Abstract
Bordetella pertussis expresses two serologically distinct fimbriae (Fim2 and Fim3) which are included in the Sanofi Pasteur 5-component acellular pertussis vaccine, and antibody responses to these antigens have been shown to be associated with protection. Studies to date have assessed the IgG response to this vaccine using a copurified mixture of Fim2 and Fim3, and the response to the individual antigens has not been characterized. We have purified separate Fim2 and Fim3 from strains that express either Fim2 or Fim3 and have used these antigens in an enzyme-linked immunosorbent assay (ELISA) to quantify IgG responses following immunization with 5-component acellular pertussis vaccine in 15-month-old, 4- to 6-year-old, and 11- to 18-year-old subjects. All individuals showed increases in Fim2 and Fim3 IgG concentrations following immunization, with 3-fold-greater Fim2 than Fim3 IgG concentrations seen in the younger two age groups. Fim2 IgG concentrations were 1.5-fold greater than Fim3 IgG concentrations in the 11- to 18-year-olds. We have also compared Fim2 and Fim3 IgG concentrations in individuals with prolonged cough who were diagnosed as having recent pertussis using a pertussis toxin (Ptx) IgG ELISA with individuals with prolonged cough but without elevated Ptx IgG concentrations. Individuals with evidence of recent pertussis had greater Fim3 IgG concentrations, consistent with the predominant serotype of isolates obtained in the United Kingdom. However, a surprising number of individuals had moderate Fim2 IgG concentrations despite very few isolates of that serotype obtained in the sampling period.
INTRODUCTION
Bordetella pertussis causes whooping cough, a highly communicable disease which continues to be a public health concern despite high levels of vaccination with either whole-cell or acellular pertussis vaccines. In the last decade, a resurgence of pertussis has been observed in highly immunized populations (10). A large number of cases are found in adolescents and adults, who can then transmit bacteria to vulnerable infants who are too young to have received a full vaccination schedule. There are many possible causes for this apparent resurgence, such as increased awareness of the disease in older patients, better diagnostic tools, and waning of vaccine-induced immunity. To tackle this problem, booster doses for older children with acellular vaccine are recommended (30). Acellular pertussis vaccines contain between one and five B. pertussis antigens: pertussis toxin (Ptx), filamentous hemagglutinin (FHA), pertactin (Prn), and fimbriae (Fim2 and Fim3). Many aspects of the pathogenesis of pertussis and vaccine correlates of protection are poorly understood. However, antibodies to Prn, Fim, and, to a lesser extent, Ptx appear to have a direct correlation with protection (3, 25). Fimbriae have been considered important vaccine components for many years in both whole-cell and acellular vaccines, with antibodies shown to block adhesion (7, 24), and studies performed >50 years ago found that protection correlated with high titers of agglutinating antibodies (17). We now know that the agglutinogens to which these antibodies were directed are fimbriae, Prn, and lipopolysaccharide (LPS) (3).
B. pertussis expresses two serologically distinct fimbriae composed of either Fim2 or Fim3 major subunits which have molecular weights of 22,500 and 22,000, respectively (18). In addition to the major subunits, the fimbriae contain a single minor fimbrial subunit, designated FimD (29); both major and minor subunits can mediate binding to heparin (5, 6). United Kingdom and most European isolates used to be a mixed population of Fim2 and Fim3, Fim2, or Fim3 strains, but following introduction of universal whole-cell or acellular pertussis vaccination, strains almost exclusively express Fim3 (13). Recently, variants of Fim2 (Fim2-1 and Fim2-2) and Fim3 (Fim3A, -B, and -C) have been documented in several countries, including the United Kingdom (19, 27).
Serological responses to copurified mixtures of Fim2 and Fim3 have been measured by enzyme-linked immunosorbent assay (ELISA) in many studies, and an interlaboratory study reported that measurement of Fim antibody responses appeared to be less precise than in other assays due to the variety of Fim preparations used as coating antigens (15). One laboratory in that study was reported to determine antibodies to both Fim2 and Fim3, but no details were provided. To date, separate Fim2 and Fim3 have not been available to study responses to the different serotypes. This is particularly relevant to understand the shifts in serotype observed in many countries, including the United Kingdom (14, 20), and determine if both Fim types in the five-component acellular vaccine are equally immunogenic. There is a suggestion that Fim3 is less immunogenic than Fim2 following whole-cell vaccination (21), and data from Sweden suggest that the acellular vaccine containing Fim2 and Fim3 may offer higher protection against Fim2 strains than Fim3 (8).
In this study, we have purified separate Fim2 and Fim3 from B. pertussis strains expressing either Fim2 or Fim3 and used these antigens to measure antibody concentrations following vaccination with five-component acellular pertussis vaccine or following recent pertussis disease.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. pertussis strains Wellcome 28 (Fim2 plus Fim3) (23), Tohama (Fim2) (23), and S3 (Fim3, kindly provided by D. Xing, National Institute for Biological Standards and Control [NIBSC], United Kingdom) were cultured on charcoal agar for 48 h at 35°C in a humid atmosphere. Growth from each plate was transferred to 50 ml CL-CD medium (12) in a 250-ml baffled shake flask. After being cultured for 48 h at 35°C with shaking, this culture was used to inoculate 3 flasks containing 50 ml CL-CD medium and incubated as before. This culture was used as the inoculum for 10 2-liter baffled flasks containing 300 ml CL-CD medium, which were incubated for 48 h at 35°C with shaking. The contents of the 10 flasks were pooled to provide the 3-liter inoculum for a 70-liter fermenter culture. The culture was grown in CL-CD medium at 35°C, controlled to pH 8.1 with hydrochloric acid, and stirred and sparged with air (24 to 26 liters/min) to maintain dissolved oxygen tension above 20%, with antifoam added sparingly. The culture was harvested following a plateau in optical density (OD) and an increase in dissolved oxygen tension. The culture was concentrated by tangential-flow filtration, and the bacteria were removed by centrifugation at 4,600 × g for 60 min. The bacteria were then washed with ice-cold water, and the cell paste was aliquoted in batches equivalent to 10 liters of culture and frozen at −70°C.
Purification of Fim2 plus Fim3 and Fim2.
