Abstract
Immunity against Vibrio cholerae O1 is serogroup specific, and serogrouping is defined by the O-specific polysaccharide (OSP) part of lipopolysaccharide (LPS). Despite this, human immune responses to V. cholerae OSP have not previously been characterized. We assessed immune responses against V. cholerae OSP in adults with cholera caused by V. cholerae O1 El Tor serotype Inaba or Ogawa in Dhaka, Bangladesh, using O1 OSP-core–bovine serum albumin (OSPc:BSA) conjugates; responses targeted OSP in these conjugates. Responses of Inaba-infected patients to Inaba OSP and LPS increased significantly in IgG, IgM, and IgA isotypes from the acute to convalescent phases of illness, and the responses correlated well between OSP and LPS (R = 0.86, 0.73, and 0.91, respectively; P < 0.01). Plasma IgG, IgM, and IgA responses to Ogawa OSP and LPS in Ogawa-infected patients also correlated well with each other (R = 0.60, 0.60, and 0.92, respectively; P < 0.01). Plasma IgM responses to Inaba OSP and Ogawa OSP correlated with the respective serogroup-specific vibriocidal antibodies (R = 0.80 and 0.66, respectively; P < 0.001). Addition of either OSPc:BSA or LPS, but not BSA, to vibriocidal assays inhibited vibriocidal responses in a comparable and concentration-dependent manner. Mucosal IgA immune responses to OSP and LPS were also similar. Our study is the first to characterize anti-OSP immune responses in patients with cholera and suggests that responses targeting V. cholerae LPS, including vibriocidal responses that correlate with protection against cholera, predominantly target OSP. Induction of anti-OSP responses may be associated with protection against cholera, and our results may support the development of a vaccine targeting V. cholerae OSP.
INTRODUCTION
Cholera is a severe diarrheal disease in humans caused by infection with either Vibrio cholerae serogroup O1 or O139, and it continues to be a major health burden in resource-limited countries worldwide. Cholera affects 3 to 5 million people annually, resulting in over 100,000 deaths (51, 53). V. cholerae is a noninvasive Gram-negative bacterium that induces rapid dehydration in patients by secreting cholera toxin (CT) at the gut mucosal surface, resulting in a profuse secretory diarrhea. Strains of V. cholerae are differentiated by the O-specific polysaccharide (OSP) portion of V. cholerae lipopolysaccharide (LPS) in the outer membrane of the bacterium. Over 200 O serogroups of V. cholerae have been characterized to date, but only serogroups O1 and O139 are known to cause epidemic cholera. The O1 serogroup can itself be classified into the El Tor and classical biotypes based on a number of genotypic and phenotypic characteristics. Globally, O1 El Tor is by far the most prevalent serogroup causing cholera. The O1 serogroup can also be subdivided into two major serotypes, Inaba and Ogawa (45), which differ only by the presence of a 2-O-methyl group in the nonreducing terminal sugar of the Ogawa OSP, which is absent from Inaba OSP (16, 17, 50).
A growing body of evidence suggests that the human immune response to V. cholerae LPS provides protective immunity against cholera (10, 15, 33). Patients with cholera respond with LPS-specific antibodies in the IgG, IgM, and IgA isotypes (35, 36, 42). The best-characterized correlate of protection against cholera has historically been the vibriocidal response, an assay that measures the ability of serum antibodies to lyse V. cholerae in the presence of complement (28). In population studies in Bangladesh, every 2-fold increase in vibriocidal titer is associated with a 44% decrease in the subsequent risk of cholera (30). In household contacts of cholera patients, the baseline vibriocidal response in contacts inversely correlates with the risk of developing cholera in the following 3 weeks (15). More recently, it has also been demonstrated that baseline plasma IgA antibody levels to LPS and circulating baseline IgG memory B cells targeting V. cholerae LPS also correlate with protection against subsequent disease in household contacts of cholera patients (15, 33). The vibriocidal response has previously been shown to be largely comprised of IgM responses targeting LPS (28).
V. cholerae LPS consists of the O-specific polysaccharide (OSP) connected through a core oligosaccharide to lipid A (endotoxin) that is integrated into the bacterial outer membrane (52). Lipid A and the core are similar across all V. cholerae serogroups (5, 6, 48), with the lipid A component of El Tor organisms undergoing glycine modification (13). The V. cholerae El Tor and O139 strains have shared lipid A cores (13). The OSPs vary widely across the 200 serogroups of V. cholerae and are the basis of serogrouping. Importantly, protective immunity against V. cholerae is serogroup specific. Infection with V. cholerae O1 provides no cross-protection from cholera caused by V. cholerae O139 and vice versa (2, 42, 49). Despite this, human immune responses to V. cholerae OSP have not previously been characterized. To assess these immune responses, we utilized O1 Ogawa and Inaba OSP-core–bovine serum albumin (OSPc:BSA) conjugates. The OSPc:BSA conjugates were prepared using squaric acid chemistry as previously described (52). Immune responses targeting OSP were then evaluated in adult patients with cholera caused by infections with the V. cholerae O1 El Tor serotypes Inaba and Ogawa in Dhaka, Bangladesh.
MATERIALS AND METHODS
Study population.
Study participants were selected from patients admitted to the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B) with severe acute watery diarrhea and with stool cultures positive for V. cholerae O1 or O139. Stool samples were plated on taurocholate-tellurite-gelatin agar and gelatin agar (Difco, Detroit, MI) overnight. Suspected Vibrio colonies were identified by slide agglutination by using monoclonal antibodies against the V. cholerae O1 and O139 serogroups (42). Stools were also analyzed to detect other enteric pathogens—e.g., enterotoxigenic Escherichia coli (37) and Salmonella, Shigella, and Campylobacter spp. (40)—and were tested by direct microscopy for cyst and vegetative forms of parasites and ova of helminths. Only patients positive for V. cholerae and negative for other bacterial pathogens were enrolled in this study. The stools of healthy controls were similarly screened. All of the cholera patients were treated with intravenous fluid resuscitation as well as with doxycycline or azithromycin (14, 18). Adults from an urban area in the city of Dhaka with demographic characteristics similar to those observed in the cholera patients and with no history of diarrhea during the previous 1 month were included as healthy controls (23).
All patients were adults 18 years of age or older. Table 1 presents basic demographic information about the patients. Stored plasma specimens from patients infected with V. cholerae O1 Ogawa (n = 17), Inaba (n = 15), and O139 (n = 15) were analyzed. Samples collected at days 2, 7, 30, 90, 180, 270, and 360 after onset of disease were analyzed for LPS, OSP, and vibriocidal antibody responses. In addition, nine patients with V. cholerae O1 Ogawa infection were enrolled from September 2011 to January 2012 to assess mucosal antibody-secreting cell (ASC) responses to Ogawa OSP, Ogawa-LPS, and cholera toxin B subunit (CtxB) in fresh cells recovered from blood on days 2, 7, and 30 after onset of cholera. Stored antibody-in-lymphocyte supernatant (ALS) samples obtained from days 2 and 7 after onset of disease from Ogawa (n = 10) and Inaba (n = 10) patients or healthy controls (n = 10) were used to further characterize mucosal responses to Ogawa OSP and LPS (18). The Institutional Review Boards of the ICDDR,B and the Massachusetts General Hospital approved this study. All study participants gave informed written consent for study enrollment.
