Abstract
Appropriate animal models are required to test medical countermeasures to bioterrorist threats. To that end, we characterized a nonhuman primate (NHP) inhalational anthrax therapeutic model for use in testing anthrax therapeutic medical countermeasures according to the U.S. Food and Drug Administration Animal Rule. A clinical profile was recorded for each NHP exposed to a lethal dose of Bacillus anthracis Ames spores. Specific diagnostic parameters were detected relatively early in disease progression, i.e., by blood culture (∼37 h postchallenge) and the presence of circulating protective antigen (PA) detected by electrochemiluminescence (ECL) ∼38 h postchallenge, whereas nonspecific clinical signs of disease, i.e., changes in body temperature, hematologic parameters (ca. 52 to 66 h), and clinical observations, were delayed. To determine whether the presentation of antigenemia (PA in the blood) was an appropriate trigger for therapeutic intervention, a monoclonal antibody specific for PA was administered to 12 additional animals after the circulating levels of PA were detected by ECL. Seventy-five percent of the monoclonal antibody-treated animals survived compared to 17% of the untreated controls, suggesting that intervention at the onset of antigenemia is an appropriate treatment trigger for this model. Moreover, the onset of antigenemia correlated with bacteremia, and NHPs were treated in a therapeutic manner. Interestingly, brain lesions were observed by histopathology in the treated nonsurviving animals, whereas this observation was absent from 90% of the nonsurviving untreated animals. Our results support the use of the cynomolgus macaque as an appropriate therapeutic animal model for assessing the efficacy of medical countermeasures developed against anthrax when administered after a confirmation of infection.
INTRODUCTION
Bacillus anthracis is a Gram-positive, rod-shaped, aerobic and/or facultative anaerobic, spore-forming bacterium that can cause human disease via the gastrointestinal, cutaneous, or inhalation (pulmonary) routes, each resulting in different clinical manifestations of disease (4, 20). The pulmonary form of B. anthracis is the most lethal, and the incubation period usually varies from 1 to 6 days, depending upon the dose received (5). After inhalation exposure, some reports suggest a delayed onset of several weeks in low-dose exposure or after the removal of therapeutic intervention (4). In inhalation anthrax, the initial clinical signs and symptoms are nonspecific and may include malaise, headache, fever, nausea, and vomiting (4). These are followed by a sudden onset of respiratory distress with dyspnea, stridor, cyanosis, and chest pain. The onset of respiratory distress is followed by shock and often death, with close to 100% mortality in untreated cases (4).
The mortality caused by B. anthracis is predominantly due to the three well-characterized virulence factors: the capsule and two toxins (23). The polyglutamate capsule prevents phagocytosis of the bacterium. Three polypeptides—protective antigen (PA), lethal factor (LF), and edema factor (EF)—interact to form the anthrax toxins (23). PA and LF combine to produce anthrax lethal toxin (LT), and the PA and EF combine to produce edema toxin (ET). PA is the binding moiety of the toxin complex and facilitates the entry of LF and EF into host cells. LF is a protease, and EF is a calcium-dependent adenylate cyclase, and both toxin components can inhibit a variety of signaling cascades needed for appropriate immune cell function (i.e., proliferation, cell cycle regulation, and innate immune cell function) (18).
Historically, documentation of clinical signs of anthrax in the nonhuman primate (NHP) has been limited by the frequency of sample collections following challenge, and the majority of published work has focused on the pathology associated with anthrax infection in rhesus macaques, chimpanzees, or African green monkeys (8–11, 21, 25). However, due to the limited availability of some of these NHP species, it is essential to develop and characterize a more accessible NHP model that can be used for testing of vaccines, postexposure prophylactics, and therapeutics for U.S. Food and Drug Administration (FDA) approval for licensure (16). The FDA Animal Rule (21 CFR 314.600 for drugs and 21 CFR 601.90 for biological products), which allows the FDA to grant marketing approval for a new drug based on adequate and well-controlled animal studies, includes three components that are applicable to developing the inhalational anthrax NHP model: (i) a reasonably well-understood pathophysiological mechanism for the toxicity of the chemical, biological, radiological, and nuclear (CBRN) substance (agent) and its amelioration or prevention by the drug, (ii) demonstration of the effect (of the drug) in more than one animal species expected to react with a response predictive for humans, and (iii) an animal study endpoint that is clearly related to the desired benefit in humans. The pathology of inhalational anthrax in cynomolgus macaques has previously been characterized (26). Therefore, we assessed clinical and physiological parameters of the disease, including body temperature, hematological parameters, antigenemia (PA detected in the blood), and bacteremia incidence in order to clearly define the clinical progression of disease after exposure to B. anthracis spores in cynomolgus macaques. The frequent assessment of clinical parameters during disease progression could potentially lead to a better understanding of the clinical and physiological changes that can be observed in the cynomolgus macaque model of inhalational anthrax. We also assessed the efficacy of a monoclonal antibody specific for PA when administered at the onset of antigenemia with the overall goal of confirming the use of cynomolgus macaques as a therapeutic model of inhalational anthrax.
MATERIALS AND METHODS
Test system.
Thirty (50% male, 50% female) cynomolgus macaques (Macaca fascicularis) younger than 5 years and weighing between 2.7 and 7.3 kg prior to challenge were utilized (Covance, Alice, TX). All NHPs were tested and verified negative for tuberculosis and confirmed seronegative for simian immunodeficiency virus, simian T-cell lymphotrophic virus type 1, and cercopithecine herpesvirus type 1 and negative for simian retrovirus by PCR. All NHPs were considered healthy prior to placement on study. Twenty-four NHPs (12 males and 12 females) were challenged with B. anthracis spores, and the remaining six animals (three males and three females) served as naive controls. During the quarantine and prechallenge period and from day 9 through day 28 postchallenge, clinical observations were recorded twice daily. Animals were observed every 6 h beginning 24 h postchallenge and ending 8 days postchallenge for abnormal clinical signs.
All animal procedures were approved by Battelle's Institutional Animal Care and Use Committee, and all exposures and assays were performed in a biosafety level 3 laboratory registered with the Centers for Disease Control and Prevention and inspected by the U.S. Departments of Defense and Agriculture.
Aerosol challenge.
NHPs were aerosol challenged with B. anthracis Ames strain spores according to previous methods (26). The average aerosol exposure dose for all 24 animals challenged was 433 ± 156 median (50%) lethal dose (LD50) equivalents. The calculated dose was based on an LD50 of 6.18 × 104 CFU for cynomolgus macaques (26). The average mass median aerodynamic diameter of the aerosolized spores was 1.15 μm, which is consistent with particle sizes capable of reaching the lower respiratory tract (4). Table 1 presents the estimated inhaled challenge dose of B. anthracis Ames LD50 equivalents for each animal. The unchallenged control animals were handled like the challenged animals throughout the course of the study except that they were not anesthetized and placed in the aerosol exposure system.
Table 1.