Cell paste from 10 liters of culture was resuspended to 10% (wt/vol) in chilled 50 mM HEPES–0.15 M NaCl (pH 8.0) buffer prior to homogenization using a Silverson L2R blender. The cell debris was removed by centrifugation at 4,600 × g for 90 min and the supernatant retained. Fimbriae were precipitated by addition of ammonium sulfate to 30% saturation at room temperature followed by static incubation at 4°C for 5 days. The precipitate was recovered by centrifugation at 25,000 × g for 2 h, resuspended in HEPES buffer, and extracted by repeated homogenization using a handheld homogenizer followed by centrifugation at 25,000 × g for 2 h, and the supernatant containing the fimbriae was retained. The supernatant was precipitated with 15% saturation ammonium sulfate and the resultant precipitate subjected to 4 rounds of extraction with HEPES buffer using a handheld homogenizer before pooling of the supernatants. The resultant final pool of supernatants was concentrated by dialysis using polyethylene glycol (PEG) 20000 (Sigma) at room temperature for 3 to 7 h until a protein concentration of >0.5 mg/ml was achieved.
Purification of Fim3.
B. pertussis strain S3 cell paste from 10 liters of culture was resuspended in phosphate-buffered saline (PBS) 4 M urea and incubated at 60°C for 30 min with gentle agitation. The supernatant was then recovered following centrifugation at 4,600 × g at 4°C for 60 min. Solid PEG 600 was added to 4% (wt/vol), left to stir for a minimum of 48 h at room temperature, and then placed at 4°C for 48 h before recovering of the fimbriae by centrifugation at 25,000 × g for 2 h at 4°C. After resuspension in 10 mM Tris–0.145 M NaCl (pH 7.4) containing 4 M urea, the fimbriae were left to equilibrate for 3 h before dialysis against 2 changes of 10 mM Tris–1 M MgCl3 (pH 7.0) at room temperature for 48 h and 12 h. The Fim3 precipitate was recovered by centrifugation (11,000 × g for 3 h) and resuspended in 10 mM Tris–0.145 M NaCl (pH 7.4). The fimbrial preparation was then applied to a 5-ml phenyl Sepharose column (GE Healthcare) and eluted with buffer without NaCl.
Fimbriae were analyzed by SDS-PAGE using Novex Tris glycine 14 or 16% acrylamide gels according to the manufacturer's instructions, and percent purity was determined using a Bio-Rad GS800 and Imagemaster software. Endotoxin concentration was determined using a chromogenic kinetic Limulus amoebocyte lysate (LAL) assay used according to the manufacturer's instructions (Lonza).
Mouse sera.
Groups of 10 6- to 8-week-old NIH/OLAHSD mice were immunized by subcutaneous injection with 1 μg of either Fim2 plus Fim3, Fim2, Fim3, or Fim2 treated with 4 M urea in 0.33% Alhydrogel (Superfoss Biosector, Denmark). Mice were immunized on days 0, 21, and 28, and terminal blood samples were taken on day 35.
Human sera.
Three panels of 30 paired serum samples taken before and after 5-component acellular pertussis vaccination were obtained from Sanofi Pasteur. These were from 15-month-old children following a fourth dose of Pentacel, 4- to 6-year-old children following a fifth dose of 5-component acellular pertussis vaccine, and adolescents 11 to 18 years old given 1 dose of Adacel. These sera were a convenience sample of paired specimens demonstrating good responses to Fim2 plus Fim3 and with sufficient volume to conduct the assays. The postvaccination sera were known to contain high concentrations of Fim antibody, but there was no selection based on responses to other pertussis antigens. All subjects were enrolled in accordance with the Declaration of Helsinki and good clinical practice guidelines, and the study protocols and informed-consent forms were reviewed and approved by appropriate institutional review boards for each study. Anonymized residual sera sent for serodiagnosis of pertussis following prolonged cough to the Health Protection Agency in 2009, 2010, and 2011 that were either seropositive or seronegative for recent pertussis by Ptx ELISA were also analyzed.
ELISA.
ELISA plates (Nunc Maxisorp) were coated with 1 μg/ml fimbriae (Fim2 plus Fim3, Fim2, or Fim3) at 100 μl per well in carbonate buffer (15 mM Na2 CO3, 35 mM NaHCO3 [pH 9.5]) for 20 h, with static incubation at 4°C. Fim2 was pretreated with 4 M urea for 2 h. Coated plates were washed with Tris-buffered saline/Brij buffer (0.137 M NaCl, 2.15 mM KCl, 1.1 mM Tris base, 9 mM Trizma HCl, 0.1% Brij 35 [pH 7.2]) and blocked with 150 μl PBS containing 5% vol/vol fetal bovine serum (FBS) and 0.1% Tween 20 for 1 h with shaking at 20°C. Duplicate serial dilutions of human sera, quality control (QC) in-house serum from a vaccinated adult volunteer, and reference standard 89/530 (NIBSC, United Kingdom, http://www.nibsc.ac.uk/documents/ifu/89-530.pdf) were prepared in blocking buffer on a separate dilution plate. One hundred microliters of each serial dilution was then transferred to the coated assay plate and incubated for 1 h with shaking at 20°C. After washing of the plates, goat anti-human IgG Fcγ fragment-specific affinity-purified antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories) was diluted 1/5,000 in blocking buffer, and 100 μl was applied to each well and incubated with shaking for 1 h at 20°C. After the plates were washed, 100 μl AP Yellow (p-nitrophenyl phosphate from BioFX) substrate was applied to each well and incubated for 1 h with shaking at 20°C before stopping the reaction by the addition of 50 μl 3 N NaOH and incubation with shaking for 5 min. For analysis of mouse sera, goat anti-mouse IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories) was used at a dilution of 1/2,000. The absorbance of each well was read using a Versamax plate reader (Molecular Devices) at 405 nm with a reference wavelength of 690 nm; the data were analyzed using SOFTmax PRO software using a predefined template which applied a 4-parameter logistic (4PL) model to the pertussis reference serum dose response data. The template then used OD values from duplicate serial dilutions of each test sample to interpolate IgG ELISA concentration units from the reference curve and multiplied the value by the appropriate (serial) dilution factor. The mean of the values for the serial dilutions was used as the final result for each test sample, and the variation across the serial dilution series was measured (as relative standard deviations [RSD], expressed as percentages) to ensure that the test sample was parallel to the reference and gave confidence in the integrity of the result obtained. Values from at least two serial dilutions were required to calculate a reportable value, and the maximum allowable RSD was 30%, although most samples tested had an RSD of <15%. An in-house QC serum with an assigned range (historical mean ± 3 standard deviations) against each antigen was tested on each assay plate to ensure consistency of assay performance. Plates with QC concentrations outside this assigned range were rejected, and samples on that plate were retested.