Table 1.
Demographic and clinical characteristics of cholera patients and healthy controls in this study
| Sample type and infectiona | No. of patients | Median age (yr) |
|---|---|---|
| Plasma | ||
| Ogawab | 17 | 29 |
| Inabab | 15 | 30 |
| O139c | 15 | 35 |
| Stool extract | ||
| Ogawad | 14 | 29 |
| Inabad | 14 | 29 |
| ALS | ||
| Ogawae | 10 | 33 |
| Inabae | 10 | 36 |
| Healthyf | 10 | 33 |
| ASC | ||
| Ogawad | 9 | 32 |
ALS, antibody-in-lymphocyte supernatant assay; ASC, antibody-secreting cell assay.
Specimens were analyzed on day 2 and on follow-up days 7, 30, 90, 180, 270, and 360.
Specimens were analyzed on days 2, 7, and 21.
Specimens were analyzed on days 2, 7, and 30.
Specimens were analyzed on days 2 and 7.
Specimens were analyzed on day 0 for specimens obtained from healthy controls.
V. cholerae LPS, OSP, and CtxB.
We prepared V. cholerae LPS, OSPc, and OSPc:BSA conjugates as previously described (52). Briefly, we obtained LPS from V. cholerae O1, Ogawa (strain X-25049) and Inaba (strain T19479) by protein denaturation by hot phenol-water extraction followed by enzymatic treatment (proteinase K, DNase I, and RNase A), and ultracentrifugation (100,000 × g for 3 h) as previously described (52). The samples were dialyzed against distilled water, freeze dried, and stored for subsequent use. The amount of protein in the purified LPS preparations ranged from 10 to 19 μg per mg (<2% by weight). To define antigens within the LPS preparations to be used in immunological analyses, we used high-resolution liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis using an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific) as previously described (4). LC-MS/MS data analysis, protein identification, and peak list generation were performed using the Proteome Discoverer (Thermo Fisher Scientific) algorithm incorporating the SEQUEST search engine and Percolator (19) as previously described (27). MS/MS data were searched using 10-ppm mass accuracy on precursor m/z and a 0.5-Da window on fragment ions. Fully enzymatic tryptic searches with up to three missed cleavage sites were allowed. Oxidized methionines were searched as a variable modification, and alkylated cysteines were searched as a fixed modification. V. cholerae databases (N16961, O395, and INDRE 9/11) were downloaded from EMBL and supplemented with common contaminants. We filtered peptides for each charge state to a false discovery rate (FDR) of 1% and then grouped peptides into proteins using Occam's razor logic.
To generate OSPc:BSA conjugates, we used a protocol previously described by us (52) that includes acid hydrolysis, chloroform-based separation and recovery of hydrolysate, size exclusion chromatography, ultracentrifugation, and dialysis. The retentate was lyophilized to afford the OSPc antigens as white solids, and analysis by 13C nuclear magnetic resonance (NMR) surface-enhanced laser desorption ionization–time of flight (SELDI-TOF) mass spectroscopy indicated that the average molecular masses of the Inaba and Ogawa OSPc antigens were ∼5,100 and ∼5,900 Da, respectively. To facilitate binding to the plastic plates and membranes used in immunologic analyses, we conjugated OSPc to bovine serum albumin to generate OSPc:BSA, as previously described (52). Briefly, we modified OSPc for conjugation using squarate derivatization engaging the single amino group present in the core (48), filtration, and dialysis (3,000-Da cutoff). The retentate was lyophilized to afford the OSPc squarate monomethyl ester as a white solid. The lyophilate was dissolved in 0.5 M pH 9 borate buffer to yield ∼2.5 mM (Inaba) or ∼4.9 mM (Ogawa) solutions with respect to OSPc; BSA was added in the amount necessary to form OSPc/carrier molar ratios of ca. 11:1 for Inaba and 22:1 for Ogawa. Conjugation was monitored by SELDI-TOF MS at 24, 48, 72, 96, and 240 h, when the reaction was terminated by addition of 300 μl of pH 7 phosphate buffer. The solution was transferred to centrifugation filtration tubes, dialyzed against 10 mM aqueous ammonium carbonate, and lyophilized. On the basis of the average molecular weight (MW) of OSPc, the Inaba core/BSA ratio of the final product was 2.8:1 and the Ogawa core/BSA ratio was 4.8:1. To assess responses targeting CtxB, we used recombinant antigen graciously supplied by A. M. Svennerholm, Gothenburg University, Sweden.
Immune responses in stored plasma and ALS specimens of study participants.
We quantified anti-LPS and anti-OSP IgG, IgM, and IgA responses in plasma using an enzyme-linked immunosorbent assay (ELISA) protocol as previously described (38, 39, 52). Briefly, we coated 96-well polystyrene plates (Nunc F) with V. cholerae O1 Ogawa or Inaba LPS (2.5 μg/ml) (36) dissolved in phosphate-buffered saline (PBS) or Ogawa or Inaba OSPc:BSA (1 μg of the conjugated product/ml) dissolved in carbonate buffer (pH 9.6). To each well, we added 100 μl of plasma (diluted 1:40 in 0.1% BSA in phosphate-buffered saline–0.05% Tween) and detected antigen-specific antibodies in the sample using horseradish peroxidase-conjugated rabbit anti-human IgA, IgG, and IgM as secondary antibodies (1:1,000 dilution; Jackson ImmunoResearch, West Grove, PA). We developed plates with 1 mg/ml ortho-phenylene diamine (Sigma, St. Louis, MO) dissolved in 0.1 M sodium citrate buffer (pH 4.5), with 0.03% H2O2 added, and determined the optical density at 450 nm by reading the plates kinetically for 5 min at 14-s intervals (18). The maximum slope for an optical density change of 0.2 U was reported as milli-optical density units per minute (mOD/min). We normalized data to ELISA units by calculating the ratio of the optical density of the test sample to that of a standard of pooled convalescent-phase plasma from patients previously infected with cholera that was included on each plate.
ALS specimens were collected after 48 h of culture as previously described (41). ELISAs using ALS were performed according to the plasma ELISA protocol as outlined above. The ALS samples were diluted 1:2 in 0.1% BSA in phosphate-buffered saline–0.05% Tween, and responses were detected using horseradish peroxidase-conjugated rabbit anti-human IgA as the secondary antibody (1:1,000 dilution; Jackson ImmunoResearch, West Grove, PA), with development as described above. A patient was considered a responder when the response on day 7 increased 2-fold or higher than that seen on day 2 after onset of diarrhea.
