Abstract
West Nile virus (WNV) is endemic throughout Africa, Eurasia, America, and Australia and has important implications for avian, horse, and human health. In these regions, dead birds are monitored for the presence of WNV through immunohistochemistry (IHC) and PCR. However, a number of the tools for IHC are inadequate owing to their cross-reactivity to other Japanese encephalitis serogroup viruses. Here we have established eight monoclonal antibodies (MAbs) to WNV. Four of them bound to the envelope protein, three of them bound to nonstructural protein 1 (NS1), and one bound to precursor membrane protein (prM), as shown by Western blot analysis. The anti-NS1 MAbs and the anti-prM MAb did not cross-react with Japanese encephalitis virus (JEV), Murray valley encephalitis virus, or St. Louis encephalitis virus in an indirect enzyme-linked immunosorbent assay. One NS1-specific MAb, SHW-32B1, and the previously reported NS1-specific MAb, SHW-7A11, were shown by IHC to specifically detect the cytoplasm of degenerated cells in the heart and brain of a WNV-infected goose. Neither of these MAbs were shown by IHC to cross-react with degenerated cells in the brain of a JEV-infected pig. These MAbs are the first reported anti-NS1 MAbs that can be used for WNV-specific IHC using formalin-fixed, paraffin-embedded sections. They may be useful for WNV research and surveillance.
INTRODUCTION
West Nile virus (WNV) is an RNA-enveloped virus of the genus Flavivirus, family Flaviviridae (14), and belongs to the Japanese encephalitis virus (JEV) serocomplex group. The JEV serocomplex group consists of JEV, WNV, St. Louis encephalitis virus (SLEV), Murray Valley encephalitis virus (MVEV), Alfuy virus, Koutango virus, Kokobera virus, Stratford virus, and Usutu virus (14). WNV is endemic throughout Africa, Eurasia, America, and Australia (12) and is spread via mosquitoes that bite and infect birds, which act as amplifying hosts for the virus (14). Birds, particularly the Corvidae species, are known to be susceptible to WNV infection (4). Thirty-eight days after the death of a wild bird infected with WNV was reported, the presence of WNV infection in humans was reported (16). Therefore, dead bird surveillance and sentinel bird surveillance are performed in areas of WNV endemicity (2). In dead bird surveillance, immunohistochemistry (IHC) and RNA detection using reverse transcription-PCR (RT-PCR) are utilized for WNV detection (21, 8).
Generally, flaviviruses have common antigenicity owing to the high similarity in the amino acid sequences between related proteins. For example, the nonstructural protein 1 (NS1), precursor membrane (prM) or membrane (M) protein, and envelope protein (E) of WNV and JEV share between 60 and 80% of their amino acid sequences. Therefore, there is a large problem with cross-reaction in many of the tests used for serodiagnosis, such as the neutralization test, IgG indirect enzyme-linked immunosorbent assay (ELISA), and hemagglutination inhibition test (1). In IHC, there are also problems with cross-reactivity due to the use of polyclonal antibodies (21). Therefore, the results generated through bird surveillance have to be considered in light of the potential cross-reaction problems in the methods used.
IHC is the most common assay used to evaluate the cause of death in birds, and consequently, it is performed on many birds suspected of dying from WNV around the world. However, as mentioned above, the JEV serogroup viruses have common antigenicity, which therefore requires the use of virus species-specific antibodies for IHC in areas where multiple flaviviruses are endemic. The scope of the problem is evident when you consider that JEV and WNV are circulating in parts of India (5, 9), SLEV and WNV are circulating in North, Central, and South America (12), and MVEV and WNV are circulating in northern parts of Australia (13). Furthermore, wild ducks have been shown to have WNV antibodies in Japan and South Korea, where JEV is endemic (18, 22). Recent research has also shown that WNV is endemic in Far East Siberia (15), demonstrating that the number of areas with multiple flaviviruses is expanding (12). This indicates the importance of developing specific assays in order to differentiate WNV from other JEV serogroup viruses.