Challenge results, time from challenge to abnormal clinical signs, and time to death for each animal
| Group and animalb | Gender | Wt (kg) | Estimated inhaled dosage (LD50 equivalents) | Time (h) toa: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIBTc | Positive PA-ECL | Positive blood culture | Abnormal white blood cell count | Abnormal neutrophil count | Abnormal N/L ratio | Treatment | Death | ||||
| Group 1 | |||||||||||
| 1 | F | 3.0 | 426 | 39.78 | 42.73 | 36.68 | 48.92 | 24.95 | 42.73 | 45.00 | NA |
| 2 | F | 3.4 | 950 | 61.52 | 48.72 | 48.72 | 36.47 | 36.47 | 48.72 | 51.00 | NA |
| 3 | M | 3.7 | 449 | 39.20 | 42.27 | 36.22 | 30.27 | 36.22 | 36.22 | 45.00 | NA |
| 4 | F | 4.3 | 386 | 60.62 | 47.45 | 47.45 | 41.22 | 50.87 | 59.42 | 51.00 | NA |
| 5 | M | 3.3 | 477 | 38.88 | 23.02 | 23.02 | 40.72 | 28.47 | 28.47 | 27.00 | NA |
| 6 | M | 3.4 | 430 | 38.18 | 27.90 | 27.9 | 52.00 | 31.18 | 40.17 | 33.00 | NA |
| 7 | M | 6.1 | 372 | 63.17 | 30.55 | 30.55 | 33.77 | 36.52 | 36.52 | 33.00 | 85.33 |
| 8 | F | 2.9 | 371 | 37.72 | 30.17 | 33.40 | 36.13 | 36.13 | 42.17 | 33.00 | NA |
| 9 | F | 3.0 | 315 | 61.07 | 41.72 | 41.72 | 65.77 | 59.60 | 59.60 | 45.00 | 84.23 |
| 10 | M | 3.4 | 369 | 37.53 | 29.20 | 23.48 | 32.32 | 35.18 | 35.18 | 33.00 | 101.93 |
| 11 | M | 4.0 | 262 | 63.93 | 52.77 | 47.12 | 55.68 | 55.68 | 71.02 | 57.00 | NA |
| 12 | F | 4.3 | 377 | 52.70 | 34.58 | 34.58 | 190.3 | 47.02 | 47.02 | 39.00 | NA |
| Group 2 | |||||||||||
| 1 | M | 5.2 | 443 | 60.92 | 42.05 | 36.05 | 54.22 | 30.10 | 36.05 | NA | 156.78 |
| 2 | M | 7.2 | 390 | 59.68 | 41.92 | 41.92 | 48.02 | 60.10 | 60.10 | NA | NA |
| 3 | F | 4.3 | 612 | 39.30 | 41.65 | 35.57 | 119.72 | 119.72 | 47.93 | NA | 173.97 |
| 4 | F | 2.9 | 678 | NA | 41.35 | 41.35 | 65.52 | 59.55 | 59.55 | NA | 93.78 |
| 5 | F | 3.1 | 601 | 37.32 | 28.82 | 23.25 | NA | NA | 47.22 | NA | 118.05 |
| 6 | M | 3.6 | 497 | 63.68 | 58.73 | 58.73 | 118.35 | 118.35 | 118.35 | NA | 220.40 |
| 7 | M | 7.3 | 198 | 36.80 | 43.03 | 43.03 | 121.13 | 121.13 | 55.08 | NA | 181.98 |
| 8 | M | 4.0 | 364 | 62.37 | 36.65 | 36.65 | 66.75 | 66.75 | 48.90 | NA | 133.57 |
| 9 | F | 3.3 | 382 | NA | 41.88 | 48.10 | 66.02 | 35.85 | 66.02 | NA | 71.12 |
| 10 | F | 2.7 | 402 | 85.20 | 28.95 | 28.95 | 65.12 | 118.82 | 58.88 | NA | 131.40 |
| 11 | M | 4.0 | 385 | 44.42 | 28.38 | 22.75 | NA | NA | NA | NA | 51.05 |
| 12 | F | 3.0 | 244 | 55.13 | 34.13 | 40.38 | 58.05 | 189.90 | 52.13 | NA | NA |
Time to each abnormal parameter, time to treatment, and time to death are indicated in hours postchallenge. NA, not applicable.
Group 1 included animals challenged with B. anthracis and treated with antibody when PA-ECL positive; group 2 included animals challenged with B. anthracis but not treated.
SIBT, significant increase in body temperature (six consecutive measurements above the temperature threshold).
Blood collection.
Blood samples were taken from a femoral artery or vein, a saphenous vein, or other appropriate vein. Baseline blood samples were collected 7 days prior to challenge. Additional samples were collected every 6 h from 24 to 72 h and on days 5, 8, 14, and 28 postchallenge. A sample was also collected just prior to treatment for each NHP in the treatment group.
Anti-PA monoclonal antibody treatment.
When an animal exhibited a positive PA level postchallenge according to the PA-electrochemiluminescence (ECL) assay, one 10-mg/kg intravenous bolus injection of fully human monoclonal antibody against B. anthracis PA was administered (19). Doses were administered on an individual animal basis dependent on real-time analysis of serum samples via the PA-ECL assay.
Body temperature.
Prior to placement on study, all NHPs were surgically implanted with TA-D70 telemetry transmitters (Data Sciences International, St. Paul, MN) using aseptic surgical techniques. Briefly, NHPs were lightly anesthetized by the intramuscular administration of ketamine (20 mg/kg of body weight), followed by balanced gaseous anesthesia (ca. 100% oxygen and 1 to 3% isoflurane) to maintain surgical anesthesia. The implantation procedure was performed at least 5 weeks prior to aerosol challenge to ensure that the animals had completely recovered from the implantation surgery at the time of challenge.
Body temperature was monitored for a 30-s interval once every hour beginning 14 days prior to challenge through 28 days postchallenge. For each NHP, a separate mean baseline temperature was computed at every hour of the day. At each hour of the study postchallenge, the change from baseline temperature was then calculated using the corresponding hourly baseline average. These changes from baseline temperatures were used in the statistical analysis and are referred to as “baseline-adjusted” temperatures. The standard deviation of all of the prechallenge, baseline-adjusted temperatures was calculated for each animal, and twice this standard deviation was used as the threshold for an elevated temperature. A postchallenge temperature was considered elevated if the baseline-adjusted temperature for the time point was greater than this threshold. NHPs were determined to have a significant increase in body temperature (SIBT) when an animal had six consecutive elevated temperature measurements.
Complete blood cell counts and C-reactive protein levels.
Complete blood cell counts were performed on whole-blood samples using an Advia 120 hematology analyzer (Siemens, Deerfield, IL). For each white blood cell (WBC) hematology parameter, the threshold for an abnormal parameter was defined as each individual animal's baseline (day −7) parameter value ± two standard deviations that were calculated separately for each parameter in an analysis of variance (ANOVA) model using the prechallenge baseline values from all animals. The change from baseline was calculated for each parameter, and then these values were normalized to the change from baseline observed in the unchallenged control animals (group 3) to adjust for diurnal trends observed in some of the hematology parameters. Animals were determined to have an abnormal parameter value when their observed value was greater than the upper threshold or less than the lower threshold for that respective parameter.
C-reactive protein (CRP) analysis (using a Siemens Advia 1200 chemistry analyzer) was performed on residual plasma collected from each whole-blood sample after processing. For CRP, a result was abnormal if the observation had a reported value greater than the limit of detection (LOD), which is 0.5 mg/dl.
Blood culture (bacteremia).
For blood cultures, ∼40 μl of each whole-blood sample collected into EDTA tubes was streaked over a blood agar plate, followed by incubation at 37°C ± 2°C for at least 48 h. Postincubation, the plates were observed, and those having at least one colony consistent with B. anthracis Ames morphology were documented as positive.