Quantification of circulating IgG, IgM, and IgA ASCs to OSP, LPS, and CtxB.
Ogawa OSP, Ogawa LPS, and CtxB-specific ASC responses were measured by enzyme-linked immunosorbent spot (ELISPOT) as previously described (14, 42, 44). Briefly, nitrocellulose-bottomed plates (Millipore, Bedford, MA) were coated with Ogawa OSPc:BSA in carbonate buffer (pH 9.6) (10 μg of conjugate/ml), Ogawa LPS (25 μg/ml), affinity-purified goat anti-human Ig (5 μg/ml; Jackson Immunology Research, West Grove, PA), monosialotetrahexosylganglioside (GM1 ganglioside) (3 nM), or keyhole limpet hemocyanin (KLH) (2.5 μg/ml) and were incubated overnight at 4°C (42). Prior to blocking, CtxB (2.5 μg/ml) was added to the GM1-coated plates, and the plates were incubated for 1 h at 37°C. All plates were then blocked for 2 h at 37°C prior to use, with RPMI 1640 containing 10% fetal bovine serum. Peripheral blood mononuclear cells (PBMCs) were harvested as previously described by centrifugation of diluted whole-blood samples on Ficoll-Isopaque (Pharmacia, Piscataway, NJ) (18). A total of 6 × 105 PBMCs/well were added to the OSPc:BSA-, LPS-, and CtxB-coated plates, while 1 × 105 PBMCs/starting well were added to the total Ig-coated plates and serially diluted. After incubation at 37°C for 3 h, the plates were washed and IgG, IgM and IgA ASCs were detected using horseradish peroxidase-conjugated mouse anti-human IgA and IgM (Hybridoma Reagent Laboratory, Baltimore, MD) and alkaline phosphatase-conjugated IgG (Southern Biotech, Birmingham, AL), both diluted 1:500. After overnight incubation at 4°C, IgG-conjugated plates were developed with 5-bromo-4-chloro-3-indolyl-phosphate–nitroblue tetrazolium. IgA- and IgM-conjugated plates were developed with 3-amino-9-ethylcarbazole. ASCs were independently quantified by two individuals using a stereomicroscope (Leica WILD M3Z). KLH (Pierce Biotechnology, Rockford, IL)-coated plates were used as a negative control. The numbers of antigen-specific IgG, IgM, and IgA ASCs were expressed per 106 PBMCs.
Vibriocidal assay and vibriocidal inhibition assay with OSP and LPS.
We assessed vibriocidal antibody responses as previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 Ogawa (X-25049) or Inaba (T19479) as the target organism (1, 14, 39, 42, 52). The vibriocidal titer was defined as the reciprocal of the highest serum dilution resulting in >50% reduction of the optical density associated with V. cholerae growth compared to that of the positive-control wells without plasma.
The vibriocidal inhibition assay was performed as previously described (9, 12, 22). In brief, after heat inactivation of day 7 plasma at 56°C for 30 min, Ogawa OSPc:BSA and LPS at concentrations of 1, 10, and 100 μg/ml were mixed with diluted plasma (1:160) and incubated at 37°C while gently shaking for 1 h. The same concentrations of BSA and CtxB were also incubated with diluted plasma as controls, in addition to plasma without any antigen added. These samples were then used to assess the vibriocidal titer of the plasma using the standard vibriocidal assay protocol described above (1, 14, 39, 42, 52).
ELISAs for IgA specific to Ogawa OSP and LPS in fecal extracts.
We used an ELISA format to determine the total IgA content in fecal samples using pooled human Swedish milk with a known IgA concentration of 1 mg/ml as the standard and with affinity-purified goat antibodies to the F(ab′)2 fragment of human immunoglobulin as the capture antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), as previously described (38, 46); detection was with goat anti-human immunoglobulin specific to IgA, conjugated to horseradish peroxidase as the secondary antibody (1:3,000 dilution; Jackson ImmunoResearch, West Grove, PA). Anti-Ogawa OSP and LPS IgA responses in fecal extracts were detected using rabbit anti-human immunoglobulin specific to IgA conjugated to horseradish peroxidase as the secondary antibody (1:1,000 dilution; Jackson ImmunoResearch, West Grove, PA). Responses were measured kinetically and expressed as milliabsorption units per minute per microgram of total IgA in fecal extracts. Patients whose fecal IgA responses to Ogawa OSP and LPS at convalescence (day 7 and/or day 30) doubled in comparison to day 2 levels were considered responders.
Statistical analyses.
We compared the magnitude of acute to convalescent phase responses using the Wilcoxon signed rank test and used the Mann-Whitney U test to compare the immune responses to OSPc:BSA, LPS, and healthy controls. All reported P values were two tailed, with a cutoff of P ≤ 0.05 considered a threshold for statistical significance. Analyses and figure preparations were performed using Graphpad Prism 5.0 (Graphpad Software, Inc., La Jolla, CA). Pearson's product moment correlation analysis was performed using SigmaStat 3.1 (Systat Software, San Jose, CA) for correlations between vibriocidal antibody and antigen-specific antibody responses.
RESULTS
Analysis of LPS and OSPc:BSA.
The LPS preparations were found to contain around 10 to 19 μg protein/mg LPS (<2% protein by weight). MS analysis of the two V. cholerae LPS preparations disclosed a total of 610 proteins, including many extracellular and membrane proteins, such as flagellin A, starvation lipoprotein Slp-like protein, a number of methyl-accepting chemotaxis proteins (VC1248 and VCA0906), and general secretion pathway proteins (EpsD, EpsI, and EpsN) (data not shown). Of the 396 proteins with functional annotations in the J. Craig Venter Institute database, the most highly represented groups included hypothetical proteins and proteins involved in cellular processes and pathogenesis and energy metabolism. The role that the identified proteins may play in mediating immune responses during cholera is unknown; however, our analysis suggests that standard preparations of LPS are heterogeneous.
OSPc:BSA is highly purified compared to LPS. SELDI analysis of Inaba OSPc:BSA after freeze-drying showed that the average molecular mass of the conjugate obtained was 81,000 Da (OSPc:BSA molar ratio of ∼2.8). The conjugate still contained ∼5% of the unchanged BSA (52). SELDI analysis of Ogawa OSPc:BSA after freeze-drying showed that the average molecular mass of the conjugate obtained was 95,000 Da (OSPc:BSA molar ratio of ∼4.8); unconjugated BSA was not detected (52).
IgG, IgM, and IgA responses to V. cholerae O1 Ogawa and Inaba OSP and LPS antigens.