Monoclonal antibodies (MAbs) are utilized in a variety of fields owing to their high specificity. A number of MAbs have been developed and used for research purposes and the diagnosis of flavivirus infections. However, their use is limited, as there are few MAbs that can distinguish WNV from other JEV serogroup viruses in immunoassays, especially in IHC of formalin-fixed tissues. In this study, anti-WNV MAbs were developed for application in WNV-specific IHC.
MATERIALS AND METHODS
Viruses.
WNV (NY99-A301 strain, g2266 strain, eg101 strain, Kunjin MRM61C strain), JEV (Nakayama NIH strain, JaNAr0102 strain), MVEV (MVE-1-51 strain), and SLEV (Parton strain) were used. Culture supernatants of these viruses were prepared using Vero cells (7). Virus antigens were prepared by sucrose gradient purification of β-propiolactone (Nacalai Tesque, Kyoto, Japan)-inactivated virus culture supernatants as previously described (7) and used as antigens for immunization, indirect ELISA, and Western blotting. All experiments using infectious viruses were approved by the Biosafety Committee of the National Institute of Animal Health in Japan and were performed in a biosafety level 3 laboratory.
Monoclonal antibody production.
The methods for MAb production and antibody purification have been previously described (7).
Western blot analysis.
Western blot analysis was performed as described previously (7). The anti-WNV NS1 antibody SHW-7A11 (7), the anti-WNV E antibody HB112 (ATCC, Manassas, VA), the anti-WNV prM antibody ab25888 (Abcam, Cambridge, MA), and WNV-infected chicken serum (plaque reduction neutralization test [PRNT] titer of 90% reduction; 1:800; kept in our laboratory) were used as reference antibodies.
Indirect ELISA.
The indirect ELISA was performed as described previously (7). All virus antigens were used at protein concentrations of 100 ng/well. The optical density (OD) values were corrected to facilitate comparison using the following formula: (OD value for each viral antigen)/(OD value for the WNV NY99 strain antigen) × 100.
Immunohistochemistry.
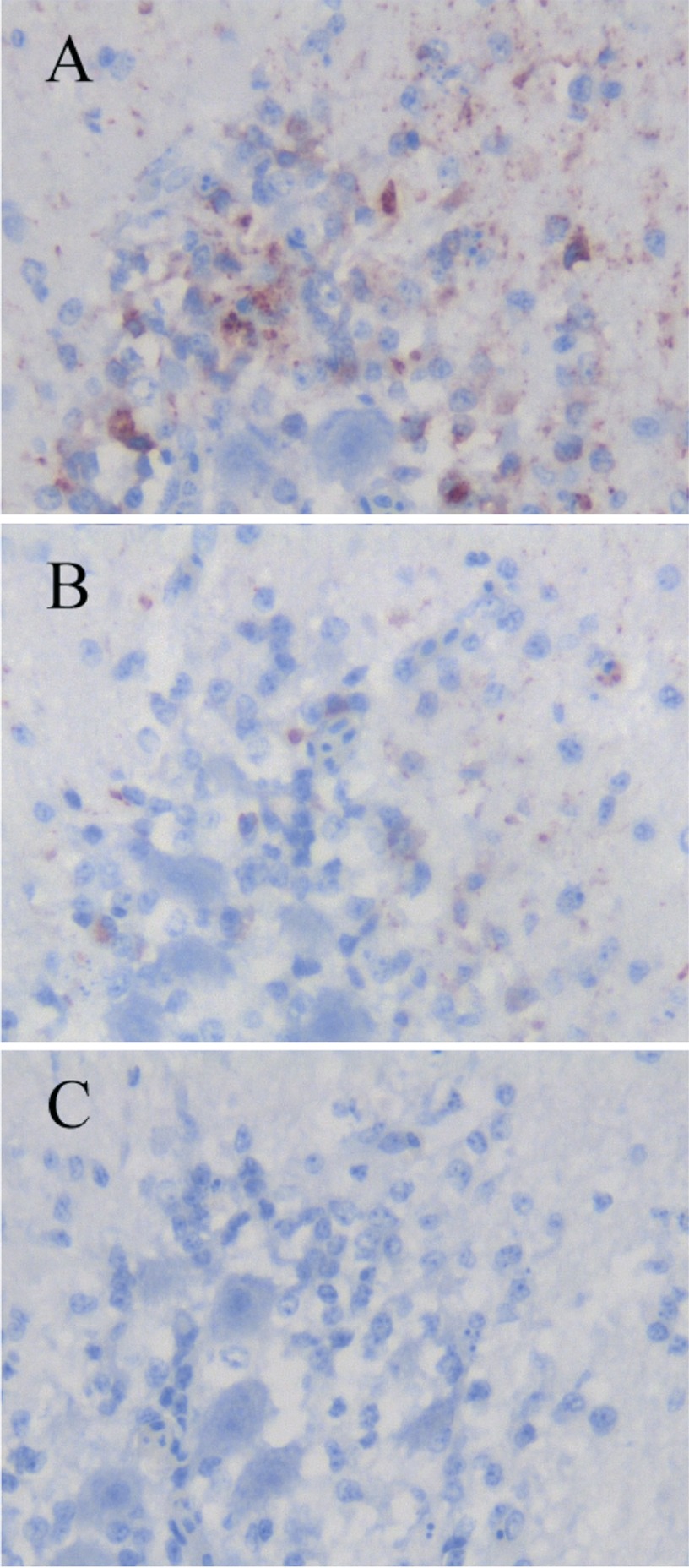