Circulating PA levels.
Circulating PA was assessed via two methods. The first method, using ECL detection, was performed in real-time on serum samples and was used to determine only the presence or absence of PA in circulation as a trigger for treatment. The second method, PA-enzyme-linked immunosorbent assay (ELISA), was performed after all of the samples had been collected to determine the quantities of circulating PA in sera.
ECL assay.
The rapid protective antigen screening assay, produced by MesoScale Discovery (Gaithersburg, MD), is a 96-well ECL assay designed to detect B. anthracis PA. Briefly, a detection antibody solution and PA-containing serum samples (i.e., unknown test samples collected from infected animals) were loaded onto a specialized 96-well microplate that contains electrodes coated with a B. anthracis anti-PA capture antibody. During a brief incubation period, the detection antibody forms a complex with the immobilized PA. After several wash steps and the addition of a read buffer, an electrical charge was applied to the microplate by the Sector PR plate reader, thereby causing any PA-bound detection antibody to emit light. The amount of light emitted (i.e., measured in ECL units) is directly proportional to the amount of PA present in the serum sample.
A sample was considered negative when the mean PA-ECL value of the test sample was less than the mean PA-ECL value of the positive control, and the sample was considered positive when the mean PA-ECL value of the test sample was greater than or equal to the mean PA-ECL value of the positive control (2.0 ng/ml).
Circulating PA-ELISA.
A double affinity-purified polyclonal, anti-PA IgG “capture antibody” (produced by Battelle Memorial Institute, Columbus, OH; purified from rPA-vaccinated rabbit serum over first protein A and then a PA column) was used to coat the wells of a 96-well plate at a concentration of 2 μg/ml. The plates were blocked with 5% skim milk and then incubated with NHP serum samples containing native PA or a reference standard and quality control samples consisting of rPA spiked differentially into naive NHP serum (lot NR-164; BEI Resources, Manassas, VA). The PA was detected by first incubating each well with diluted whole goat PA antiserum, followed by incubation (37°C) with a bovine anti-goat horseradish peroxidase-conjugated secondary antibody for 60 min (Santa Cruz Biologicals, Santa Cruz, CA) and then an ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (37°C, 30 min) and a stop solution (both from Kirkegaard and Perry Laboratories, Gaithersburg, MD). The plates were read (optical density at 405 nm), and the data were analyzed using a four-parameter logistical-log (4PL) model to fit the eight-point calibration curve. The concentrations of PA in unknown samples were determined by computer interpolation from the plot of the reference standard curve data (Softmax Pro; Molecular Devices, Sunnyvale, CA). An LOD of 2.4 ng/ml was used for this assay, based upon final qualification results. Levels greater than the LOD were considered abnormal.
Pathology.
Gross necropsy was performed on all NHPs that were found dead or that were euthanized during the study. Sections of target tissues, including brain/meninges, lungs, liver, spleen, kidney, and mediastinal lymph nodes, as well as all gross lesions, were preserved in 10% neutral buffered formalin until histopathology analysis. Tissues were cut to approximately 5-μm sections, deparaffinized, rehydrated, stained with hematoxylin and eosin, and examined by a board-certified veterinary pathologist. All microscopic findings were graded semiquantitatively according to the following scale: minimal (grade 1), the least detectible lesion; mild (grade 2), an easily discernible lesion; moderate (grade 3), a change affecting a large area of the represented tissue; and marked (grade 4), a lesion that approached maximal. The associated numerical score was used to calculate the average severity grades for each lesion by group.
Statistical methods. (i) Survival.
All statistical analyses were conducted with SAS (version 9.1). A one-sided Fisher exact test was performed to compare the survival rates between the untreated challenge group and the treated challenge group. The time-to-death data combined with overall survival was analyzed to determine whether the challenged groups differed in a model that also takes into account length of survival. A log-rank test was performed to determine whether the differences between the groups was statistically significant. Since the animals in group 3 were not challenged, they were excluded from all survival analyses.
(ii) Time to onset of clinical signs.
The time from challenge until an abnormal or positive value was determined for temperature, hematology, bacteremia, PA-ELISA, and PA-ECL. The criteria for abnormal values are described above. Since one group of challenged NHPs was not treated, the proportion of animals from this group that became abnormal at the same blood draw or prior to the first abnormal PA-ECL blood draw was calculated for each parameter. This was done in order to compare the proportion of the antibody-treated NHPs that became abnormal for each parameter to the proportion of untreated NHPs exposed to B. anthracis that became abnormal. The untreated, B. anthracis-exposed NHPs had one less possible blood draw to become abnormal before treatment since these NHPs did not undergo a prior-to-treatment blood draw.
The Pearson correlation coefficients for the time from challenge until an abnormal value between PA-ECL and temperature (SIBT), white blood cells, neutrophils, CRP, bacteremia, and PA-ELISA parameters were calculated. For each parameter, two-sample t tests were used to test for significant differences in the time from challenge until an abnormal parameter between the two challenged groups was observed.
(iii) Group and parameter comparisons.
An ANOVA model was fitted at each time point to determine whether there were statistically significant differences between groups for hematology parameter levels. In conjunction with the ANOVA model, Tukey's multiple comparisons were performed to determine which pairs of groups had significant differences in the hematology parameter levels.
A two-sided Fisher exact test was utilized to compare the proportion of animals exhibiting each clinical observation between all pairs of groups, and a two-sided t test was used to compare the geometric mean PA-ELISA values between the two challenged groups at each time point.
RESULTS
Survival.
Ten of the twelve NHPs in the untreated control group succumbed to inhalational anthrax with a mean time from challenge to death of 133.21 h (range, 51.05 to 220.40 h). Seventy-five percent (9/12) of the animals treated with the monoclonal anti-PA antibody at the first positive PA-ECL result survived a lethal inhalational exposure to B. anthracis. The average time to death for the three treated animals that died was 90.5 h postchallenge, and individual times ranged from 84.23 to 101.93 h (Table 1).
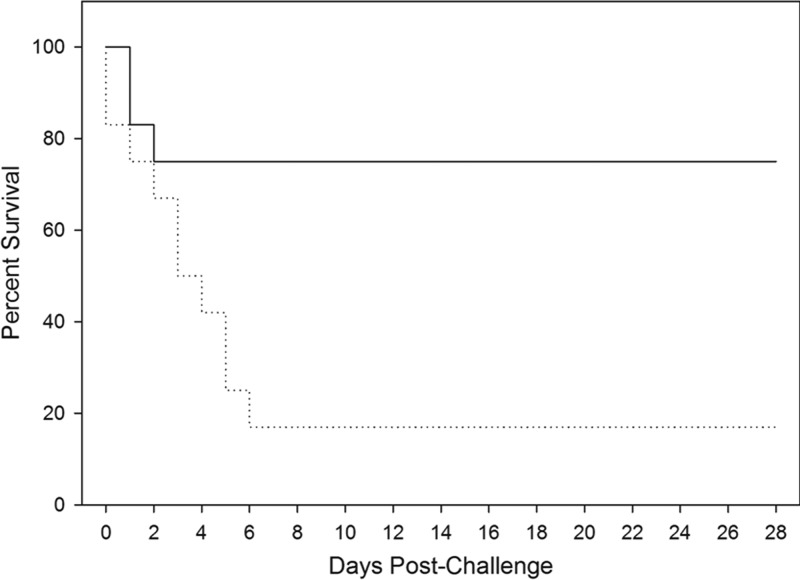
Treated animals had a significantly greater survival rate than untreated animals (P = 0.006; Fisher Exact test). When the time to death is taken into account, treated animals had significantly greater protection than the untreated control animals (P = 0.013; log-rank test). Figure 1 illustrates the Kaplan-Meier curves that show the survival and time-to-death for each group of animals.