Statistical methods.
Geometric mean antibody concentrations with 95% confidence intervals (95% CIs) were calculated for Fim2 plus Fim3, Fim2, and Fim3, and geometric mean fold ratios comparing Fim2 and Fim3 were calculated with 95% confidence intervals. Reverse cumulative distribution curves of Fim2 and Fim3 antibody concentrations in sera positive or negative for pertussis diagnosis by pertussis toxin ELISA were plotted.
RESULTS
Purification of fimbriae.
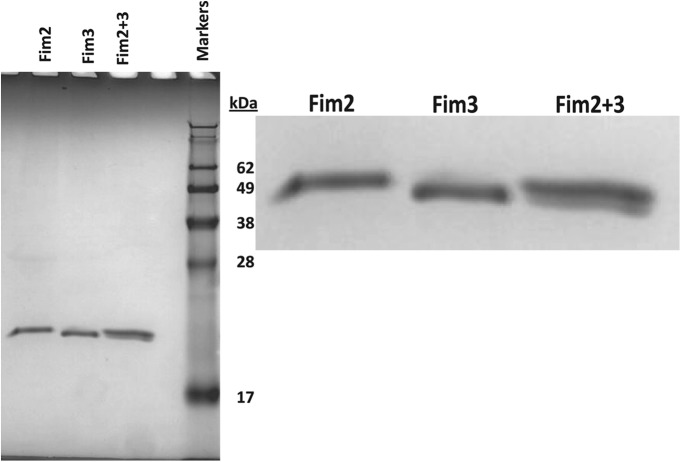
The purification methods for Fim2 plus Fim3, Fim2, and Fim3 were adapted from those described by Robinson et al. (23), and yields of approximately 9, 1.2, and 0.3 mg/liter, respectively, were obtained. No Fim3 was obtained with the homogenization method used for Fim2 plus Fim3 and Fim2, which resulted in the development of an alternative method using 4 M urea for isolation of Fim3. SDS-PAGE analysis showed these preparations to be highly pure (Fig. 1), with undetectable levels of contaminating proteins by scanning densitometry. Fim2-Fim3 preparations contained 57% Fim2 and 43% Fim3 as a percentage of total protein. The endotoxin concentrations of the Fim2-Fim3, Fim2, and Fim3 preparations were determined as 1.1 × 105, 4.8 × 105, and <6 × 103 EU/mg, respectively.
Fig 1.
Purified Fim2 plus Fim3, Fim2, and Fim3 analyzed by SDS-PAGE (3 μl protein was added per well).
Serotype specificity of the immune response to purified fimbriae.
Groups of 10 mice were immunized with purified Fim2 plus Fim3, Fim2, and Fim3 and the sera characterized for recognition of homologous or heterologous fimbrial preparations. As Fim3 is purified using buffers containing 4 M urea, Fim2 was also treated in this buffer before being used to coat the ELISA plate. All the sera recognized Fim2 plus Fim3 with a high titer, whereas sera raised against native or urea-treated Fim2 only reacted with plates coated with Fim2-containing preparations (Fig. 2). Urea treatment of immunizing antigen or the ELISA coating antigen did not affect the specificity or magnitude of the IgG response. Serum raised against Fim3 was also exquisitely serotype specific, with no cross-reaction to Fim2 treated with urea and only a slight reaction with native Fim2 detected (Fig. 2). Thus, we confirm that the antigen preparations contain undetectable levels of heterologous antigens and are suitable for use in a highly specific Fim2 or Fim3 antibody detection ELISA.
Fig 2.
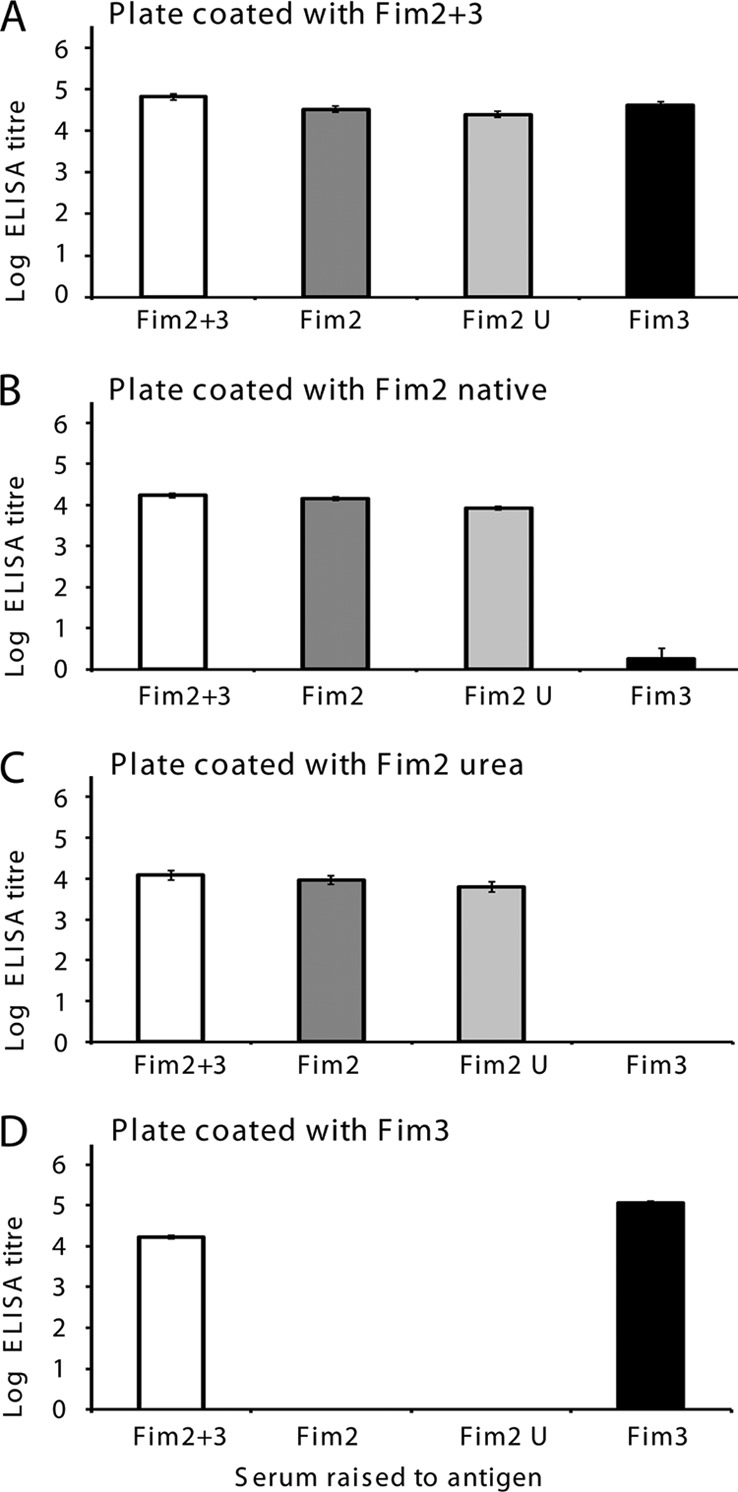