IHC examination was performed using the heart and brain of a WNV-infected white Embden goose and the brain of a JEV-infected pig. Tissues were fixed in 10% neutral buffered formalin for several days. Formalin-fixed tissues were trimmed, embedded in paraffin, and sectioned at 3 μm. Immunolabeling and visualization were performed as described previously with the universal immunoenzyme polymer method using a Histofine Simple Stain MAX-PO kit (Nichirei Co., Tokyo, Japan) (19). In brief, the serial sections were pretreated with 0.1% actinase for 20 min at 37°C, and endogenous peroxidase activity was blocked using 3% H2O2 in methanol for 30 min at room temperature. Each of the established MAbs was applied as the primary antibody for 60 min at room temperature. The sections were lightly counterstained with hematoxylin and assessed using light microscopy. An anti-WNV E protein MAb (7H2; BioReliance, Rockville, MD), an anti-WNV rabbit polyclonal antibody (BioReliance), and an anti-JEV rabbit polyclonal antibody (JEV AS6 strain immunized sera) were used in IHC as reference antibodies. The MAbs and antiserum were checked for their nonspecific binding on uninfected tissues of goose and pig, and none of them showed any binding on these tissues. The MAbs and polyclonal antibodies were used at the dilutions indicated in Table 1. The selection criteria of antibody reactivity in Table 1 are indicated in Fig. 1.
Table 1.
Immunohistochemistry of WNV-infected goose section and JEV-infected pig section
| IDa | Antibody concn (μg/ml) | Antibody dilution (1/×) | IHC result forb: |
|||||
|---|---|---|---|---|---|---|---|---|
| WNV-infected white Embden goose |
WNV-uninfected goose |
JEV-infected pig brain | JEV-uninfected pig brain | |||||
| Heart | Brain | Heart | Brain | |||||
| SHW-32B1 | 16 | 256 | ++ | ++ | − | − | − | − |
| 8 | 1,024 | ++ | ++ | − | − | − | − | |
| 4 | 4,096 | ++ | + | − | − | − | − | |
| SHW-7A11 | 16 | 256 | ++ | ++ | − | − | − | − |
| 8 | 1,024 | ++ | ++ | − | − | − | − | |
| 4 | 4,096 | ++ | + | − | − | − | − | |
| Anti-WNV monoclonal antibody 7H2 | 125 | 8 | − | − | ND | ND | − | ND |
| 31 | 32 | − | − | ND | ND | − | ND | |
| 8 | 128 | − | − | ND | ND | − | ND | |
| Anti-WNV polyclonal antibody | 512 | ++ | ++ | − | − | ++ | − | |
| 2,048 | + | + | − | − | − | − | ||
| 8,192 | + | + | − | − | − | − | ||
| Anti-JEV polyclonal antibody | 256 | − | − | − | − | ++ | − | |
| 1,024 | − | − | − | − | + | − | ||
ID, antibody identification.