We found prominent reactivity of convalescent-phase sera from patients recovering from cholera using OSPc:BSA, but not unconjugated OSPc, presumably through improved binding of the polysaccharide-conjugate antigens to assay wells (8). We measured plasma IgG, IgM, and IgA antibody responses to Ogawa and Inaba OSPc:BSA and LPS antigens over a 1-year period postinfection in patients infected with V. cholerae O1 Ogawa and Inaba and responses in O139 patients to Ogawa OSPc:BSA over a 21-day period. As described below, we found prominent responses targeting O1 OSPc:BSA in patients recovering from O1 serogroup cholera, but we did not detect such responses in patients recovering from O139 serogroup cholera, despite the fact that these serogroups share identical core oligosaccharides (5, 6, 48), suggesting that the measured responses were specific to the OSP component of the conjugates and not to the core or protein components. Henceforth, we refer to the responses in immunologic assays using OSPc:BSA as targeting OSP.
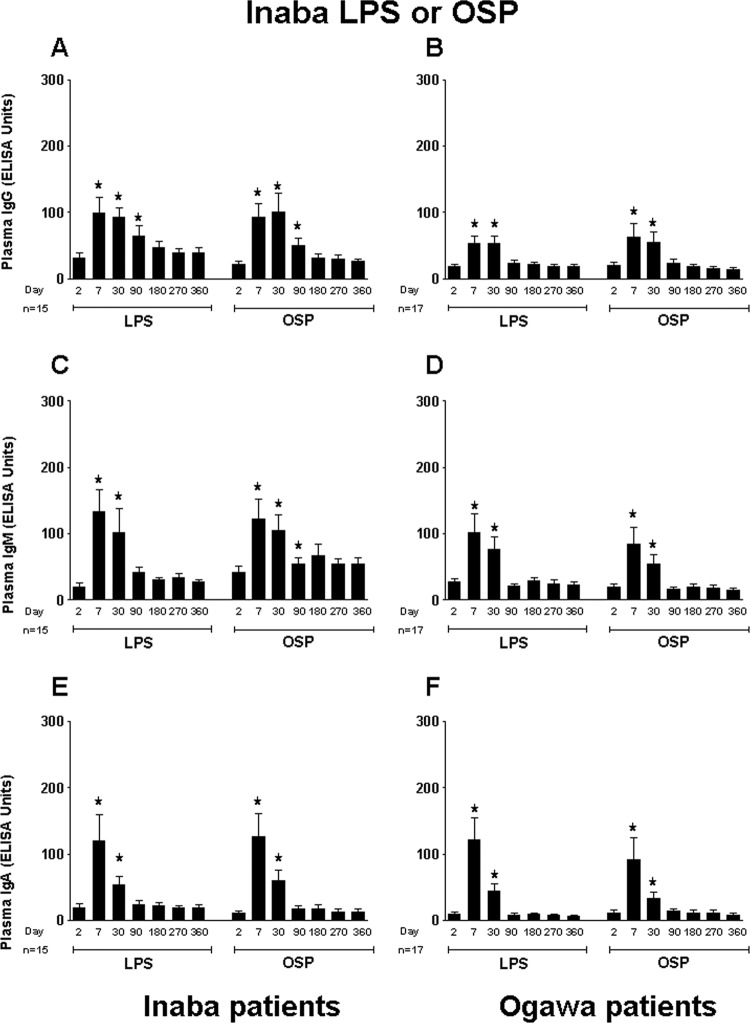
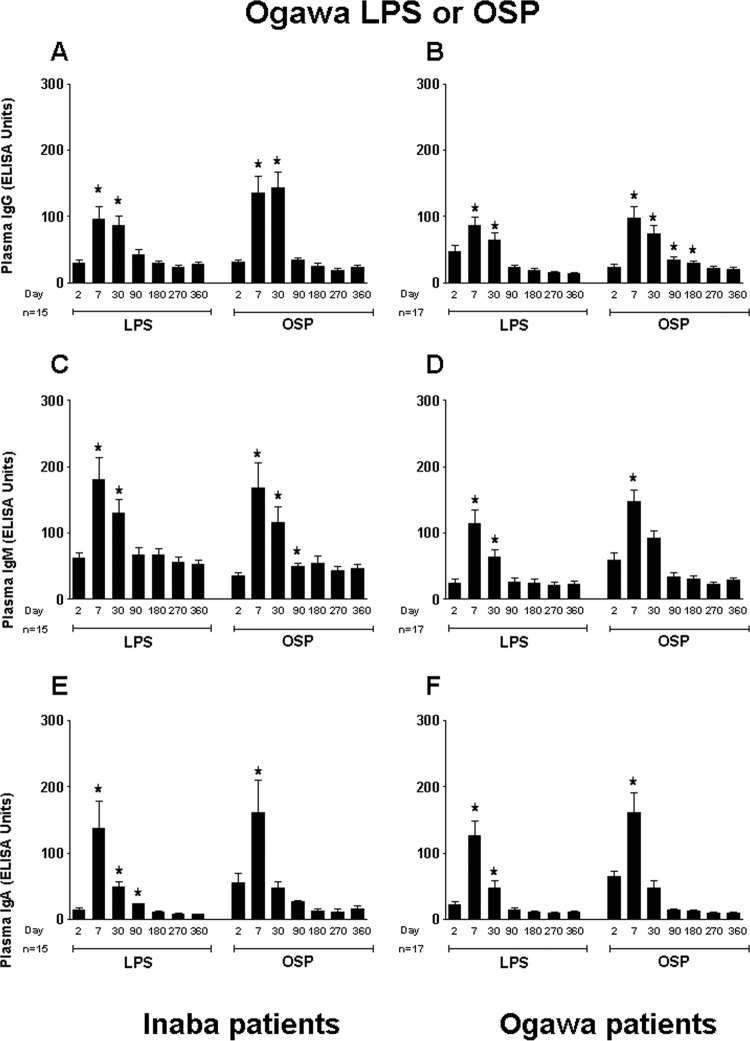
We assessed responses in Ogawa- and Inaba-infected patients to both the homologous and heterologous OSP and LPS O1 antigens. We found that the IgG, IgM, and IgA antibody responses to Inaba OSP and LPS were similar among the Ogawa- and Inaba-infected patient groups (Fig. 1). Both patient groups showed significantly elevated antibody responses to Inaba OSP and LPS at day 7 after onset of disease compared to baseline. Correlations between antibody responses to Inaba OSP and LPS in Inaba-infected patients over the 1-year follow-up period were strong (IgG, R = 0.86, IgM, R = 0.73, and IgA, R = 0.91; P < 0.01). Likewise, Ogawa- and Inaba-infected patients demonstrated similar antibody responses to Ogawa OSP and LPS (Fig. 2). Correlations between antibody responses to Ogawa OSP and LPS in Ogawa-infected patients over the 1-year follow-up period mirrored those seen in Inaba-infected patients (IgG, R = 0.60, IgM, R = 0.60, and IgA, R = 0.92; P < 0.01).
Fig 1.