Log ELISA titers of mouse sera raised against Fim2 plus Fim3 (A), Fim2 (B), Fim2 treated with 4 M urea (C), and Fim3 (D) against each of these antigens. Two groups of 5 mice were immunized with each antigen. Pooled sera from each group were analyzed, and the geometric mean and standard error from 3 replicates is presented.
Assignment of ELISA antibody concentration units to pertussis reference serum 89/530 for separate Fim2 and Fim3.
Pertussis reference serum 89/530 (NIBSC, United Kingdom) is a pool of sera from 2 adults recently recovered from pertussis and 3 adults immunized with a 5-component pertussis vaccine. It was assigned a value of 280 ELISA units of anti-Fim2-Fim3 antibody per vial in a collaborative study using U.S. standard pertussis antiserum lot 3. This was diluted in 3.3 ml 50% glycerol to make a working stock containing 84.8 units/ml. To assign ELISA antibody concentration units for separate Fim2 and Fim3, wells of an ELISA plate were coated with either a Fim2-Fim3 mixture (1 μg/ml of Fim2 plus 1 μg/ml Fim3), Fim2 alone (1 μg/ml), or Fim3 alone (1 μg/ml). An ELISA was performed using duplicate serial dilutions of the 89/530 reference serum across the whole plate. A 4PL curve was fitted to the data for the Fim2-Fim3 mixture, and this was used as the reference curve with an assigned value of 84.8 (Fig. 3). The wells coated with Fim2 or Fim3 were treated as test samples, and the absorbance values for each serum dilution were used to interpolate ELISA concentration units from the reference curve for each antigen. The final assigned ELISA concentration units used were the means of 4 repeat plates and were 72.8 for Fim2 and 14.8 for Fim3, with relative standard deviations of 12.9 and 14.3%, respectively. Thus, we observed that a higher proportion of the Fim antibody in 89/530 recognized Fim2 than Fim3. The lower limits of detection were calculated from at least 18 replicate 8-point doubling dilutions of the 89/530 reference as 0.8, 1.7, and 0.2 for Fim2 plus Fim3, Fim2, and Fim3, respectively. We also investigated the use of the first international standard for pertussis antiserum (31), but it has a lower concentration of anti-Fim antibodies and we did not obtain ELISA dilution curves for Fim2 plus Fim3, Fim2, and Fim3 that allowed assignment of values to the test sera.
Fig 3.
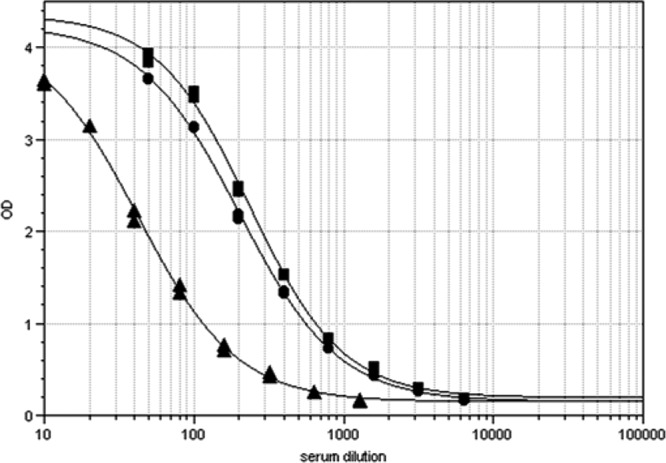
ELISA dose response curves of pertussis standard serum NIBSC 89/530 with a mixture of separate Fim2 and Fim3 (■) (coating concentration, 1 μg each per ml), Fim2 (●), or Fim3 (▲) (coating concentration, 1 μg/ml).
Quantification of Fim2-Fim3, Fim2, and Fim3 IgG concentrations in human sera following vaccination with 5-component acellular pertussis vaccine.