Fig 1.
Kaplan-Meier curves representing time to death from challenge and mortality data for each group. The time to death from challenge and mortality data for both challenged groups are shown. Solid line, group challenged with B. anthracis and then treated with antibody when ECL positive; dotted line, group challenged with B. anthracis and untreated.
Anthrax-specific indications of infection.
Positive B. anthracis blood culture and circulating PA are considered confirmation of anthrax infection; therefore NHPs were monitored for these parameters frequently over the first 3 days postchallenge and then approximately weekly thereafter.
Bacteremia.
All of the challenged animals were bacteremic after challenge. Positive blood cultures were observed between 24 and 60 h postchallenge (Table 1). B. anthracis was cultured from the blood of all animals that succumbed to infection (data not shown). In addition, all NHPs in the treated group (group 1) were bacteremic prior to passive immunization. The average times from challenge until positive bacteremia were 35.90 and 38.06 h for the treated and untreated groups, respectively (P = 0.59; Student t test). Generally, positive blood cultures persisted to death for all of the untreated animals. The two surviving untreated animals had positive cultures at 42 h postchallenge, which resolved by the blood collection at 72 h.
Circulating PA (PA-ECL and PA-ELISA).
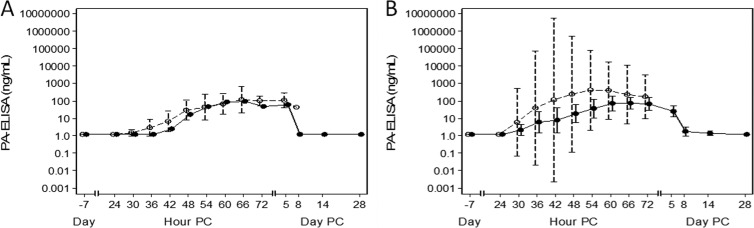
Circulating PA was detected in real-time by the PA-ECL at time points ranging from 24 to 60 h postchallenge with all animals positive for PA by ECL by 60 h postchallenge (Table 1). The mean times to positive PA-ECL for treated and untreated animals were 37.59 and 38.69 h, respectively (P = 0.71; Student t test). All samples collected immediately prior to treatment were positive for PA via ECL, which correlated with the bacteremia results. Quantitative levels of PA in circulation were determined by the PA-ELISA. Figure 2 illustrates the PA levels over the course of the study in treated or untreated survivors (Fig. 2). The geometric mean PA levels at the time of treatment were 26.6 ng/ml, with the geometric mean levels in the treatment group reaching 641.7 ng/ml by 48 h postchallenge. The highest geometric mean level of PA observed in the untreated group was 680.6 at 66 h postchallenge (Fig. 2). The PA levels in samples obtained from NHPs that died during the study ranged from 205 to 27,452 ng/ml. Interestingly, there was no statistical difference in the PA levels for the treated and untreated groups, except for the day 5 postchallenge time point (P = 0.01; Student t test).
Fig 2.
Circulating levels of PA. The circulating PA levels as assessed by ELISA are presented for the untreated animals (open circles, average of nonsurvivors; closed circles, average of survivors) (A) and treated animals (open circles, average of nonsurvivors; closed circles, average of survivors) (B).
Clinical evaluation.
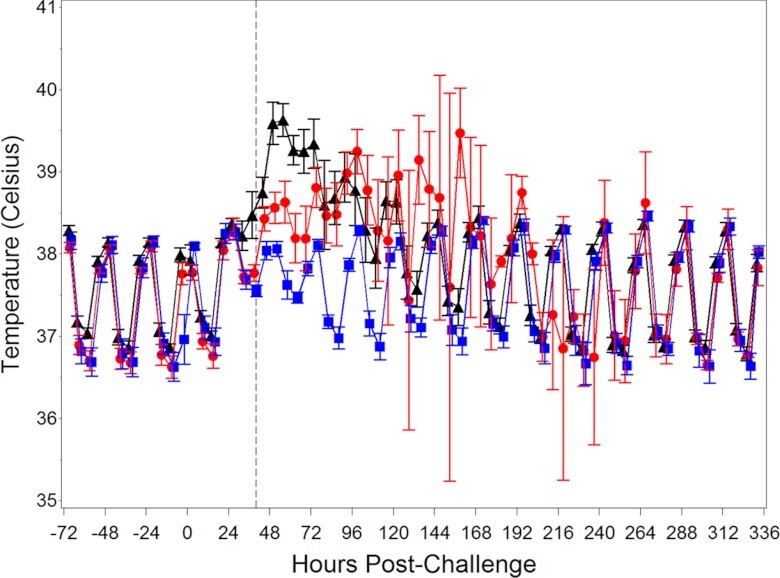
In addition to monitoring anthrax-specific indicators of infection, the NHPs were evaluated for changes in several nonspecific clinical parameters that could help support a profile for the cynomolgus macaque inhalational anthrax model. Figure 3 shows the mean body temperatures for the treated and untreated challenged groups, as well as for the naive control group. The treated and untreated groups show a loss of diurnal rhythm and an elevated body temperature starting 30 h postchallenge. The diurnal pattern returned for the surviving treated animals by ∼96 h postchallenge, whereas the body temperature pattern for the two untreated survivors remained disrupted until 8 days postchallenge. The diurnal rhythm for the unchallenged group was slightly interrupted during the frequent animal manipulation time period (30 to 72 h postchallenge), but not to the extent of the challenged groups.
Fig 3.
Kinetics of body temperature for challenged and unchallenged animals. The mean body temperatures (± the standard errors of the mean) for B. anthracis-challenged and treated (black), B. anthracis-challenged and untreated (red), and unchallenged (blue) groups are shown. The dotted line represents the average time to treatment for treated animals.
While six consecutive elevated body temperature readings (baseline adjusted) were chosen to identify a significant increase in body temperature (SIBT), analysis showed that two of the unchallenged animals exhibited SIBT. One of the unchallenged animals did not exhibit SIBT until 685.12 h (∼28 days) postchallenge, and the other unchallenged animal exhibited SIBT at 39.12 h postchallenge. The cause of the febrile response in these naive animals is not known.
The time to an SIBT for each challenged animal is shown in Table 1. The average times from challenge to SIBT for the treated and untreated groups were 49.53 and 54.48 h, respectively (P = 0.40; Student t test).
NHPs challenged with B. anthracis showed clinical signs of disease, including inappetence, stool abnormalities (e.g., soft stool, no stool, or diarrhea), posture changes (hunched or lying down in cage), inactivity or lethargy, signs of respiratory abnormalities (e.g., coughing, wheezing, and labored respirations), and unresponsiveness. When the challenged animals were compared to the unchallenged animals, certain clinical signs were significantly more prevalent in the challenged animals, while other clinical signs were nonspecific and observed in all three groups of animals (Table 2). In addition, while respiratory abnormalities were observed for 58% (7/12) of the untreated animals, these moderate to severe observations (i.e., wheezing, coughing, labored breathing, and respiratory distress) were documented for only 17% (2/12) of treated animals. None of the unchallenged animals exhibited a respiratory abnormality. The soft stool and rare hunched posture observations in the unchallenged NHPs occurred during the high frequency manipulation period (the first 72 h postchallenge) and may likely be due to stress (Table 2).