Mean normalized IgG, IgM, and IgA responses in plasma of patients infected with V. cholerae O1 serotype Ogawa or Inaba to Inaba OSPc:BSA (OSP) and lipopolysaccharide (LPS). Plasma IgG, IgM, and IgA responses to Inaba OSP and LPS in Inaba-infected patients are shown in panels A, C, and E, and those in Ogawa-infected patients are shown in panels B, D, and F. Asterisks indicate a statistically significant difference (P ≤ 0.05) from baseline (day 2) levels within a particular antigen group.
Fig 2.
Mean normalized IgG, IgM, and IgA responses in plasma of patients infected with V. cholerae O1 serotype Ogawa or Inaba to Ogawa OSPc:BSA (OSP) and lipopolysaccharide (LPS). Plasma IgG, IgM, and IgA responses to Ogawa OSP and LPS in Inaba-infected patients are shown in panels A, C, and E, and those in Ogawa-infected patients are shown in panels B, D, and F. Asterisks indicate a statistically significant difference (P ≤ 0.05) from baseline (day 2) levels within a particular antigen group.
IgG, IgM, and IgA antibody responses in Inaba-infected patients were cross-reactive to the Ogawa OSP antigen when plasma specimens were tested (Fig. 2A, C, and E). Similarly, antibodies from Ogawa-infected patients were cross-reactive to the Inaba OSP antigen (Fig. 1B, D, and F). However, as mentioned above, cholera patients infected with V. cholerae O139 showed no responses to Ogawa OSPc:BSA or LPS (data not shown); we did not assess responses in O139 patients to Inaba OSPc:BSA or LPS.
Comparison of plasma antibody responses to OSP and LPS with vibriocidal antibody responses.
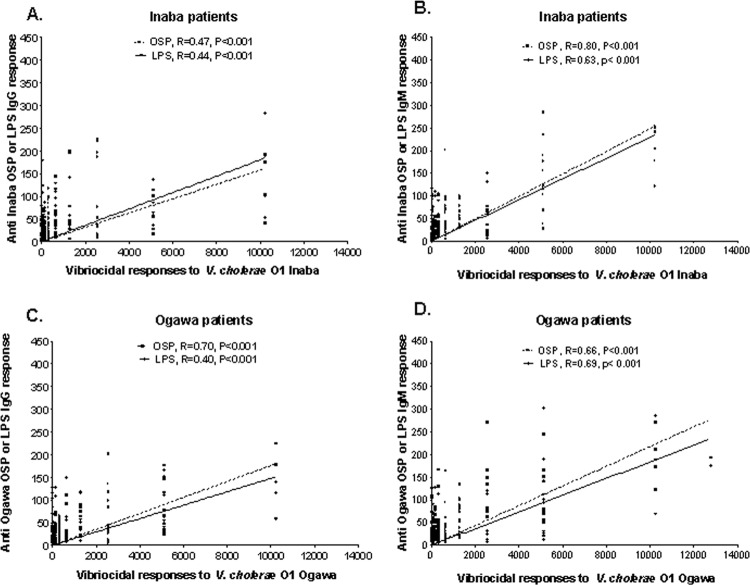
We compared vibriocidal antibody responses in 15 Inaba-infected patients with plasma IgM and IgG responses to Inaba OSP and LPS (Fig. 3A and B). We found that the IgM responses to Inaba OSP and LPS strongly correlated with vibriocidal responses (R = 0.80 and 0.63, respectively; P < 0.001) (Fig. 3B). Inaba OSP and LPS responses in the IgG isotype were less strongly correlated with vibriocidal responses (R = 0.47 and 0.44, respectively; P < 0.001) (Fig. 3A). We also compared vibriocidal responses in 17 Ogawa-infected patients to plasma IgM and IgG responses to Ogawa OSP and LPS (Fig. 3C and D). We found that IgM responses in these patients to Ogawa OSP and LPS correlated with vibriocidal responses (R = 0.66 and 0.69, respectively; P < 0.001) (Fig. 3D); the IgG responses similarly correlated (R = 0.70 for OSP and R = 0.40 for LPS; P < 0.001) (Fig. 3C).
Fig 3.
Correlation between vibriocidal antibody responses and plasma IgG or IgM antibody responses to OSPc:BSA (OSP) and lipopolysaccharide (LPS). The lines indicate the correlations between the different responses to OSP and LPS and the vibriocidal antibody response.
Mucosal immune responses to V. cholerae O1 Ogawa OSP and LPS antigens.
Antibody-secreting cell (ASC) and antibody-in-lymphocyte supernatant (ALS) responses are considered to be surrogate markers of mucosal immunity (7) and reflect transient circulation of mucosal lymphocytes in blood that are rehoming to mucosal surfaces as they mature; these responses peak on day 7 after mucosal stimulation (41). We assessed IgG, IgM, and IgA ASC responses to Ogawa OSP, LPS, and CtxB in peripheral blood of nine V. cholerae O1-infected patients. ASC responses to both OSP and LPS were comparable (Fig. 4A). The responses of all three antibody isotypes to OSP and LPS peaked on day 7 and returned to baseline by day 30. IgA and IgG CtxB ASC responses were elevated at day 7. The levels of responses to LPS or OSP at days 2 and 30 were very low and thus are not shown in the figure (Fig. 4A). The level of IgM responses to CtxB at day 7 was also low and comparable to those of the LPS or OSP responses on days 2 and 30 and is not shown in the figure.
Fig 4.
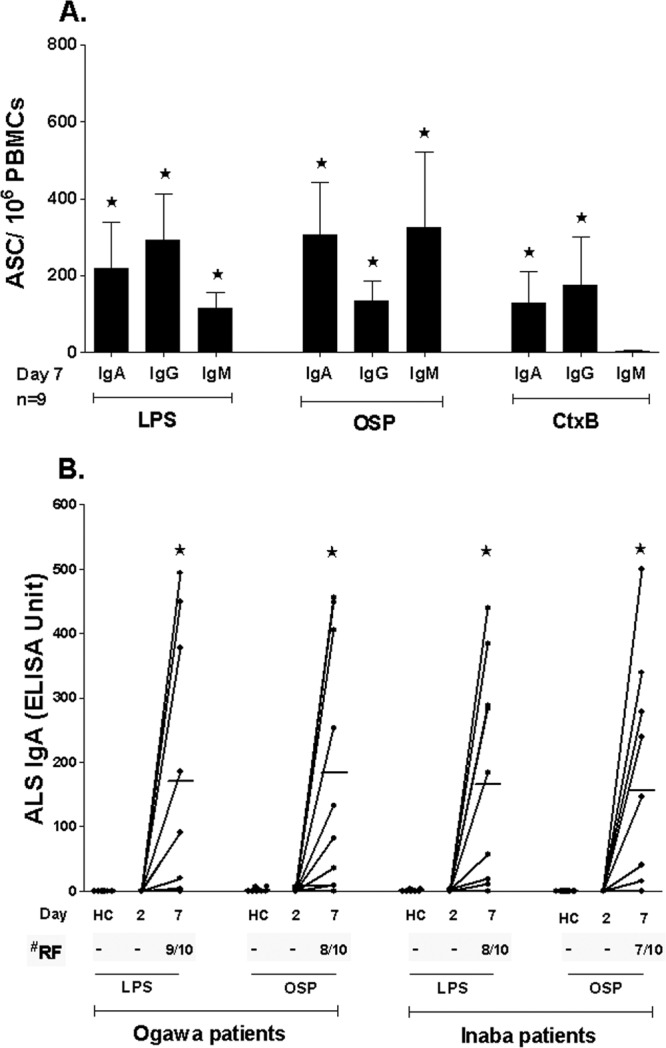
Mucosal immune responses to Ogawa OSPc:BSA (OSP) and lipopolysaccharide (LPS). Asterisks indicate a statistically significant difference (P ≤ 0.05) from baseline (day 2). (A) Mean circulating antigen-specific IgG, IgM, and IgA ASC responses to Ogawa OSP, LPS, and CtxB with standard error bars. (B) ALS IgA responses to Ogawa OSP and LPS in healthy controls (HC) and Ogawa- and Inaba-infected patients. RF, responder frequencies.