Subjective grading scheme of antibody reactivity: −, no reactivity; +, weak reactivity; ++, strong reactivity. The selection criteria of antibody reactivity are indicated in Fig. 1. ND, not done.
Fig 1.
Selection criteria of antibody reactivity in immunohistochemical staining in Table 1. Formalin-fixed, paraffin-embedded brain sections of a WNV-infected goose were immunohistochemically stained using an anti-WNV rabbit polyclonal antibody. (A) Example of “++.” Strong staining is seen in positively stained cells. (B) Example of “+.” Weak staining is seen in positively stained cells. (C) Example of “−.”
Animal welfare.
All experiments using living animals were approved by the Ethics Committee of the National Institute of Animal Health in Japan.
RESULTS
Characterization of monoclonal antibodies.
The anti-WNV MAb-secreting hybridomas SHW-2D8, SHW-6F10, SHW-7D12, SHW7G8, SHW-11A9, SHW-18C10, SHW-25C1, and SHW-32B1 were obtained. The immunoglobulin classes and specificity are summarized in Table 2.
Table 2.
Immunoglobulin class and specificity of monoclonal antibodies
| Clone name | Immunoglobulin class | Virus specificity in an indirect ELISA | Virus component specificity in a Western blota |
|---|---|---|---|
| SHW-2D8 | IgM | WNV | NS1 |
| SHW-25C1 | IgM | WNV | NS1 |
| SHW-32B1 | IgG1 | WNV | NS1 |
| SHW-6F10 | IgG1 | JEV serogroup flavivirus | E |
| SHW-7D12 | IgG2a | JEV serogroup flavivirus | E |
| SHW-7G8 | IgG1 | JEV serogroup flavivirus | E |
| SHW-18C10 | IgA | JEV serogroup flavivirus | E |
| SHW-11A9 | IgG1 | WNV | prM |
| SHW-7A11b | IgG1 | WNV | NS1 |
NS1, nonstructural protein 1; E, envelope protein; prM, precursor membrane protein.
SHW-7A11 was previously developed and evaluated elsewhere (7).
Western blot analysis was performed to determine the reactivity of the MAbs. SHW-2D8, SHW-25C1, and SHW-32B1 reacted with NS1, SHW-7D12, SHW-6F10, SHW-7G8, and SHW-18C10 reacted with the E protein, and SHW-11A9 reacted with prM (Fig. 2).
Fig 2.
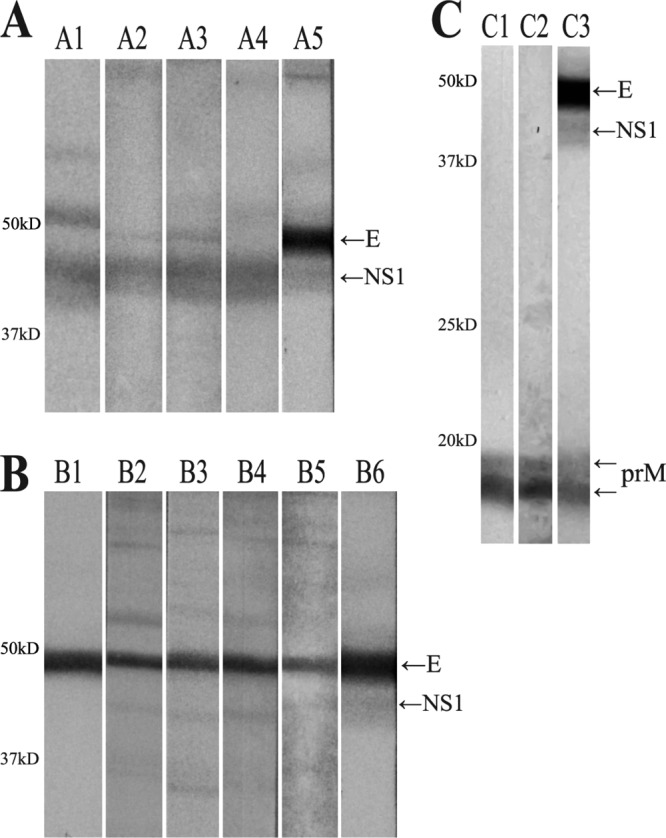
Western blots using the established monoclonal antibodies and reference antibodies. Sucrose gradient-purified WNV particles were used as antigens in Western blots. (A) A1, anti-NS1 monoclonal antibody (SHW-7A11); A2, SHW-2D8; A3, SHW-25C1; A4, SHW-32B1; A5, WNV-infected chicken serum. (B) B1, anti-E protein monoclonal antibody (HB112); B2, SHW-6F10; B3, SHW-7D12; B4, SHW-7G8; B5, SHW-18C10; B6, WNV-infected chicken serum. (C) C1, anti-prM polyclonal antibody (ab25888); C2, SHW-11A9; C3, WNV-infected chicken serum. The following proteins are indicated: E, envelope protein; NS1, nonstructural protein 1; prM, precursor membrane protein.