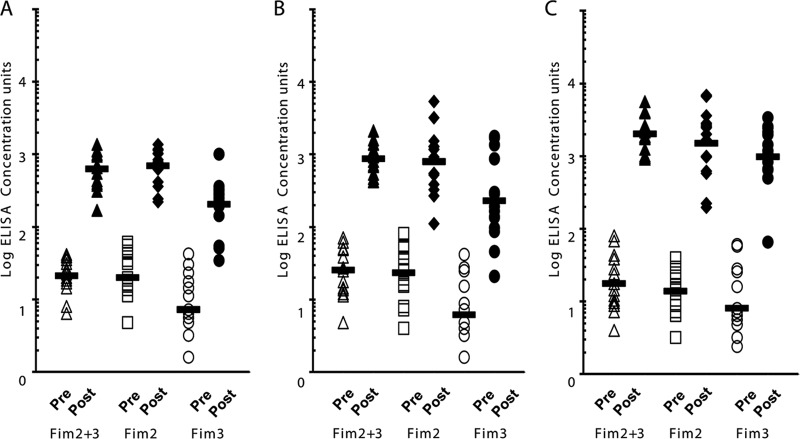
Three panels of paired serum samples taken before and after 5-component acellular pertussis vaccination were supplied by Sanofi Pasteur from 15-month-old children, 4- to 6-year-old children, and 11- to 18-year-olds. The 15-month-old children and the 4- to 6-year-old children were primed with 5-component acellular pertussis vaccine, but the vaccination history of the 11- to 18-year-olds is unknown. These sera were analyzed using the copurified Fim2-Fim3, Fim2, and Fim3 ELISAs, and dot plots of the antibody concentrations obtained are shown in Fig. 4. Geometric mean antibody concentrations and 95% confidence intervals are given in Table 1. Prevaccination levels of antibody to fimbriae were low in all 3 groups, but all individuals showed a rise in antibody concentration to Fim2 plus Fim3 and to the separate Fim2 and Fim3 following vaccination with the 5-component vaccine. Similar geometric mean concentrations of Fim2 IgG were seen following vaccination in the younger two age groups and similar Fim3 IgG concentrations were also observed, while higher antibody concentrations against both antigens were seen in the 11- to 18-year-old subjects. Fim2 geometric mean antibody concentrations were determined to be 3.4- and 3.5-fold greater than Fim3 geometric mean antibody concentrations in 15-month-old and 4- to 6-year-old subjects, respectively, while in the sera from the 11- to 18-year-olds this ratio was 1.5, although this difference was not significant (95% CI includes 1.0) (Table 1). There was an approximately 30-fold rise in concentration of antibody to Fim2 plus Fim3, Fim2, and Fim3 following vaccination in the younger children, while a >100-fold rise was observed following vaccination in the 11- to 18-year-olds (Table 1).
Fig 4.
Dot plot of ELISA IgG concentrations for Fim2 plus Fim3, Fim2, and Fim3 pre- and postvaccination (with 5-component acellular pertussis vaccine) serum pairs in 15-month-olds after the 4th dose (A), 4- to 6-year-olds after the 5th dose (B), and 11- to 18-year-olds after the adolescent dose (C). n = 15 for each group. Bars indicate the geometric mean IgG concentrations.
Table 1.
Geometric mean Fim2-Fim3, Fim2, and Fim3 ELISA IgG concentrations in vaccineesa
| Age group | Geometric mean IgG ELISA concn units (95% CI) |
Fim2/Fim3 ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Fim2 + Fim3 |
Fim2 |
Fim3 |
|||||
| Pre | Post | Pre | Post | Pre | Post | ||
| 15 mo old, after 4th dose of aP5 (n = 15) | 21 (16, 29) | 634 (459, 875) | 20 (13, 31) | 695 (504, 958) | 7 (4, 13) | 206 (126, 335) | 3.4 (2.0, 5.6) |
| 4–6 yr old, after 5th dose of aP5 (n = 15) | 26 (17, 40) | 871 (661, 1,145) | 23 (15, 37) | 801 (464, 1,383) | 6 (3, 11) | 230 (111, 475) | 3.5 (1.5, 8.2) |
| 11–18 yr old, after adolescent dose of aP5 (n = 15) | 18 (11, 29) | 2027 (1,502, 2,735) | 14 (10, 20) | 1,509 (828, 2,751) | 8 (4, 16) | 980 (571, 1,683) | 1.5 (0.6, 3.9) |
Pre and post, before and after vaccination, respectively; aP5, 5-component acellular pertussis vaccine.
Fim antibody concentrations in individuals following recent disease.
B. pertussis isolates in recent years received by the United Kingdom Pertussis Reference Laboratory have almost exclusively only expressed Fim3 (Table 2). However, 32% of isolates received in 2011 expressed Fim2 and 27% expressed Fim2 in the first 5 months of 2012, suggesting that a change in the predominant serotype may be occurring. We were interested to determine if the Fim2 or Fim3 antibody concentrations detected in individuals diagnosed with recent pertussis reflected the predominant serotype of B. pertussis isolates. Thus, we analyzed residual sera sent to the Pertussis Reference Laboratory for pertussis serodiagnosis using a Ptx antibody ELISA. Sera were obtained from 30 individuals with prolonged cough and Ptx IgG antibody concentrations over the diagnostic threshold of 70 IU/ml in 2009, 2010, and 2011. Fim antibody concentrations were also determined in sera from 30 individuals with prolonged cough but low Ptx IgG concentrations submitted for analysis in 2010 and 2011. The geometric mean Ptx IgG concentrations and 95% confidence intervals are given in Table 3. The median (ranges are in parentheses) ages of individuals seropositive for recent pertussis in 2009, 2010, and 2011 were 35 (6 to 70), 41 (3 to 70), and 36 (7 to 74) years, respectively. Those for seronegative individuals in 2010 and 2011 were 40 (5 to 84) and 45 (2 to 69) years, respectively. Individuals with low Ptx IgG concentrations generally had low Fim antibody concentrations (Table 3). The geometric mean Fim3 IgG concentrations determined in Ptx-positive individuals were greater than for Fim2 in all 3 years, consistent with the predominant serotype of B. pertussis isolates. However, it was surprising that some individuals had relatively high Fim2 IgG concentrations, indicating recent exposure to B. pertussis expressing this serotype. Reverse cumulative distribution curves of Fim2 and Fim3 antibody concentrations are shown in Fig. 5, and the superiority of Fim3 IgG concentrations is clear. It can also be seen that a small number of individuals show clear responses to Fim2. Figure 5 demonstrated that Fim2 antibody concentrations were greater in 2010 and 2011 than in 2009, with at least 20% of individuals with Fim2 IgG concentrations of 29, 137, and 81 in 2009, 2010, and 2011, respectively.
Table 2.