Table 2.
Incidence of clinical signs post challenge
| Clinical sign | No. of animals with clinical signs/no. of animals per group (%)c |
||
|---|---|---|---|
| Treated (group 1) | Untreated (group 2) | Unchallenged (group 3) | |
| Lethargy | 9/12 (75) | 12/12 (100)* | 2/6 (33) |
| Stool abnormalitiesa | 7/12 (58) | 7/12 (58) | 3/6 (50) |
| Inappetence | 12/12 (100) | 11/12 (92) | 6/6 (100) |
| Hunched posture | 12/12 (100) | 12/12 (100) | 5/6 (83) |
| Vomiting | 5/12 (42) | 2/12 (17) | 0/6 (0) |
| Respiratory abnormalitiesb | 2/12 (17) | 7/12 (58)* | 0/6 (0) |
| Moribund | 1/12 (8) | 1/12 (8) | 0/6 (0) |
Stool abnormalities included soft stool, no stool, diarrhea, or mucous stool.
Respiratory abnormalities included respiratory distress, labored respirations, wheezing, or coughing.
*, P < 0.05 compared to the unchallenged (group 3).
Challenged animals showed changes in certain clinical hematology parameters and CRP. In contrast, hematology parameters remained unaffected throughout the study period for unchallenged animals.
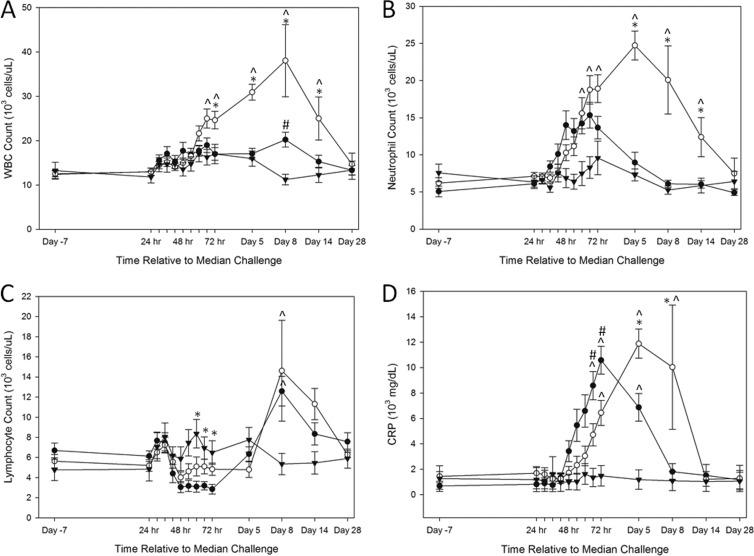
Figure 4A shows elevations in WBC counts after challenge compared to prechallenge levels. In addition, the untreated group had significantly higher WBC counts compared to the unchallenged group and the treated group at certain time points (Fig. 4A). By 8 days postchallenge, the WBC counts returned to baseline levels in treated animals, while the untreated animals showed an increase that did not return to baseline levels until day 28 (in the two survivors). In addition, the two untreated survivors exhibited remarkably high total WBC count levels by day 8 postchallenge (44,300 and 47,880 cells/μl, respectively).
Fig 4.
Kinetics of clinical hematology and CRP for challenged and unchallenged animals. Average WBC count (A), neutrophil count (B), lymphocyte count (C), and CRP (D) trends are depicted for treated, untreated, and unchallenged animals. Closed circles, animals challenged with B. anthracis and treated with antibody; open circles, animals challenged with B. anthracis and untreated; closed triangles, unchallenged animals. Group averages with standard errors of the mean are shown. ^, P < 0.05 compared to the unchallenged group; *, P < 0.05 compared to the treated group; #, P < 0.05 compared to the untreated group.
The average times from challenge to an abnormal WBC count for treated and untreated animals were 55.30 h and 78.29 h, respectively (P = 0.17; Student t test). One treated animal did not exhibit an abnormal WBC count until 190.30 h (statistical outlier), and a Student t test determined a significant difference in the time to abnormal WBC count (P = 0.001; Student t test) between treated and untreated animals when this animal was excluded.
Challenged animals showed elevations in neutrophil counts at 42 h postchallenge (Fig. 4B). The neutrophil response for the treated animals occurred earlier relative to a change in total WBC count, whereas the neutrophil change for the untreated animals appeared to be later than the time to an altered WBC count. Interestingly, the two untreated survivors had the highest neutrophil counts at 48 h postchallenge compared to the other untreated animals. The average times from challenge until abnormal neutrophil count were 39.86 and 92.02 h for treated and untreated animals, respectively (P = 0.002; Student t test).
Unlike the neutrophil count and total WBC count, lymphocyte counts decreased 48 h after challenge and then became elevated on day 8 postchallenge (Fig. 4C). This peak resolved in surviving animals by the end of the study. The average times from challenge until an abnormal lymphocyte count for the treated and untreated groups were 49.63 and 54.43 h, respectively (P = 0.25; Student t test).
CRP levels were elevated for 23 of the 24 challenged animals (96%) (Fig. 4D). All 12 treated animals exhibited an increase in CRP with an average time postchallenge of 39.68 h, and 11 of the 12 untreated animals exhibited an increase in CRP on average of 35.25 h postchallenge (P = 0.47; Student t test). Importantly, three unchallenged animals had increased CRP levels compared to baseline levels.
Correlation between onset of clinical parameters.
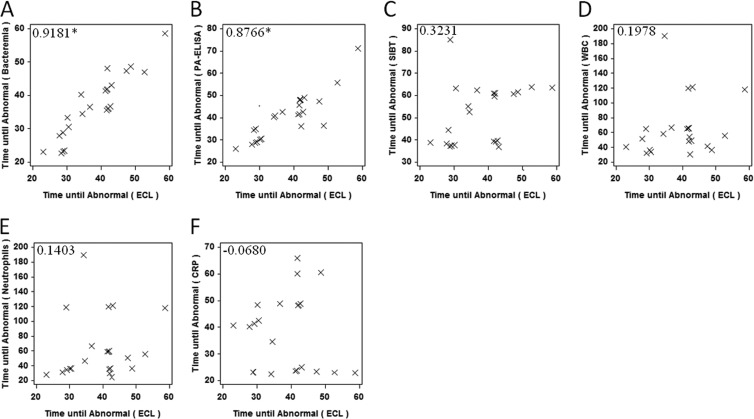
Correlations between the onset of several clinical signs are shown in Fig. 5. Importantly, the time until an abnormal (positive) PA-ECL was significantly correlated with the time until abnormal for other anthrax-specific parameters, such as positive blood culture, and PA-ELISA. However, nonspecific parameters, such as SIBT, abnormal WBCs, neutrophils, and CRP, were not significantly correlated with time to a positive PA-ECL result.
Fig 5.
Correlation for the onset of clinical parameters. The correlations for the onset for a positive/abnormal ECL with bacteremia (A), PA-ELISA (B), SIBT (C), WBC (D), neutrophils (E), and CRP (F) are shown in scatter plots. The correlation coefficient for each comparison is included. *, P < 0.05.