The cross-reactivity of the established MAbs was examined against that of other JEV serocomplex flaviviruses using an indirect ELISA. SHW-2D8, SHW25C1, SHW-32B1, and SHW-11A9 were highly reactive for the WNV NY99 antigen and also reacted with the WNV group (Kunjin, g2266, and Eg-101 [data not shown]). Importantly, they showed no cross-reactivity with JEV, SLEV, and MVEV. The four other MAbs showed cross-reactivity with JEV, SLEV, and MVEV (Fig. 3).
Fig 3.
Monoclonal antibody cross-reactivity in an indirect ELISA. Each bar indicates the percentage of the optical density (OD) value obtained for the indicated monoclonal antibody for each JEV serocomplex virus antigen. OD values were calculated using the following formula: (OD value for each viral antigen)/(OD value for the WNV NY99 strain antigen) × 100. All virus antigens were used at protein concentrations of 100 ng/well.
The developed MAbs did not neutralize WNV in PRNT (data not shown).
Immunohistochemistry.
SHW-32B1, SHW-7A11, and the anti-WNV rabbit polyclonal antibody strongly reacted with the cytoplasm of degenerated cells in the heart (Fig. 4A, D, and G) and brain (Fig. 4B, E, and H) of a WNV-infected goose. The staining patterns of each antibody were very similar. Neither SHW-32B1 nor SHW-7A11 showed any positivity when used to stain the degenerated cells in the brain of a JEV-infected pig (Fig. 4C and F). In contrast, anti-WNV rabbit polyclonal antibody showed positive staining of the degenerated cells in the brain of a JEV-infected pig (Fig. 4I), indicating cross-reactivity. The staining pattern was similar to that seen with the anti-JEV rabbit polyclonal antibody (data not shown). These results indicate that we have successfully established MAbs that specifically recognize WNV antigens in formalin-fixed tissue sections.
Fig 4.
Immunohistochemical staining for WNV and JEV using SHW-32B1, SHW-7A11, and an anti-WNV rabbit polyclonal antibody. Formalin-fixed, paraffin-embedded heart sections (A, D, G, and J) and brain sections (B, E, H, and K) of a WNV-infected goose and the brain from a JEV-infected pig (C, F, I, and L) were immunohistochemically stained using SHW-32B1 (A, B, and C), SHW-7A11 (D, E, and F), and an anti-WNV rabbit polyclonal antibody (G, H, and I). The sections without primary antibodies were also stained (J, K, and L). The MAbs and polyclonal antibody were applied at 1,024× and 512× dilutions, respectively. Bar, 50 μm.
DISCUSSION
Here we have described the establishment of eight MAbs against WNV, four of which were specific to WNV and four which also reacted with other JEV serocomplex flaviviruses. Flaviviruses are constructed of three structural proteins (the E, prM/M, and capsid proteins) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (3, 14). The established MAbs reacted with the NS1, E, and prM proteins of WNV. Among the four WNV-specific MAbs, three reacted with NS1 and 1 reacted with prM. All four of the JEV serocomplex flavivirus-reactive MAbs reacted with the E protein. And the developed MAbs did not react with antigen in Western blot analysis under reduced conditions (data not shown); consequently, their epitopes are thought to be conformational.