Serotypes of B. pertussis isolates received by the UK Pertussis Reference Laboratorya
| Yr | No. (%) with indicated serotype |
No. (%) nontypeable | Total | ||
|---|---|---|---|---|---|
| 1,2 | 1,3 | 1,2,3 | |||
| 2008 | 3 (2) | 135 (95) | 0 | 4 (3) | 142 |
| 2009 | 2 (2) | 92 (98) | 0 | 0 | 94 |
| 2010 | 3 (8) | 36 (90) | 0 | 1 (2) | 40 |
| 2011 | 50 (32) | 106 (68) | 0 | 0 | 156 |
| 2012 | 69 (33) | 133 (64) | 1 | 3 (1) | 206 |
Data represent isolates received for each calendar year from 2008 to 2011 and for the first 7 months of 2012. Nontypeable isolates autoagglutinate, and thus a serotype cannot be assigned.
Table 3.
Geometric mean Ptx, Fim2-Fim3, Fim2, and Fim3 IgG concentration in sera from individuals seropositive or seronegative for pertussis by Ptx ELISAa
| Yr of serum collection | Geometric mean IgG ELISA concn units (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ptx |
Fim2 + Fim3 |
Fim2 |
Fim3 |
|||||
| Ptx− | Ptx+ | Ptx− | Ptx+ | Ptx− | Ptx+ | Ptx− | Ptx+ | |
| 2009 | ND | 299 (202, 443) | ND | 65 (27, 161) | ND | 12 (5, 29) | ND | 132 (51, 339) |
| 2010 | 2 (1, 1) | 442 (333, 586) | 6 (4, 11) | 78 (34, 179) | 5 (3, 9) | 36 (18, 71) | 5 (3, 9) | 103 (48, 218) |
| 2011 | 5 (3, 8) | 275 (186, 405) | 7 (3, 14) | 102 (55, 188) | 6 (3, 11) | 16 (7, 35) | 8 (4, 16) | 99 48, 206) |
IgG concentrations were determined in sera from 30 seropositive and 30 seronegative individuals in each year (no sera for Ptx-negative individuals were available for 2009). ND, not done.
Fig 5.
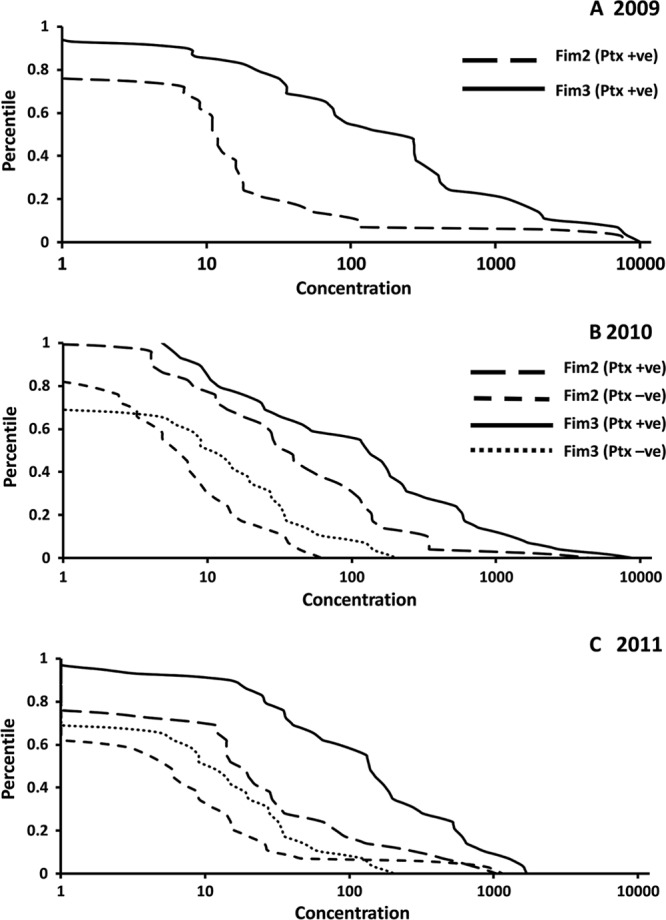
Reverse cumulative distribution curves of Fim2 and Fim3 ELISA IgG concentrations in sera from individuals seropositive or seronegative for pertussis by Ptx IgG ELISA (n = 30 per group) obtained in 2009 (A), 2010 (B), and 2011 (C).
DISCUSSION
B. pertussis fimbriae have been shown to be important components of acellular pertussis vaccines, with a significant association between clinical protection in household exposure studies and the presence of preexposure antibodies to Fim2 plus Fim3 (3, 25). Many studies have quantified the IgG response to fimbriae following immunization with pertussis vaccines, but copurified mixtures of Fim2 and Fim3 have been used which do not contain a defined ratio of antigens. In this study, we have purified separate Fim2 and Fim3 from Fim2- or Fim3-expressing B. pertussis strains so that the immune response to the individual fimbrial antigens could be quantified. The purification of these antigens has been previously described (23), but we were not able to replicate these methods successfully. Despite modification of the methods, yields of separate antigens remained low, and large fermenter cultures were required to obtain sufficient purified protein. Fim2 was purified from a serotype Fim2 strain by homogenization and repeated ammonium sulfate precipitation in the same way as Fim2 plus Fim3 from a strain expressing both antigens. Fim3 cannot be isolated using this method, and extraction with buffer containing 4 M urea was required, as previously described (23). The proteins appeared highly pure by SDS-PAGE analysis, although Fim2 plus Fim3 and Fim2 contained large amounts of LPS. In contrast, Fim3 prepared using 4 M urea extraction contained low levels of LPS. The exquisite serotype specificity of the antigens following immunization in mice (Fig. 2) demonstrates that this LPS contamination does not cause cross-reaction of the antibody response to heterologous antigen preparations and so is unlikely to contribute to the antibody responses seen with Fim2 plus Fim3 and Fim2 in human subjects, although this cannot be completely discounted and will be the subject of investigation in future studies. The lack of availability of separate Fim2 and Fim3 was noted by Xu et al. (32) and prompted development of recombinant Fim2 and Fim3. These antigens had the predicted subunit molecular weight on SDS-PAGE and were recognized by specific Fim2 and Fim3 monoclonal antibodies. However, these antigens did not provide protection in a mouse intranasal infection model as described for native antigens (22), suggesting the lack of protective epitopes in the recombinant antigens.