We also assessed mucosal immune responses to Ogawa OSP and LPS using stored ALS specimens from patients infected with O1 Ogawa and O1 Inaba V. cholerae. IgA responses targeting OSP and LPS in these ALS specimens were similar, with significant increases seen at 7 days postinfection compared to those from day 2 after illness onset and compared to those seen in healthy controls (Fig. 4B). We considered a patient to be a responder when the response at day 7 increased 2-fold or higher than the level seen at day 2. The responder frequency was between 70% and 90% for the Ogawa- and Inaba-infected cholera patients (Fig. 4B).
We further assessed mucosal responses by assessing antigen-specific responses on days 2, 7, and 30 postinfection in fecal extracts, comparing anti-Ogawa OSP and LPS responses in patients infected with V. cholerae O1 Ogawa or Inaba. Eighty-six percent of Ogawa-infected patients had a detectable IgA response to homologous LPS and 78% to homologous OSP by convalescence (day 7 or 30 postinfection). For patients infected with O1 Inaba, 57% had detectable responses to Ogawa LPS and 50% had detectable responses to Ogawa OSP. We did not assess responses using Inaba LPS or OSPc:BSA.
Vibriocidal inhibition assay using OSP and LPS antigens.
We used a vibriocidal inhibition assay to assess whether antibody contributing to the vibriocidal response was specific to OSP, using Ogawa OSPc:BSA antigen for absorption of plasma from Ogawa-infected patients. Our results indicate that increasing concentrations of both Ogawa OSPc:BSA and Ogawa LPS inhibited the vibriocidal assay in concentration-dependent manners that were similar for the two antigens (Fig. 5). Preincubation of patient serum with CtxB or the negative-control antigen BSA had no effect on the vibriocidal assay. We did not assess the inhibition assay using Inaba antigens.
Fig 5.
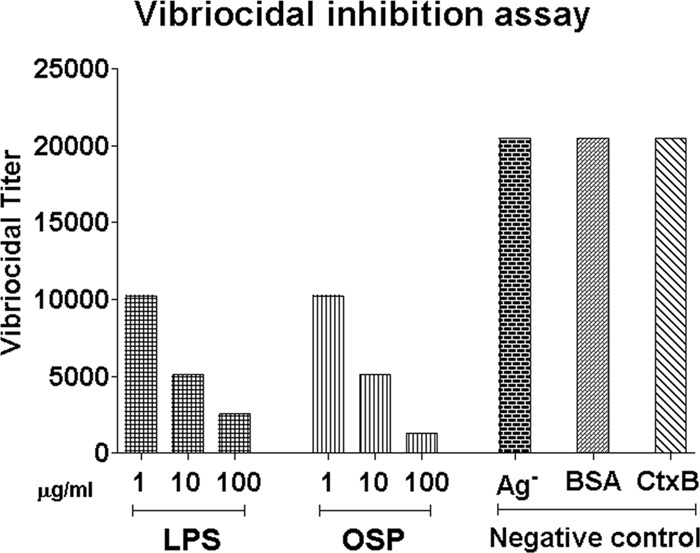
Antigen-specific inhibition of vibriocidal antibody assays using OSPc:BSA (OSP) and lipopolysaccharide (LPS). Ag− represents patient plasma not incubated with antigen. BSA and recombinant CtxB antigens were also assayed at concentrations of 1, 10, and 100 μg/ml, and no inhibition was detected. V. cholerae O1 Ogawa OSPc:BSA and Ogawa LPS were used for these experiments.
DISCUSSION
Studies in healthy North American volunteers challenged with V. cholerae O1, as well as epidemiological studies in areas of the world where cholera is endemic have shown that natural infection confers protective immunity to symptomatic disease on reexposure for at least 3 years and perhaps as long as 10 years (21, 26). However, currently available vaccines provide protection for only 3 years in adults and even shorter periods in younger children. The mechanism underlying the longer protection afforded by natural infection with cholera is not yet fully understood, although memory responses particularly to LPS may play a part (14, 18, 20, 33). Interestingly, current oral killed-cholera vaccines do not induce memory B-cell responses targeting LPS, while wild-type disease does, even in young children (1, 24, 25).
Currently, the best-studied indirect marker of protective immunity to cholera is the vibriocidal antibody, a complement-dependent serum bactericidal antibody of patients who have previously had cholera. This complement-dependent antibody is thought to be largely derived from plasma IgM antibody and directed at V. cholerae LPS (10, 15, 28, 43). Although the vibriocidal response is currently the most used marker of immunity to cholera, it is believed to be a surrogate marker for another yet to be fully defined immune response at the mucosal surface (10, 43).
In previous studies, we have shown that there are significant IgA antibody-secreting cells specific for LPS in duodenal biopsy specimens of patients out to day 180 after infection, suggesting a persisting mucosal immune response to this antigen (47). Furthermore, following cholera, patients develop a circulating memory B-cell response to LPS that persists out to days 180 to 270 after infection but wanes by 1 year postinfection. B-cell responses to LPS are T-cell independent, and this may explain waning memory B-cell responses at 1 year, even while memory B-cell responses are still present to T-cell-dependent protein antigens (14). Patients who undergo vaccination with Dukoral (Crucell, Sweden), an inactivated-whole-cell V. cholerae vaccine combined with recombinant nontoxic CtxB, develop lower LPS-specific memory B-cell responses following vaccination than patients recovering from natural infection. This difference in the longevities of the memory B-cell responses to LPS may underlie the relatively short-term protection afforded by oral killed-cholera vaccine administration (1). More recently, our group has found that memory B-cell responses directed against LPS present on exposure in household contacts of cholera patients protect against subsequent infection (33).