NS1 is expressed on the cell surface (23) or secreted into virus-infected animal blood at high concentrations (11), and it is reported that flavivirus NS1 was found in membrane, organelle, nucleus, and cytoskeletal cell fractions (17). These observations suggest that a lot of NS1 will be present in the cells of birds that have died owing to WNV infection. Thus, it is expected that detection of NS1 would be very useful when surveying birds that have died owing to WNV infection. However, currently there is no available anti-NS1 MAb that can be used for IHC. WNV surveillance in dead birds is extremely important for public health, and IHC is one of the main diagnostic methods. IHC of WNV has been performed utilizing commercially available anti-WNV rabbit polyclonal antibody or E-specific MAbs (20). The anti-WNV rabbit polyclonal antibody strongly cross-reacts with degenerated cells in the brain of a JEV-infected pig (Fig. 4). This cross-reactivity would be a problem for WNV surveillance in areas in which JEV is endemic. On the other hand, the anti-E MAb did not react with heart sections from a WNV-infected white Embden goose. It is reported that the anti-E MAb-based IHC shows low sensitivity compared with that of IHC that utilizes polyclonal antibodies (20). For example, approximately 50% of heart sections from WNV-infected American crows were judged as negative using the anti-E MAb-based IHC (20). SHW-32B1 and SHW-7A11 showed strong staining of tissue sections from a WNV-infected bird, with the strength of staining similar to that for the polyclonal antibody, although the anti-E MAb did not show any staining. Strong staining of the cytoplasm of degenerated cells in the heart of a WNV-infected goose was observed in IHC using the anti-NS1 MAbs, SHW-32B1 and the previously reported SHW-7A11 (7), and an anti-WNV polyclonal antibody. Furthermore, the patterns of staining were very similar. Neither of the established MAbs reacted with JEV, unlike the WNV polyclonal antibody. These results strongly indicate that SHW-32B1 and SHW-7A11 are good candidates for WNV-specific IHC.
SHW-11A9 reacted with prM of WNV in Western blot analysis. The high specificity of the anti-prM SHW-11A9 MAb against WNV indicates the existence of a WNV-specific epitope on prM. This finding suggests that this MAb would be useful for WNV research.
The E protein is the major surface protein of flaviviruses, and it plays a critical role in cell attachment and membrane fusion (14). Therefore, the E protein is a principal target of neutralizing antibodies. There are a number of WNV-specific anti-E protein MAbs that have been previously developed (6, 10), but all of our developed anti-E protein MAbs showed cross-reactivity with the E protein from SLEV, MVEV, and JEV in an indirect ELISA.
In summary, 3 WNV-specific anti-NS1 MAbs were developed, with one of the MAbs, SHW-32B1, and the previously developed MAb, SHW-7A11, being suitable for use in IHC of formalin-fixed, paraffin-embedded sections for the specific detection of WNV. Further investigation is required to determine the applicability of SHW-32B1 and SHW-7A11 in actual WNV surveillance by IHC, but they are, however, good candidates for this application. This study is the first report showing that antibodies to NS1 can be used for WNV IHC of formalin-fixed, paraffin-embedded sections. We further showed that the SHW-11A9 MAb to prM was highly specific for WNV. These MAbs would be useful for WNV surveillance and research.
ACKNOWLEDGMENTS
The WNV-infected white Embden goose formalin-fixed, paraffin-embedded tissue blocks were generously provided by David E. Swayne (U.S. Department of Agriculture). We thank M. Kobayashi and M. Shimada for histopathological assistance.
This study was supported by a grant-in-aid from the Zoonoses Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan.
