 (22) |
| High | 3 | 0 | 0 | 3 (8) | 17 | 2 | 2 | 21 (66) |
| Total | 21 | 13 | 3 | 37 (100) | 26 | 3 | 3 | 32 (100) |
No. of weeks after rash onset.
Unknown, unknown date of rash onset.
Assay applicability.
The applicability of the measles avidity assay in an elimination setting was tested with 18 samples collected in the United States in 2006 during a confirmed measles outbreak. The assay was able to further confirm 2 acute measles cases in IgM-positive samples: one patient with classic measles had received an outbreak response immunization dose 1 day before rash onset, and in all respects, this patient was considered unvaccinated; the other patient had no record of vaccination. Among 11 patients with modified measles, avidity testing classified 3 (27%; 4 samples tested) as SVF and 7 (64%; 11 samples tested) as suspect SVF, since they had high-avidity results but no record of vaccination, while 1 (9%; 1 sample tested) with intermediate avidity and IgM-negative results could not be classified.
DISCUSSION
Regions involved in maintaining measles elimination refer specimens from many cases of rash illness and fever for laboratory confirmation of measles, typically by IgM enzyme immunoassay and less frequently by other serological or molecular-based tests (4, 41). However, due to inherent limitations of the methods used in measles diagnostics, not all cases can be resolved (1, 4, 11, 21). For confirmed cases in vaccinees, vaccine failure classification would normally ensue by determination of IgG avidity. In these regions, the primary use of avidity testing is in the classification of vaccine failures. However, a well-validated avidity assay can also support confirmation of clinically ambiguous cases.
As an approach to solving some of the current problems with measles diagnostics, a rapid (<2 h) and accurate measles-specific avidity assay was developed by adding a DEA wash step to the protocol of a commercially available platform. The utility of this measles avidity assay was illustrated by the analysis of samples collected during a measles outbreak in an elimination setting. This outbreak was originally confirmed by the detection of measles-specific IgM, the preferred confirmatory test. However, with the current paucity of commercial IgM assays and the limitations of current confirmatory assays, there is a need to find alternatives to help confirm the diagnosis of measles and help detect and control measles outbreaks. In our study, the majority of low-avidity results were obtained in immunologically naive IgM-positive individuals during the first 3 weeks after exposure to measles virus. Thus, in the event that IgM assays are not commercially available, this IgG-based avidity method could help confirm measles in those suspected cases with sufficient measles-specific IgG for testing. Furthermore, because specific antibodies are of low avidity for several weeks after classic rash onset, low-avidity control samples should be easier to obtain after recovery from classic measles and are expected to be more readily available than controls for IgM assays.
The central role of avidity testing is vaccine failure classification, which is important for both control and surveillance purposes (3, 31, 32). In current elimination settings, where surveillance is case based, vaccine failure classification can be helpful in characterizing the frequency and the symptoms of modified measles and in investigating the role of SVF cases in measles transmission. Additionally, cases with unknown vaccination status and high-avidity results can be classified as suspect SVF. High measles IgG avidity and very high neutralization titers appear to correlate with SVF cases upon recent exposure to measles. Together, these two parameters have recently been proposed as biomarkers for the confirmation of SVF cases, and with appropriate validation, they could be used to confirm suspected SVF cases (19). The everyday application of this diagnostic approach was further investigated with the analysis of suspected cases referred to our laboratory from 2009 to 2011 (data not shown). Among 15 confirmed cases, avidity testing classified 10 as SVF and 5 as suspected SVF. Of the 10 SVF cases, 3 had PRN titers of ≥81,916 mIU/ml, or 54 to 163 times the mean (1,525 mIU/ml) observed in unexposed vaccinated individuals (the mean was calculated by averaging the PRN geometric mean titer values of four previously described studies in unexposed vaccinated [1 or 2 doses] individuals) (19). One of these three SVF had not been confirmed by IgM or reverse transcription (RT)-PCR assays, only by an epidemiological link, and it is an example of the potential application of elevated high-avidity IgG titers as a biomarker for SVF. Interestingly, none of these 3 cases transmitted measles to others (34). Furthermore, although widespread 2-dose coverage has made PVF a rarity, identifying PVF cases in young children vaccinated once can expedite control measures.