Pathology.
Gross lesions at necropsy were consistent with anthrax-related lesions previously observed in untreated cynomolgus macaques, including body cavity effusions, red lung discoloration, liver foci, red or dark foci in the brain, enlargement and mottling of the ovary, and enlargement and/or dark color of multiple lymph nodes (26). Lesions of vascular damage (hemorrhage, edema, and parenchymal necrosis) in multiple organ systems were typical of anthrax. All three animals in the treated group that succumbed to disease exhibited gross findings in the brain, but only one of these animals exhibited gross findings in the other tissues assessed at necropsy. Alternatively, only 2 of the 10 animals in the untreated control group showed lesions in the brain upon necropsy, whereas lesions were observed in many of the other tissues assessed in the untreated control animals.
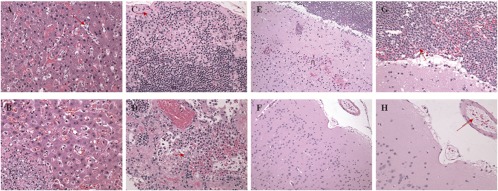
Microscopic findings considered consistent with inhalational anthrax in untreated cynomolgus macaques were present in all animals that died or became moribund during the study (26). Table 3 shows the incidence of anthrax-related microscopic observations, with average severity scores for these animals. Lesions typical of anthrax included acute inflammation (suppurative), hemorrhage, edema, fibrin exudation, and/or necrosis and the presence of large rod-shaped bacteria consistent with B. anthracis in multiple organs evaluated histologically. Representative anthrax-related lesions in the livers, lymph nodes, and brains of treated and untreated animals are shown in Fig. 6.
Table 3.
Incidence summary of anthrax-related microscopic observations with average severity scores in treated and untreated NHPs
| Tissue | Observation(s) | Treatment after anthrax challenge | Total no. of animals examineda | Lesion incidence (no. of animals) | Avg lesion severityb |
|---|---|---|---|---|---|
| Brain | Bacteria | Antibody | 3 | 3 | 3.0 |
| Untreated | 10 | 8 | 1.3 | ||
| Acute inflammation | Antibody | 3 | 3 | 3.7 | |
| Untreated | 10 | 1 | 0.4 | ||
| Hemorrhage | Antibody | 3 | 3 | 2.3 | |
| Untreated | 10 | 1 | 0.4 | ||
| Necrosis | Antibody | 3 | 3 | 1.0 | |
| Untreated | 10 | 1 | 0.1 | ||
| Liver | Bacteria | Antibody | 3 | 1 | 0.7 |
| Untreated | 10 | 8 | 1.5 | ||
| Acute inflammation | Antibody | 3 | 0 | 0.0 | |
| Untreated | 10 | 4 | 0.7 | ||
| Necrosis | Antibody | 3 | 0 | 0.0 | |
| Untreated | 10 | 6 | 1.2 | ||
| Sinusoidal leukocytosis | Antibody | 3 | 3 | 1.0 | |
| Untreated | 10 | 9 | 1.8 | ||
| Lymph node, mediastinal | Bacteria | Antibody | 3 | 1 | 0.3 |
| Untreated | 10 | 9 | 1.1 | ||
| Acute inflammation | Antibody | 3 | 1 | 0.7 | |
| Untreated | 10 | 5 | 0.6 | ||
| Depletion/necrosis, lymphocytes | Antibody | 3 | 2 | 1.3 | |
| Untreated | 10 | 10 | 2.5 | ||
| Edema | Antibody | 3 | 1 | 0.3 | |
| Untreated | 10 | 4 | 0.5 | ||
| Hemorrhage | Antibody | 3 | 0 | 0.0 | |
| Untreated | 10 | 8 | 1.6 |
Only animals that died on study were assessed for histopathology.
Lesions were graded on a scale of 1 to 4: 1, minimal (the least detectable lesion); 2, mild (easily discernible lesion); 3, moderate (change affecting a large area of represented tissues with potential to be relevant); and 4, marked (lesion that approached maximal).
Fig 6.
Anthrax-related lesions in the livers, lymph nodes, and brains of treated and untreated NHPs. (A) Image at ×40 magnification of liver section from a monoclonal antibody-treated NHP that died 3 days after challenge. Mild sinusoidal leukocytosis and a few circulating B. anthracis organisms (arrow) are evident. (B) Image at ×40 magnification of a liver section from an untreated NHP that died 7 days after challenge. A focus of hepatocellular necrosis, fibrin exudation, suppurative inflammation, and numerous intralesional B. anthracis organisms can be seen in the lower left portion of the image. (C) Image at ×40 magnification of mediastinal lymph node from monoclonal antibody-treated NHP that died 3 days after challenge. Mild lymphoid depletion, subcapsular edema, minimal hemorrhage, and a few B. anthracis organisms (arrow) are apparent. (D) Image at ×40 magnification of mediastinal lymph node from an untreated NHP that died 5 days after challenge. Marked lymphoid necrosis, fibrin exudation, hemorrhage, and intralesional B. anthracis organisms (arrow) can be seen. (E) Image at ×20 magnification of the cerebral cortex from a monoclonal antibody-treated NHP that died 4 days after challenge. Suppurative meningitis, vasculitis, hemorrhage, and necrosis (n) are visible. (F) Image at ×20 magnification of the meninges and cerebral cortex from an untreated NHP that died 3 days after challenge. Note the typical lack of notable microscopic lesions. (G) Image at ×40 magnification of the cerebral meninges from a monoclonal antibody-treated NHP that died 4 days after challenge. Suppurative meningitis and hemorrhage with intralesional B. anthracis organisms (arrow) are evident. (H) Image at ×40 magnification of the cerebral meninges from an untreated NHP that died 3 days after challenge. Note the presence of intravascular B. anthracis organisms (arrow) but no other microscopic lesions. hematoxylin and eosin stain was used for all tissues.
Although only three animals in the antibody-treated group succumbed to disease, all three of these animals had considerable lesions consistent with anthrax in the brain. Specifically, these animals had acute suppurative inflammation affecting the meninges (meningitis) with extension into Virchow-Robbins' space and lesser direct penetration of adjacent neuropil. Necrosis was seen in the brain of a few animals and was characterized by rarefaction of neuropil, pyknosis of neuron and glial cell nuclei, and an overall local decrease in number of glia. This lesion is consistent with early liquefactive necrosis and was always located in close proximity to inflamed meninges, affecting the superficial cerebral cortex or cerebellar folia within ∼3 mm of the surface. Hemorrhaging was found in the same localizations as inflammation and necrosis in the brain, affecting the meninges and outer neutrophil. In contrast to the three treated animals that did not survive to day 28, only one of the 10 untreated control animals that died during the study had substantial lesions in the brain.
DISCUSSION
The 2001 intentional anthrax release emphasized the need for appropriate animal models to evaluate the efficacy and safety of potential medical countermeasures against B. anthracis. NHPs have been used as a model for infectious diseases due to their similar disease progression and susceptibilities to humans, including anthrax infection (8–11, 21, 25). Therefore, we set out to further characterize the cynomolgus macaque model of inhalational anthrax to support three of the four components of the FDA Animal Rule. The fourth component of the Animal Rule, pharmacokinetics (PK) and pharmacodynamics (PD) analysis of a product (drug), is not applicable here since this study was not designed to assess the PK/PD for a specific drug.