We developed an ELISA to determine IgG concentrations to separate Fim2 and Fim3 using the assigned concentration of NIBSC standard 89/530, which has been calibrated against U.S. reference pertussis antiserum lot 3 and has been used in many studies (16). The NIBSC 89/530 serum was a pool that contained serum from individuals immunized with 5-component acellular pertussis vaccine or following recent pertussis disease and thus had detectable responses to both Fim2 and Fim3. The Fim units assigned were obtained in studies using copurified mixtures of Fim2 and Fim3 where the ratios of Fim2 to Fim3 were not known. The Fim2-Fim3 preparation obtained in this study was approximately 60% Fim2 and 40% Fim3 (Fig. 1) and thus was not used to assign concentration values for Fim2 and Fim3, as this would have skewed the Fim2 and Fim3 antibody binding ratio. Thus, we used Fim2 and Fim3 at a defined 1:1 ratio of as the coating antigens, which showed that 89/530 contains a higher proportion of antibodies that recognize Fim2 than Fim3 (Fig. 3). We have shown that 4 M urea-treated fimbriae make suitable coating antigens. Fim2 was treated with 4 M urea before use in the ELISA to match the Fim3 preparation available. Also, sera from mice immunized with Fim2 treated with urea produced an IgG response similar to that produced by native Fim2 (Fig. 2), and use of urea-treated Fim2 as a coating antigen did not affect the magnitude of the response seen with sera raised against native or urea-treated Fim2. In addition, the avidity of the anti-Fim2 IgG population for its antigen is assumed to be the same as that for anti-Fim3 population and Fim3. This was shown to be the case, as the slopes of the Fim2 and Fim3 dose response curves are similar (Fig. 3). The higher result assigned to 89/530 for Fim2 compared to Fim3 is thus a true reflection of how much antibody is present for each serotype.
This ELISA was used to quantify Fim2 plus Fim3, Fim2, and Fim3 IgG concentrations in small panels of sera from 15-month-old children, 4- to 6-year-old children, and 11- to 18-year-olds immunized with 5-component acellular pertussis vaccine. All individuals showed rises in IgG concentration to both Fim2 and Fim3, confirming that both components of the copurified Fim2-Fim3 mixture in the vaccine are immunogenic. Higher geometric mean responses to Fim2 than Fim3 were seen in the younger two age groups, but this difference was reduced in the 11- to 18-year-old subjects. Responses to both proteins were greater in the older vaccinees, indicating a strong boost response to these antigens. The significance of the lower IgG concentrations seen in response to Fim3 following vaccination of young children is not known. Two studies performed in Sweden suggest that 5-component acellular pertussis vaccine may be less effective against Fim3 strains. Hallander et al. (8) report a higher percentage of Fim3 breakthrough strains among children who received 5-component acellular vaccine than in an unvaccinated control group. This was also seen in the 1992 Swedish study, with a higher percentage of Fim3 breakthrough strains seen in the 5-component acellular and whole-cell vaccine groups, compared to an equal ratio of serotypes between two-component acellular vaccine and control groups (26).
We have also used the Fim2 and Fim3 ELISA to determine IgG concentrations in individuals with evidence of recent pertussis by Ptx IgG ELISA. It has been observed previously that individuals with anti-Ptx IgG above the diagnostic threshold were more likely to have high levels of anti-FHA and anti-Fim2-Fim3 IgG (1), but responses to individual Fim2 and Fim3 have not previously been analyzed. Because United Kingdom B. pertussis isolates have been almost exclusively Fim3 serotype during the time period examined, it would be expected that individuals would show increased Fim3 IgG concentrations following disease, and this was demonstrated in panels of sera from 2009, 2010, and 2011. However, the number of individuals with moderate Fim2 IgG concentrations in 2009 and 2010 was surprising given the predominant serotypes of isolates obtained in those years (Table 2). This could mean either that Fim2 strains were circulating and not represented in the small number of isolates obtained or that B. pertussis may express both Fim types in vivo. This was suggested by Heikkinen et al. (11), who used a monoclonal antibody blocking ELISA, due to the unavailability of separate Fim2 and Fim3, to determine serotype-specific antibody responses following infection. They found that patients infected by Fim3 strains had antibodies which blocked the binding of monoclonal antibodies to Fim3 but not to Fim2. However, one-third of the Fim2 strain-infected patients developed antibodies capable of blocking binding of both Fim2 and Fim3 monoclonal antibodies, indicating a difference in fimbrial expression in vivo and in vitro. The expression of fimbriae is known to be dependent on a run of C residues in the promoter regions of fim2 and fim3 which renders the genes capable of phase variation through spontaneous variation in the poly(C) tract length (2, 28). As strains appear to possess both fimbrial genes, it is possible that variable expression could occur during disease. However, despite this genetic flexibility, the predominant serotypes are stable over a number of years in most countries, with periodic switching (13, 14, 20), and we present preliminary evidence from 2011 that this may be occurring in the United Kingdom. An additional possibility for the high Fim2 ELISA IgG concentrations seen in some individuals may be anti-LPS antibodies reacting with the higher level of LPS in the Fim2 ELISA coating antigen, and this will be the subject of further study.
The strong anti-Fim2 and -Fim3 IgG responses seen following disease may be, in part, influenced by previous vaccination, as individuals have been shown to produce strong responses to fimbriae if they had received prior fimbria-containing pertussis vaccine (9). In addition, blunted responses to antigens not contained in pertussis vaccines previously received have been observed (4). United Kingdom individuals in this study are likely to have received whole-cell pertussis vaccination which primed for response to both Fim2 and Fim3.
Thus, in this study, we have purified separate Fim2 and Fim3 and characterized the immune response to these antigens following vaccination, showing that both proteins are immunogenic. In addition, the anti-Fim IgG response following recent disease was greater for Fim3, consistent with the predominant serotype of circulating B. pertussis isolates. However, a surprising number of individuals have moderate responses to Fim2, raising a number of questions about the serotype of circulating strains or in vivo expression of fimbriae. With the resurgence of pertussis seen in a number of countries with high levels of vaccine coverage, improved vaccines are required that may reduce transmission of the pathogen, and improved responses to both Fim2 and Fim3 may be important to achieve this aim.