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Beaty BJ, Calisher CH, Shope RE. 1995. Arboviruses, p 189–212 In Lennette EH, Lennette DA, Lennette ET. (ed), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th ed American Public Health Association, Washington, DC [Google Scholar]
- 2. Carney RM, et al. 2011. Early warning system for West Nile virus risk areas, California, USA. Emerg. Infect. Dis. 17:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambers TJ, McCourt DW, Rice CM. 1990. Production of yellow fever virus proteins in infected cells: identification of discrete polyprotein species and analysis of cleavage kinetics using region-specific polyclonal antisera. Virology 177:159–174 [DOI] [PubMed] [Google Scholar]
- 4. Eidson M, Kramer L, Stone W, Hagiwara Y, Schmit K. 2001. Dead bird surveillance as an early warning system for West Nile virus. Emerg. Infect. Dis. 7:631–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulati BR, et al. 2011. Serosurveillance for Japanese encephalitis virus infection among equines in India. J. Vet. Sci. 12:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall RA, Burgess GW, Kay BH, Clancy P. 1991. Monoclonal antibodies to Kunjin and Kokobera viruses. Immunol. Cell Biol. 69:47–49 [DOI] [PubMed] [Google Scholar]
- 7. Hirota J, Shimoji Y, Shimizu S. 2012. New sensitive competitive enzyme-linked immunosorbent assay using a monoclonal antibody against nonstructural protein 1 of West Nile virus NY99. Clin. Vaccine Immunol. 19:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jozan M, et al. 2003. Detection of West Nile virus infection in birds in the United States by blocking ELISA and immunohistochemistry. Vector Borne Zoonotic Dis. 3:99–110 [DOI] [PubMed] [Google Scholar]
- 9. Khan SA, et al. 2011. West Nile virus infection, Assam, India. Emerg. Infect. Dis. 17:947–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, et al. 2008. Characterization and application of monoclonal antibodies specific to West Nile virus envelope protein. J. Virol. Methods 154:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macdonald J, et al. 2005. NS1 protein secretion during the acute phase of West Nile virus infection. J. Virol. 79:13924–13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 10:S98–S109 [DOI] [PubMed] [Google Scholar]
- 13. Mackenzie JS, Williams DT. 2009. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health 56:338–356 [DOI] [PubMed] [Google Scholar]
- 14. Monath TP, Heinz FX. 1996. Flaviviruses, p 961–1034 In Fields BN, Howley PM. (ed), Fields virology, vol 1, 3rd ed Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 15. Murata R, et al. 2011. Seroprevalence of West Nile virus in wild birds in far eastern Russia using a focus reduction neutralization test. Am. J. Trop. Med. Hyg. 84:461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Leary DR, et al. 2004. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 4:61–70 [DOI] [PubMed] [Google Scholar]
- 17. Poungsawai J, Kanlaya R, Pattanakitsakul SN, Thongboonkerd V. 2011. Subcellular localizations and time-course expression of dengue envelope and non-structural 1 proteins in human endothelial cells. Microb. Pathog. 51:225–229 [DOI] [PubMed] [Google Scholar]
- 18. Saito M, Osa Y, Asakawa M. 2009. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: risk assessment of invasive flaviviruses. Vector Borne Zoonotic Dis. 9:253–258 [DOI] [PubMed] [Google Scholar]
- 19. Shimizu C, et al. 2010. Lawsonia intracellularis and virulent Rhodococcus equi infection in a thoroughbred colt. J. Comp. Pathol. 143:303–308 [DOI] [PubMed] [Google Scholar]
- 20. Smedley RC, et al. 2007. Sensitivity and specificity of monoclonal and polyclonal immunohistochemical staining for West Nile virus in various organs from American crows (Corvus brachyrhynchos). BMC Infect. Dis. 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steele KE, et al. 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 37:208–224 [DOI] [PubMed] [Google Scholar]
- 22. Yeh JY, Park JY, Ostlund EN. 2011. Serologic evidence of West Nile virus in wild ducks captured in major inland resting sites for migratory waterfowl in South Korea. Vet. Microbiol. 154:96–103 [DOI] [PubMed] [Google Scholar]
- 23. Youn S, Cho H, Fremont DH, Diamond MS. 2010. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J. Virol. 84:9516–9532 [DOI] [PMC free article] [PubMed] [Google Scholar]