Victims of the 2001 anthrax attack exhibited fevers, fatigue, lethargy, cough, nausea, vomiting, and dyspnea. In addition, these patients were observed with elevated WBC counts, neutrophilia, tachycardia, and abnormal chest X-rays (14). In the present study, increases in body temperature were identified for nearly all of challenged animals; two unchallenged animals also presented with fevers. This variable febrile response was also observed with anthrax-infected African green monkeys but may be model dependent since a fever was observed for rhesus macaques exposed to anthrax (6, 8, 14, 21).
Consistent with what has been observed in human inhalational anthrax cases (3, 14), the majority of NHPs in the present study had increased total WBC counts and alterations in WBC differentials following aerosol exposure. The increase in WBC and neutrophil counts, followed by the later increase in lymphocytes for surviving challenged animals, may be due to an initial innate response, followed by the cell-mediated immune response elicited by the infection. In addition, the difference in the time to an abnormal neutrophil count for the treated and untreated groups may be due to the antibody blocking the immunomodulating effects of LT since the treated animals had abnormal neutrophil counts within a relatively short time frame after receiving treatment, whereas an abnormal neutrophil count for the untreated animals did not occur until later. The lymphocyte counts from the challenged NHPs in the present study suggest that lymphocytes may be recruited out of circulation after challenge, since the levels drop in both the treated group and the untreated group ∼48 h after challenge. While the changes in the hematology parameters were limited to the challenged animals, three of the six unchallenged animals also exhibited elevations in CRP. Since CRP is a nonspecific indicator of inflammation and is also induced by stress, its levels could have been affected by the frequent handling of the animals, suggesting that CRP may be less beneficial in defining illness than other clinical parameters.
The clinical signs observed here are similar to the lethargy, respiratory abnormalities, and decrease in responsiveness observed in chimpanzees exposed to an inhalational dose of 40,000 or 65,000 B. anthracis spores and the “flu-like” symptoms associated with anthrax-infected humans or rhesus macaques (1, 2, 4, 7, 8, 24, 25). Similar to human data, but differing from the published African green monkey anthrax model, lethargy and respiratory abnormalities were observed in the present study for the majority of challenged untreated animals (12, 21).
In addition to further characterizing the disease progress in cynomolgus macaques, we also set out to evaluate the use of antigenemia as a suitable trigger for treatment in a therapeutic model of inhalational anthrax. Medical countermeasures can be classified into three general indications: (i) general use prophylaxes (given prior to exposure), (ii) postexposure prophylaxes (given after exposure, but prior to the onset of symptoms), and (iii) therapeutics (given once the subject has presented with symptoms). In animal models, a positive blood culture before treatment has been considered a requirement for initiating administration of medical countermeasures in a therapeutic fashion (13). In the present study, we utilized a real-time diagnostic platform to confirm the presence of PA in circulation prior to initiating treatment on an individual basis. Since blood cultures require ∼24 h of incubation prior to assessing culture results, we were able to retrospectively confirm that all animals were bacteremic prior to treatment. Importantly, the time to a positive PA-ECL result significantly correlated with the onset of bacteremia. These results are consistent with blood culture and circulating PA correlations observed for the New Zealand White rabbit and African green monkey (15, 21).
Although the time to an abnormal nonspecific parameter (i.e., WBC count) did not correlate with the time to a positive PA-ECL result, this is not unexpected since a delay in nonspecific indicators of infection compared to positive culture and circulating PA levels is consistent with a recent cynomolgus macaque anthrax aerosol treatment study (17). The nonspecific clinical parameters can still be used in conjunction with bacteremia and antigenemia to monitor infection.
A statistically significant survival benefit was observed for the treated group, supporting the use of the cynomolgus macaque as a model for assessing potential therapeutics. Two untreated control animals did not succumb to disease following the aerosol challenge; however, they were infected with B. anthracis, as evidenced by persistent bacteremia cultures, circulating PA levels, alterations in hematology, and increases in body temperature. Although it was somewhat unexpected that these animals did not succumb to anthrax, the results suggest that inhalational anthrax is not 100% lethal in the cynomolgus macaque inhalational anthrax model, which is consistent with two rhesus macaque inhalational anthrax studies (11, 22). Moreover, it can be speculated that an early robust neutrophil response, followed by a marked total WBC response (including lymphocytes), might have been adequate to promote survival for these two untreated control animals. Additional data would need to be collected to evaluate whether untreated survivors have physiological or genetic differences compared to the nonsurvivors.
An important factor that should be considered when evaluating the efficacy of medical countermeasures against any infectious agent is the safety of such products in conjunction with disease. The gross and microscopic lesions observed in untreated cynomolgus macaques that died or were euthanized during the study were consistent with previous cynomolgus macaque anthrax pathology (26). In addition, the higher incidence and severity of microscopic lesions in the brain in monoclonal antibody-treated nonsurvivors versus untreated animals is consistent with findings from nonsurviving cynomolgus macaques that were administered a different monoclonal antibody (13). The pathology finding suggests that antibody treatment is able to prevent infection or enhance clearance of bacteria from non-central nervous system (CNS) tissues and that CNS infection is somewhat refractory. This is an interesting finding considering that the untreated animals showed more systemic pathological findings (i.e., lymph nodes, spleens, livers, lungs, etc.) on gross and microscopic evaluations compared to the treated animals. These data suggest that neutralizing monoclonal antibody to PA limits the systemic tissue pathology seen in untreated animals but may in some way be less protective of the brain or may not be distributed into the brain to an effective level. Anthrax toxins subdue an immune response in the infected host, and one might infer that mitigating the toxin (i.e., promoting immune response) may be beneficial to the host. This may be true in the systemic tissue, but it might also be somewhat detrimental to the host should an immune response be targeted to bacteria residing near a compromised blood-brain barrier. Since the number of animals examined in the treatment group was limited to only three, further research needs to be conducted to determine whether there is a difference in the incidence of brain lesions in treated and untreated NHPs.