ACKNOWLEDGMENTS
This work was supported by an unrestricted grant from Sanofi Pasteur.
Assistance with statistical analysis was provided by Nick Andrews, Health Protection Agency Statistics Unit, Colindale, London, United Kingdom.
Footnotes
Published ahead of print 5 September 2012
REFERENCES
- 1. Baughman AL, et al. 2004. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin. Diagn. Lab. Immunol. 11:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Q, Decker KB, Boucher PE, Hinton D, Stibitz S. 2010. Novel architectural features of Bordetella pertussis fimbrial subunit promoters and their activation by the global virulence regulator BvgA. Mol. Microbiol. 77:1326–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherry JD, Gornbein J, Heininger U, Stehr K. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901–1906 [DOI] [PubMed] [Google Scholar]
- 4. Cherry JD, et al. 2010. Antibody response patterns to Bordetella pertussis antigens in vaccinated (primed) and unvaccinated (unprimed) young children with pertussis. Clin. Vaccine Immunol. 17:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geuijen CA, et al. 1998. Identification and characterization of heparin binding regions of the Fim2 subunit of Bordetella pertussis. Infect. Immun. 66:2256–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geuijen CA, Willems RJ, Mooi FR. 1996. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect. Immun. 64:2657–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorringe AR, Ashworth LAE, Irons LI, Robinson A. 1985. Effect of monoclonal antibodies on the adherence of Bordetella pertussis to Vero cells. FEMS Microbiol. Lett. 26:5–9 [Google Scholar]
- 8. Hallander HO, Advani A, Donnelly D, Gustafsson L, Carlsson RM. 2005. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J. Clin. Microbiol. 43:2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hallander HO, et al. 2009. Should fimbriae be included in pertussis vaccines? Studies on ELISA IgG anti-Fim2/3 antibodies after vaccination and infection. APMIS 117:660–671 [DOI] [PubMed] [Google Scholar]
- 10. He Q, Mertsola J. 2008. Factors contributing to pertussis resurgence. Future Microbiol. 3:329–339 [DOI] [PubMed] [Google Scholar]
- 11. Heikkinen E, et al. 2008. Bordetella pertussis isolates in Finland: serotype and fimbrial expression. BMC Microbiol. 8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. 1983. Effect of heptakis (2,6-O-dimethyl) beta-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect. Immun. 41:1138–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kallonen T, He Q. 2009. Bordetella pertussis strain variation and evolution postvaccination. Expert Rev. Vaccines 8:863–875 [DOI] [PubMed] [Google Scholar]
- 14. Litt DJ, Neal SE, Fry NK. 2009. Changes in genetic diversity of the Bordetella pertussis population in the United Kingdom between 1920 and 2006 reflect vaccination coverage and emergence of a single dominant clonal type. J. Clin. Microbiol. 47:680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynn F, Reed GF, Meade BD. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meade BD, et al. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570–575 [PubMed] [Google Scholar]
- 17. Medical Research Council 1956. Vaccination against whooping cough. Relation between protection in children and results of laboratory tests. Br. Med. J. 2:454–462 [PMC free article] [PubMed] [Google Scholar]
- 18. Mooi FR, Avest A, van der Heide HG. 1990. Structure of the Bordetella pertussis gene coding for the serotype 3 fimbrial subunit. FEMS Microbiol. Lett. 54:327–331 [DOI] [PubMed] [Google Scholar]
- 19. Packard ER, Parton R, Coote JG, Fry NK. 2004. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J. Med. Microbiol. 53:355–365 [DOI] [PubMed] [Google Scholar]
- 20. Poolman JT, Hallander HO. 2007. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev. Vaccines 6:47–56 [DOI] [PubMed] [Google Scholar]
- 21. Preston NW, Carter EJ. 1992. Serotype specificity of vaccine-induced immunity to pertussis. Commun. Dis. Rep. CDR Rev. 2:R155–R156 [PubMed] [Google Scholar]
- 22. Robinson A, Ashworth LA, Baskerville A, Irons LI. 1985. Protection against intranasal infection of mice with Bordetella pertussis. Dev. Biol. Stand. 61:165–172 [PubMed] [Google Scholar]
- 23. Robinson A, Gorringe AR, Funnell SG, Fernandez MM. 1989. Serospecific protection of mice against intranasal infection with Bordetella pertussis. Vaccine 7:321–324 [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez ME, Hellwig SM, Pérez Vidakovics ML, Berbers GA, van de Winkel GJ. 2006. Bordetella pertussis attachment to respiratory epithelial cells can be impaired by fimbriae-specific antibodies. FEMS Immunol. Med. Microbiol. 46:39–47 [DOI] [PubMed] [Google Scholar]
- 25. Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907–1916 [DOI] [PubMed] [Google Scholar]
- 26. Tiru M, Askelöf P, Granström M, Hallander HO. 1997. Bordetella pertussis serotype of clinical isolates in Sweden during 1970–1995 and influence of vaccine efficacy studies. Dev. Biol. Stand. 89:239–245 [PubMed] [Google Scholar]
- 27. Tsang RS, et al. 2004. Polymorphisms of the fimbria fim3 gene of Bordetella pertussis strains isolated in Canada. J. Clin. Microbiol. 42:5364–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willems R, Paul A, van der Heide HG, Avest AR, Mooi FR. 1990. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 9:2803–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willems R, et al. 1993. Isolation of a putative fimbrial adhesin from Bordetella pertussis and the identification of its gene. Mol. Microbiol. 9:623–634 [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization 2010. Pertussis vaccines: WHO position paper. Wky. Epidemiol. Rec. 85:385–400 [PubMed] [Google Scholar]
- 31. Xing D, et al. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin. Vaccine Immunol. 16:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y, et al. 2009. Production and characterization of recombinant pertactin, fimbriae 2 and fimbriae 3 from Bordetella pertussis. BMC Microbiol. 9:274. [DOI] [PMC free article] [PubMed] [Google Scholar]