Taken together, these data implicate LPS-specific immune responses as important in protective immunity to cholera and suggest that LPS, or an antigenic component of LPS, might be an attractive target for vaccine design. However, analysis of the immune responses to V. cholerae LPS is complicated by the heterogeneous nature of standard LPS preparations. In the present study, we used mass spectrometry to analyze the V. cholerae O1 LPS preparation and found that the sample contained over 600 V. cholerae proteins in addition to polysaccharide and lipid components.
As the O-specific polysaccharide (OSP) of V. cholerae LPS defines serogroup specificity, this polysaccharide most likely contributes significantly to the observed immune responses to V. cholerae LPS. Despite this, the immune responses to V. cholerae OSP have not yet been characterized. Recently, our group developed both O1 Ogawa and O1 Inaba OSPc:BSA conjugates using squaric acid chemistry (52). BSA was conjugated to the OSPc antigen to facilitate binding of the polysaccharides to ELISA and ELISPOT wells for immunological analysis. In this study, we used these conjugates to characterize immune responses to the Ogawa and Inaba OSP in infected individuals and compared these responses to the corresponding responses to LPS.
Our results show that the OSP component of V. cholerae LPS is sufficient to detect immune responses comparable to those against LPS in patients infected with cholera. The Inaba-infected patients in our study demonstrated very similar plasma IgG, IgM, and IgA antibody responses to homologous Inaba OSP or LPS. The plasma antibody responses to OSP and LPS increased significantly at convalescence compared to at the acute phase and correlated well with each other for all three antibody isotypes over the year of follow-up. These plasma antibody responses also correlated well with the vibriocidal antibody response in the same patients, indicating that responses against LPS, and more specifically OSP, mediate the observed vibriocidal antibody responses. Ogawa patients showed similar IgG, IgM, and IgA plasma antibody responses to homologous Ogawa OSP and LPS that also strongly correlated with each other, as well as with the vibriocidal antibody responses from the same cohort of patients. The OSP- and LPS-specific IgG responses showed a strong relationship to each other in an earlier study (52). Taken together, these results indicate that the human immune response to infection following cholera specifically targets the OSP component of V. cholerae O1 LPS and that this response likely contributes substantially to the observed vibriocidal antibody response. The results of the vibriocidal inhibition assays we performed with the Ogawa OSP and LPS antigens further highlight the contribution of antibodies to V. cholerae LPS to the vibriocidal antibody response (9, 10, 12, 32). These results also indicate that the OSP portion of O1 LPS may be a specific target of vibriocidal antibody.
The immune response at the surface of the gut is believed to play a critical role in mediating protection from cholera, and previously infected patients may have an anamnestic immune response by mucosal lymphocytes (1, 47). After initial interaction with pathogen, antigen-specific lymphocytes from the gut can be transiently detected in the circulation while in transit to other mucosal organs. These lymphocytes are considered a marker of a mucosal response and of recent exposure to cholera (42). In our study, we found that circulating levels of ASCs specific to OSP and LPS in the IgG, IgM, and IgA isotypes increased similarly at day 7 and that these ASC responses then returned to baseline levels by day 30. The number of ASCs specific to LPS as well as to CtxB was consistent with those in previous studies (14, 42, 44). Mucosal immune responses to OSP and LPS were also comparable using ALS and in fecal extracts from infected patients.
Previous work has shown cross-reactivity between Inaba and Ogawa LPS following infection with either Ogawa or Inaba serogroup organisms, respectively, although responses are usually highest to the serotype of the infecting strain (3, 11, 12, 29, 31). Similarly, vibriocidal responses are measurable against both serotypes after infection, although once again, the titer is usually highest against the serotype of the infecting strain (43). A recent study showed that infection with V. cholerae O1 Inaba lowered the subsequent risk of both Ogawa and Inaba symptomatic infection, but that patients infected with V. cholerae O1 Ogawa were only protected from infection with the Ogawa serotype (3). In our study, we examined immune responses in patients to the heterologous OSP. We wanted to determine if immune responses to the heterologous serotype were more durable after infection with Inaba V. cholerae than after Ogawa infection. However, while patients did have substantial immune responses to the heterologous OSP, these heterologous responses were not more durable after initial Inaba infection than Ogawa infection.
Previous O1 infection with either serotype does not induce responses that react with V. cholerae O139 LPS, nor does it induce cross-reacting vibriocidal responses to this serogroup (2, 11, 12, 42, 49). We included specimens from patients infected with V. cholerae O139 in our study as a control and to determine the specificity of responses to the O1 OSPc:BSA conjugate. We found no immunoreactivity against the O1 OSP conjugates in patients infected with V. cholerae O139, highlighting the specificity of the OSP conjugate antigens used. Given the data that anti-LPS immune responses appear to mediate protective immunity to V. cholerae infection, that immune responses to LPS and OSP are largely cross-reactive, and that anti-OSP responses are serogroup specific, the OSP-conjugate may be a useful tool for examining immune responses specific to the sugar component of LPS, in the absence of contaminating V. cholerae proteins, for future immunological studies.
For several other diseases, conjugations between polysaccharides and proteins have led to vaccines that induce responses that are T-cell dependent and that induce longer-lasting immunity and improved immunogenicity in young children (34). We are currently characterizing immune responses, including memory responses, following immunization with a model OSP-conjugate vaccine in an animal model of cholera to see if conjugation of the OSPc part of LPS to a protein carrier confers the same immunological properties as conjugation of other sugar molecules to protein carriers. Since individuals in areas where cholera is endemic are frequently exposed to V. cholerae in the environment, it will also be essential to characterize the boosting effects of oral or parenteral OSP-derived conjugates after mucosal priming with intact V. cholerae or a killed-cholera vaccine, using the prime-boost method for improving responses. A cholera vaccine that improves immunogenicity in young children and generates memory sufficient to increase the duration of protection would be a significant advance in preventing a disease that can lead to death if not promptly treated.
ACKNOWLEDGMENTS
This research was supported by the ICDDR,B and by the Intramural Research Program of the National Institutes of Health, NIDDK, and extramural grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (U01 AI058935 [S.B.C. and E.T.R.], R03 AI063079 [F.Q.], U01 AI077883 [E.T.R.], K08 AI100923 and K08 AI089721 [R.C.C.]), the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 [T.U., M.M.A., and F.Q.]), an American Recovery and Reinvestment Act (ARRA) Postdoctoral Fellowship in Global Infectious Diseases (TW05572 [D.T.L.]), Career Development Awards (K01 TW07409 [J.B.H.] and TW07144 [R.C.L.]), and a Fogarty International Clinical Research Scholars Award (R24 TW007988 [R.A.J. and T.U.]), as well as by the Swedish International Development Cooperation Agency (F.Q.), a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (R.C.L.), a Postdoctoral Fellowship in Tropical Infectious Diseases from the American Society for Tropical Medicine & Hygiene—Burroughs Wellcome Fund (D.T.L.), and the Harvard Institute for Global Health Postdoctoral Fellowship in Global Infectious Diseases (D.T.L).