In the measles laboratory diagnostic toolbox, the measles avidity assay is a specialized tool that can be used to help confirm suspected measles cases when information from routine assays is inconclusive (Table 6). Avidity results can be applied as follows to rule in cases. First, low-avidity results provide support to confirm measles cases, similar to the identification of recent primary infections with rubella virus, cytomegalovirus, human herpesvirus 6 and 7, and HIV (5, 26, 39, 40). In measles, low-avidity results are especially useful to rule in sporadic cases appearing outside an outbreak and when a false IgM-positive result is suspected. For example, two cases were described with IgM-positive and low-avidity IgG results. They involved a person born before the vaccine era who would generally be assumed immune and a person born outside the United States who was unvaccinated. Moreover, low-avidity results are helpful when a serum specimen is collected late and a false IgM-negative result is suspected. The high sensitivity of this measles avidity assay in the determination of primary infections provides reassurance of IgM-positive results obtained from sporadic cases, especially those suspected cases of children presenting with febrile rash illness, unknown exposure to confirmed cases, unknown vaccination status, and absence of international travel. Second, high-avidity results support the notion that elevated PRN titers in modified measles cases derive from the activation of memory responses to measles virus. In the future, high-avidity results, together with elevated measles antibody titers, could assist in confirming SVF (19). In investigating SVF cases, intermediate-avidity results could be used to support the confirmation of some cases (see the supplemental material). In contrast to congenital rubella, cytomegalovirus infection, and toxoplasmosis diagnostics, high-avidity results cannot be applied to rule out cases (5, 25, 26). In our laboratory, high-avidity results have been observed in an SVF case with classic measles disease and in an unvaccinated confirmed case with modified measles (data not shown) (19, 34).
Table 6.
Interpretation and application of measles avidity results obtained in the absence of vaccination within 10 days of rash onset and from samples collected within the first 8 weeks
| Avidity | IgM | Clinical and epidemiological information |
Application | ||
|---|---|---|---|---|---|
| Symptoms | Exposure | Infection history | |||
| Lowa | Pb or Nc | Classic | Wild type | Unvaccinated; no history | Confirms as measles |
| Low | P or N | Classic | Wild type | Unvaccinated; wild typed | Confirms as measles |
| Low | P | Classic | Unknown | Vaccinatede; no history | Confirms as measles |
| Low | P | Classic | Wild type | Vaccinated | Classifies as PVF |
| Highf | P or N | Classic | Wild type | Vaccinated | Classifies as SVF |
| High | P or N | Modified | Wild type | Vaccinated | Classifies as SVF |
| High | N | Fever; rash | Recent MMR2g | Vaccinated | Confirms previous exposure |
| High | P or N | Modified | Wild type | Previous wild-type exposureh | Confirms previous exposure |
Low avidity is interpreted as primary immune response and is diagnostic for acute classic measles.
P, positive result. IgM is usually positive from days 3 to 28.
N, negative result. Avidity testing extends the opportunity to identify cases up to 8 weeks.
May have been misdiagnosed initially; consider contagious.
Vaccination in the distant past (at least 1 year).
High avidity is interpreted as a secondary immune response.
Symptoms are side effects from second MMR vaccine dose.
Rare event; likely not contagious.
To correctly interpret the results of measles avidity assays, it is critical to have good records of vaccination status, with the number of doses and dates of administration, date of birth, time of rash onset and sample collection, and symptoms. This information is especially useful when investigating cases with modified-measles presentations. Additionally, results obtained from young children can be misinterpreted due to the presence of maternal IgG, which is generally of high avidity. For example, avidity results for a nonimmune infant infected with measles virus can initially be of high avidity. As the infant's immune response to measles virus progresses, maternal high-avidity IgG antibodies will be replaced with low-avidity antibodies from the infant, which will likely be detected in a second sample collected later. In this study, this was observed in a 9-month-old measles case with a high-avidity result in a sample collected 5 days after rash onset and a low-avidity result in a sample collected 6 days later. It was also observed in two infants recently vaccinated with their first measles-mumps-rubella vaccine (MMR) dose (Table 5).
The described measles avidity assay has limitations. First, a minimum level of measles virus-specific IgG is required; samples must be IgG positive by the Capita assay. Second, the results of the assay cannot be used in isolation; the interpretation of the results relies on accurate medical history and epidemiological information and should be considered together with other laboratory results. Third, during our validation, low-avidity results were observed in sera collected up to 9 weeks after vaccination with a first dose of MMR but were not observed later (data not shown). Therefore, low-avidity results are difficult to interpret if vaccination has occurred recently, and the results cannot be used to distinguish vaccine from wild-type infections. Fourth, intermediate-avidity results are complex to interpret, and more data are needed to understand their diagnostic relevance and ultimate value. Finally, the assay cannot rule out false IgM-positive results; the presence of high-avidity measles virus IgG does not rule out measles as a diagnosis.
The evaluation of the assay was limited in that samples were selected according to the clinical course of measles alone without considering underlying conditions, as in HIV infection, that appear to have slower measles-specific IgG avidity maturation (28). A low-avidity result in samples collected in a timely fashion from HIV-infected unvaccinated individuals would still confirm measles (6, 28). Because an evaluation was not performed on known HIV-infected measles cases, this avidity assay should not be used in the classification of vaccine failures in HIV-infected, measles-vaccinated cases.