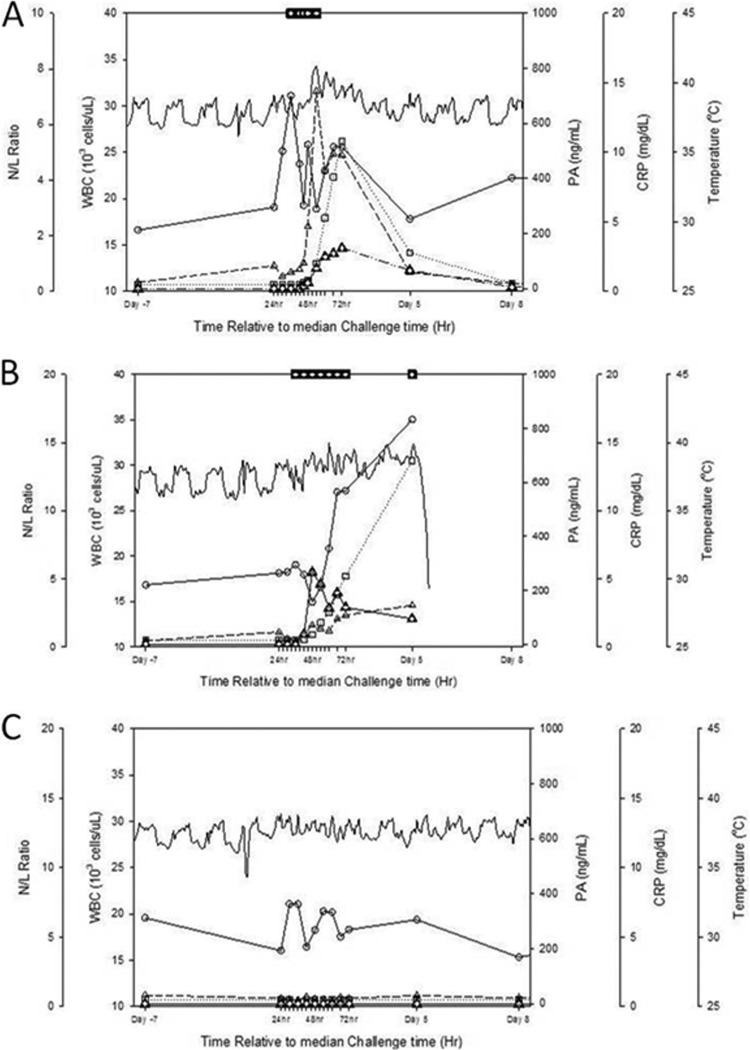
The data presented here identify distinct clinical profiles (Fig. 7) that are observed when unchallenged, challenged, and challenged-treated animals are compared, suggesting there are multiple clinical and physiological measurements consistent with clinical findings in humans that could be used to assess the disease progression of B. anthracis-challenged animals. Bacteremia and antigenemia comprise the two major components of the cynomolgus macaque inhalational anthrax model since these parameters confirm anthrax infection, whereas nonspecific parameters, such as alterations in WBC and neutrophil counts, body temperature, and clinical signs can be used in conjunction with the specific parameters to assess anthrax infection over the course of a study. Further, the detection of circulating PA as an indication for treatment resulted in antibody being administered in a therapeutic fashion, since the animals were confirmed to be bacteremic prior to passive immunization. Treatment at the first indication of antigenemia also resulted in a significant increase in survival compared to untreated control animals. Taken together, these data suggest that the cynomolgus macaque inhalational anthrax therapeutic model may be utilized for testing potential anthrax therapeutics in accordance with the FDA Animal Rule.
Fig 7.
Clinical profiles for treated (A), untreated (B), and unchallenged (C) animals. For panel A, the animal was challenged with B. anthracis and treated with antibody. For panel B, the animal was challenged with B. anthracis but not treated. Panel C shows the results for an unchallenged animal. Lines and symbols: solid lines with open circles, white blood cell count; dashed lines with open triangles, neutrophil/lymphocyte (N/L) ratio; dotted lines with squares, CRP levels; solid lines, body temperature; solid lines with open triangles, PA level; squares, positive blood culture.
ACKNOWLEDGMENTS
This study was supported by National Institute of Allergy and Infectious Disease (NIAID) contract N01-AI-30061.
We thank Amy Simmons, Phyllis Herr-Calomeni, Kevin Sayers, Andrew Puttmann, Neil Gibson, Frances Rabon, and Kristin Clement for outstanding technical assistance. We thank Roy Barnewall and David Fisher for contributions to the aerosol exposures. We thank Tracy Macgill, Judy Hewitt, and Raymond Slay for technical input and support. Finally, we thank Oscar A. Bermeo Blanco for implanting the telemetry units and James Rogers for critical review of the manuscript.
The reagent Anthrax Protective Antigen (NR-164) was obtained through the Biodefense and Emerging Infections Research Resources Repository, NIAID, National Institutes of Health.
Footnotes
Published ahead of print 5 September 2012
REFERENCES
- 1. Albrink WS. 1961. Pathogenesis of inhalation anthrax. Bacteriol. Rev. 25:268–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albrink WS, Goodlow RJ. 1959. Experimental inhalation anthrax in the chimpanzee. Am. J. Pathol. 35:1055–1065 [PMC free article] [PubMed] [Google Scholar]
- 3. Barakat LA, et al. 2002. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA 287:863–868 [DOI] [PubMed] [Google Scholar]
- 4. Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N. Engl. J. Med. 341:815–826 [DOI] [PubMed] [Google Scholar]
- 5. Friedlander AM. 1997. Anthrax, p 467–478 In Sidell F, Takafuji E, Franz D. (ed), Medical aspects of chemical and biological warfare. Office of the Surgeon General, Washington, DC [Google Scholar]
- 6. Friedlander AM. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335–349 [PubMed] [Google Scholar]
- 7. Friedlander AM, et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239–1243 [DOI] [PubMed] [Google Scholar]
- 8. Fritz DL, et al. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Invest. 73:691–702 [PubMed] [Google Scholar]
- 9. Gleiser CA. 1967. Pathology of anthrax infection in animal hosts. Fed. Proc. 26:1518–1521 [PubMed] [Google Scholar]
- 10. Gleiser CA, Berdjis CC, Hartman HA, Gochenour WS. 1963. Pathology of experimental respiratory anthrax in Macaca mulatta. Br. J. Exp. Pathol. 44:416–426 [PMC free article] [PubMed] [Google Scholar]
- 11. Henderson DW, Peacock S, Belton FC. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. (Lond.) 54:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holty JE, et al. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 144:270–280 [DOI] [PubMed] [Google Scholar]
- 13. Human Genome Sciences 2009. Raxibacumab: treatment of inhalational anthrax, BLA 125349. Anti-Infectives Advisory Committee Meeting briefing document Human Genome Sciences, FDA, Bethesda, MD [Google Scholar]
- 14. Jernigan JA, et al. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobiler D, et al. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 74:5871–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipscomb MF, Hutt J, Lovchik J, Wu T, Lyons CR. 2010. The pathogenesis of acute pulmonary viral and bacterial infections: investigations in animal models. Annu. Rev. Pathol. 5:223–252 [DOI] [PubMed] [Google Scholar]
- 17. Migone TS, et al. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135–144 [DOI] [PubMed] [Google Scholar]
- 18. Moayeri M, Leppla SH. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson JW, et al. 2007. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect. Immun. 75:3414–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pile JC, Malone JD, Eitzen EM, Friedlander AM. 1998. Anthrax as a potential biological warfare agent. Arch. Intern. Med. 158:429–434 [DOI] [PubMed] [Google Scholar]
- 21. Rossi CA, et al. 2008. Identification of a surrogate marker for infection in the African green monkey model of inhalation anthrax. Infect. Immun. 76:5790–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saile E, et al. 2011. Antibody responses to a spore carbohydrate antigen as a marker of nonfatal inhalation anthrax in rhesus macaques. Clin. Vaccine Immunol. 18:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tournier JN, Quesnel-Hellmann A, Cleret A, Vidal DR. 2007. Contribution of toxins to the pathogenesis of inhalational anthrax. Cell Microbiol. 9:555–565 [DOI] [PubMed] [Google Scholar]
- 24. Twenhafel NA. 2010. Pathology of inhalational anthrax animal models. Vet. Pathol. 47:819–830 [DOI] [PubMed] [Google Scholar]
- 25. Twenhafel NA, Leffel E, Pitt ML. 2007. Pathology of inhalational anthrax infection in the African green monkey. Vet. Pathol. 44:716–721 [DOI] [PubMed] [Google Scholar]
- 26. Vasconcelos D, et al. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 83:1201–1209 [DOI] [PubMed] [Google Scholar]