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Alam MM, et al. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin. Vaccine Immunol. 18:844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169:709–710 [DOI] [PubMed] [Google Scholar]
- 3. Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J. Infect. Dis. 204:912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charles RC, et al. 2009. Comparative proteomic analysis of the PhoP regulon in Salmonella enterica serovar Typhi versus Typhimurium. PLoS One 4:e6994 doi:10.1371/journal.pone.0006994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox AD, Brisson JR, Varma V, Perry MB. 1996. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr. Res. 290:43–58 [DOI] [PubMed] [Google Scholar]
- 6. Cox AD, Perry MB. 1996. Structural analysis of the O-antigen-core region of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr. Res. 290:59–65 [DOI] [PubMed] [Google Scholar]
- 7. Czerkinsky C, et al. 1987. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc. Natl. Acad. Sci. U. S. A. 84:2449–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fattom A, et al. 1993. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 61:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finkelstein RA. 1962. Vibriocidal antibody inhibition (VAI) analysis: a technique for the identification of the predominant vibriocidal antibodies in serum and for the detection and identification of Vibrio cholerae antigens. J. Immunol. 89:264–271 [Google Scholar]
- 10. Glass RI, et al. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236–242 [DOI] [PubMed] [Google Scholar]
- 11. Gupta RK, Szu SC, Finkelstein RA, Robbins JB. 1992. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified by lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect. Immun. 60:3201–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta RK, Taylor DN, Bryla DA, Robbins JB, Szu SC. 1998. Phase 1 evaluation of Vibrio cholerae O1, serotype Inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect. Immun. 66:3095–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc. Natl. Acad. Sci. U. S. A. 109:8722–8727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris AM, et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris JB, et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221 doi:10.1371/journal.pntd.0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hisatsune K, et al. 1993. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem. Biophys. Res. Commun. 196:1309–1315 [DOI] [PubMed] [Google Scholar]
- 17. Ito T, Higuchi T, Hirobe M, Hiramatsu K, Yokota T. 1994. Identification of a novel sugar, 4-amino-4,6-dideoxy-2-O-methylmannose in the lipopolysaccharide of Vibrio cholerae O1 serotype Ogawa. Carbohydr. Res. 256:113–128 [DOI] [PubMed] [Google Scholar]
- 18. Jayasekera CR, et al. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 198:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kall L, Canterbury JD, Weston J, Noble WS, MacCoss MJ. 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4:923–925 [DOI] [PubMed] [Google Scholar]
- 20. Kendall EA, et al. 2010. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect. Immun. 78:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 22. Kossaczka Z, et al. 2000. Vibrio cholerae O139 conjugate vaccines: synthesis and immunogenicity of V. cholerae O139 capsular polysaccharide conjugates with recombinant diphtheria toxin mutant in mice. Infect. Immun. 68:5037–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuchta A, et al. 2011. Vibrio cholerae O1 infection induces proinflammatory CD4+ T-cell responses in blood and intestinal mucosa of infected humans. Clin. Vaccine Immunol. 18:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leung DT, Chowdhury F, Calderwood SB, Qadri F, Ryan ET. 2012. Immune responses to cholera in children. Expert Rev. Anti Infect. Ther. 10:435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung DT, et al. 2012. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin. Vaccine Immunol. 19:690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine MM, et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 27. Lopez MF, et al. 2011. Mass spectrometric discovery and selective reaction monitoring (SRM) of putative protein biomarker candidates in first trimester Trisomy 21 maternal serum. J. Proteome Res. 10:133–142 [DOI] [PubMed] [Google Scholar]
- 28. Losonsky GA, et al. 1996. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect. Immun. 64:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mosley WH, Aziz KM, Rahman AS, Chowdhury AK, Ahmed A. 1973. Field trials of monovalent Ogawa and Inaba cholera vaccines in rural Bangladesh—three years of observation. Bull. World Health Organ. 49:381–387 [PMC free article] [PubMed] [Google Scholar]
- 30. Mosley WH, McCormack WM, Ahmed A, Chowdhury AK, Barui RK. 1969. Report of the 1966–67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population—the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bull. World Health Organ. 40:187–197 [PMC free article] [PubMed] [Google Scholar]
- 31. Mosley WH, et al. 1970. The 1968–1969 cholera-vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. J. Infect. Dis. 121(Suppl):121–129 [DOI] [PubMed] [Google Scholar]
- 32. Neoh SH, Rowley D. 1970. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J. Infect. Dis. 121:505–513 [DOI] [PubMed] [Google Scholar]
- 33. Patel SM, et al. 2012. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection in household contacts of cholera patients in Bangladesh. Clin. Vaccine Immunol. 19:842–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pollard AJ, Perrett KP, Beverley PC. 2009. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9:213–220 [DOI] [PubMed] [Google Scholar]
- 35. Provenzano D, Kováč P, Wade WF. 2006. The ABCs (antibody, B cells, and carbohydrate epitopes) of cholera immunity: considerations for an improved vaccine. Microbiol. Immunol. 50:899–927 [DOI] [PubMed] [Google Scholar]
- 36. Qadri F, et al. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qadri F, et al. 2000. Enterotoxin-specific immunoglobulin E responses in humans after infection or vaccination with diarrhea-causing enteropathogens. Infect. Immun. 68:6077–6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qadri F, et al. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin. Diagn. Lab. Immunol. 4:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qadri F, et al. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab Immunol. 2:685–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qadri F, et al. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qadri F, et al. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qadri F, et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saha D, et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318–2322 [DOI] [PubMed] [Google Scholar]
- 44. Shamsuzzaman S, et al. 2009. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine 27:1386–1392 [DOI] [PubMed] [Google Scholar]
- 45. Stroeher UH, Karageorgos LE, Morona R, Manning PA. 1992. Serotype conversion in Vibrio cholerae O1. Proc. Natl. Acad. Sci. U. S. A. 89:2566–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Svennerholm AM, et al. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884–893 [DOI] [PubMed] [Google Scholar]
- 47. Uddin T, et al. 2011. Mucosal immunologic responses in cholera patients in Bangladesh. Clin. Vaccine Immunol. 18:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vinogradov EV, Bock K, Holst O, Brade H. 1995. The structure of the lipid A-core region of the lipopolysaccharides from Vibrio cholerae O1 smooth strain 569B (Inaba) and rough mutant strain 95R (Ogawa). Eur. J. Biochem. 233:152–158 [DOI] [PubMed] [Google Scholar]
- 49. Waldor MK, Colwell R, Mekalanos JJ. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. U. S. A. 91:11388–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J, et al. 1998. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J. Biol. Chem. 273:2777–2783 [DOI] [PubMed] [Google Scholar]
- 51. WHO 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 52. Xu P, et al. 2011. Simple, direct conjugation of bacterial O-SP-core antigens to proteins: development of cholera conjugate vaccines. Bioconjug. Chem. 22:2179–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zuckerman JN, Rombo L, Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]