In conclusion, this paper introduces a new measles avidity assay that is ready to be used in elimination settings. It is highly accurate, precise, and reproducible, as well as sensitive and specific. The assay is able to detect measles-specific antibody maturation over time, in line with previous observations (14, 37). Besides vaccine failure classification, avidity testing can provide very valuable information in confirming those cases with RT-PCR-negative results or with questionable IgM results. It must be emphasized that the assay can only be used to help rule in measles cases, but not to rule them out. With appropriate assay evaluation, it may be possible to adapt other commercially available measles IgG platforms to avidity testing by using the DEA elution method presented here. This avidity method worked well with another commercial plate coated with whole measles virus antigen (data not shown). Finally, the endpoint titration method was used because it is independent of the IgG concentration and it is considered superior to the single-dilution method. However, in situations where time or resources are limited, the avidity assay could be performed using a single dilution (see the supplemental material).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Cedric Brown for analyzing the effect of paired samples in the ROC analysis, for comparing diethylamine treatments, and for statistical advice. We thank Jennifer Rota and Susan Redd for their support with epidemiological information. We are grateful to Jeff Priest, Victoria Olson, Scott Schmid, Dean Erdman, and Bharat Parekh for their time, constructive comments, and critical review of an earlier version of the manuscript.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names and commercial sources are for identification purposes only and do not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services.
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print 12 September 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Afzal MA, et al. 1998. Absence of detectable measles virus genome sequence in inflammatory bowel disease tissues and peripheral blood lymphocytes. J. Med. Virol. 55:243–249 [PubMed] [Google Scholar]
- 2. Atrasheuskaya AV, et al. 2005. Measles in Minsk, Belarus, 2001–2003: clinical, virological and serological parameters. J. Clin. Virol. 34:179–185 [DOI] [PubMed] [Google Scholar]
- 3. Atrasheuskaya AV, Kulak MV, Neverov AA, Rubin S, Ignatyev GM. 2008. Measles cases in highly vaccinated population of Novosibirsk, Russia, 2000–2005. Vaccine 26:2111–2118 [DOI] [PubMed] [Google Scholar]
- 4. Bellini WJ, Helfand RF. 2003. The challenges and strategies for laboratory diagnosis of measles in an international setting. J. Infect. Dis. 187(Suppl. 1):S283–S290 [DOI] [PubMed] [Google Scholar]
- 5. Böttiger B, Jensen IP. 1997. Maturation of rubella IgG avidity over time after acute rubella infection. Clin. Diagn. Virol. 8:105–111 [DOI] [PubMed] [Google Scholar]
- 6. Brunell PA, et al. 1995. Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:540–548 [PubMed] [Google Scholar]
- 7. CDC 2012. Measles—United States, 2011. MMWR Morb. Mortal. Wkly. Rep. 61:253–257 [PubMed] [Google Scholar]
- 8. CDC 2011. Notes from the field: measles outbreak—Hennepin County, Minnesota, February-March 2011. MMWR Morb. Mortal. Wkly. Rep. 60:421. [PubMed] [Google Scholar]
- 9. CDC 2011. Notes from the field: measles outbreak—Indiana, June–July 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1169. [PubMed] [Google Scholar]
- 10. CLSI 2004. Evaluation of precision performance of quantitative measurement methods: approved guideline-2nd ed. CLSI document EP5-A2. CLSI, Wayne, PA [Google Scholar]
- 11. Dietz V, Rota J, Izurieta H, Carrasco P, Bellini W. 2004. The laboratory confirmation of suspected measles cases in settings of low measles transmission: conclusions from the experience in the Americas. Bull. World Health Organ. 82:852–857 [PMC free article] [PubMed] [Google Scholar]
- 12. Edmonson MB, et al. 1990. Mild measles and secondary vaccine failure during a sustained outbreak in a highly vaccinated population. JAMA 263:2467–2471 [PubMed] [Google Scholar]
- 13. Eisen H, Siskind G. 1964. Variation in affinities of antibodies during immune response. Biochemistry 3:996. [DOI] [PubMed] [Google Scholar]
- 14. El Mubarak HS, et al. 2004. Measles virus protein-specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. J. Med. Virol. 72:290–298 [DOI] [PubMed] [Google Scholar]
- 15. Greiner M, Pfeiffer D, Smith RD. 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 45:23–41 [DOI] [PubMed] [Google Scholar]
- 16. Hamkar R, et al. 2006. Distinguishing between primary measles infection and vaccine failure reinfection by IgG avidity assay. East Mediterr. Health J. 12:775–782 [PubMed] [Google Scholar]
- 17. Hanley JA, McNeil BJ. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36 [DOI] [PubMed] [Google Scholar]
- 18. Hedman K, Lappalainen M, Söderlund M, Hedman L. 1993. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 4:123–129 [Google Scholar]
- 19. Hickman C, et al. 2011. Laboratory characterization of measles virus infection in previously vaccinated and unvaccinated individuals. J. Infect. Dis. 204(Suppl. 1):S549–S558 [DOI] [PubMed] [Google Scholar]
- 20. Hummel KB, Erdman DD, Heath J, Bellini WJ. 1992. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J. Clin. Microbiol. 30:2874–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyde TB, et al. 2009. Laboratory confirmation of measles in elimination settings: experience from the Republic of the Marshall Islands, 2003. Bull. World Health Organ. 87:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenum PA, Stray-Pedersen B, Gundersen AG. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuby J. 1997. Immunology, 3rd ed W. H. Freeman, New York, NY [Google Scholar]
- 24. Kutty P, Rota J, Bellini W, Redd SB. 17 April 2012, posting date Chapter 7, Measles. In Manual for the Surveillance of Vaccine-Preventable Diseases, 5th ed CDC, Atlanta, GA: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html [Google Scholar]
- 25. Lappalainen M, Hedman K. 2004. Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity. Ann. Ist Super Sanita 40:81–88 [PubMed] [Google Scholar]
- 26. Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41:192. [DOI] [PubMed] [Google Scholar]
- 27. Nair N, et al. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J. Infect. Dis. 196:1339–1345 [DOI] [PubMed] [Google Scholar]
- 28. Nair N, et al. 2009. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J. Infect. Dis. 200:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obuchowski NA, Lieber ML, Wians FH., Jr 2004. ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin. Chem. 50:1118–1125 [DOI] [PubMed] [Google Scholar]
- 30. Orenstein WA, Papania MJ, Wharton ME. 2004. Measles elimination in the United States. J. Infect. Dis. 189(Suppl. 1):S1–S3 [DOI] [PubMed] [Google Scholar]
- 31. Pannuti CS, et al. 2004. Identification of primary and secondary measles vaccine failures by measurement of immunoglobulin G avidity in measles cases during the 1997 Sao Paulo epidemic. Clin. Diagn. Lab. Immunol. 11:119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paunio M, Hedman K, Davidkin I, Peltola H. 2003. IgG avidity to distinguish secondary from primary measles vaccination failures: prospects for a more effective global measles elimination strategy. Expert Opin. Pharmacother. 4:1215–1225 [DOI] [PubMed] [Google Scholar]
- 33. Paunio M, et al. 2000. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol. Infect. 124:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rota J, et al. 2011. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J. Infect. Dis. 204(Suppl. 1):S559–S563 [DOI] [PubMed] [Google Scholar]
- 35. Siskind GW, Benacerraf B. 1969. Cell selection by antigen in the immune response. Adv. Immunol. 10:1. [DOI] [PubMed] [Google Scholar]
- 36. Thomas HI, Barrett E, Hesketh LM, Wynne A, Morgan-Capner P. 1999. Simultaneous IgM reactivity by EIA against more than one virus in measles, parvovirus B19 and rubella infection. J. Clin. Virol. 14:107–118 [DOI] [PubMed] [Google Scholar]
- 37. Tuokko H. 1995. Detection of acute measles infections by indirect and mu-capture enzyme immunoassays for immunoglobulin M antibodies and measles immunoglobulin G antibody avidity enzyme immunoassay. J. Med. Virol. 45:306–311 [DOI] [PubMed] [Google Scholar]
- 38. Vardas E, Kreis S. 1999. Isolation of measles virus from a naturally-immune, asymptomatically re-infected individual. J. Clin. Virol. 13:173–179 [DOI] [PubMed] [Google Scholar]
- 39. Ward KN. 2005. The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J. Clin. Virol. 32:183–193 [DOI] [PubMed] [Google Scholar]
- 40. Wei X, et al. 2010. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res. Hum. Retrovir. 26:61–71 [DOI] [PubMed] [Google Scholar]
- 41. WHO 2007. Manual for the laboratory diagnosis of measles and rubella virus infection, 2nd ed http://www.who.int/immunization_monitoring/LabManualFinal.pdf Accessed 23 May 2012
- 41a. Williams RL. 2008. Taylor series linearization. In Lavrakas PJ. (ed), Encyclopedia of survey research methods. Sage Publications, Inc., Thousand Oaks, CA [Google Scholar]
- 42. Zhou X-H, Obuchowski NA, McClish DK. 2002. Statistical methods in diagnostic medicine. Wiley-Interscience, New York, NY [Google Scholar]
- 43. Zweig MH, Campbell G. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561–577 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.