Abstract
Artificially passive immunization has been demonstrated to be effective against Yersinia pestis infection in animals. However, maternal antibodies' protective efficacy against plague has not yet been demonstrated. Here, we evaluated the kinetics, protective efficacy, and transmission modes of maternal antibodies, using mice immunized with plague subunit vaccine SV1 (20 μg of F1 and 10 μg of rV270). The results showed that the rV270- and F1-specific antibodies could be detected in the sera of newborn mice (NM) until 10 and 14 weeks of age, respectively. There was no antibody titer difference between the parturient mice immunized with SV1 (PM-S) and the caesarean-section newborns (CSN) from the PM-S or between the lactating mice immunized by SV1 (LM-S) and the cross-fostered mice (CFM) during 3 weeks of lactation. The NM had a 72% protection against 4,800 CFU Y. pestis strain 141 challenge at 6 weeks of age, whereas at 14 weeks of age, NM all succumbed to 5,700 CFU of Y. pestis challenge. After 7 weeks of age, CFM had an 84% protection against 5,000 CFU of Y. pestis challenge. These results indicated that maternal antibodies induced by the plague subunit vaccine in mother mice can be transferred to NM by both placenta and lactation. Passive antibodies from the immunized mothers could persist for 3 months and provide early protection for NM. The degree of early protection is dependent on levels of the passively acquired antibody. The results indicate that passive immunization should be an effective countermeasure against plague during its epidemics.
INTRODUCTION
Plague is a zoonotic disease caused by the Gram-negative bacterium Yersinia pestis, which is usually transmitted to humans from infected rodents via the bite of an infected flea (14). Historically, plague was an awful infectious disease that afflicted human populations. Plague has been recently classified as a reemerging disease by the World Health Organization (31) and has attracted considerable attention because of its potential misuse as an agent of biological warfare or bioterrorism (19).
To prevent this disease, both live attenuated vaccines (LAV) and killed whole-cell vaccines (KCV) against plague have been used in humans since the early part of the 20th century. However, KCV provides only short-term protection against bubonic plague (21). The LAV EV76 is effective against bubonic and pneumonic plagues, but it has side effects of varying severity and is not being used in the Western world at present (6, 13, 15). Recent studies have focused on the development of acellular vaccines containing F1 and LcrV antigens (21). The F1 antigen is encoded by the caf1 operon, which is a capsule-like protein around the bacterium and has antiphagocytic properties (1, 9). The LcrV antigen is a multifunctional virulence protein of the type III secretion system encoded by pCD1 plasmid, which affords both plague protection and immunosuppressive properties (12). The DNA vaccine based on Y. pestis F1 and LcrV antigens alone or in combination was efficacious against both bubonic plague and pneumonic plague (9, 11). However, DNA vaccines usually elicit lower and slower immune responses than conventional vaccines, and gene gun immunization that delivers DNA-coated particles into the dermis of the skin needs to be used for improving immune responses (4, 9). In contrast, subunit vaccines have obvious advantages over the traditional vaccines (killed whole-cell vaccine and live attenuated vaccine) in terms of safety or efficacy and are being developed currently (17, 21, 23, 24). It has been demonstrated that F1 and LcrV antigens alone or in combination can protect mice against bubonic and pneumonic plague, but the mice vaccinated with F1 antigen alone fail to provide protection against F1-negative Y. pestis strains (3) and the vaccine based solely on LcrV cannot protect against some Y. pestis strains producing variants of LcrV (20). Thus, to provide effective protection against plague, it is desirable that at least F1 and LcrV antigens should be administered together (10).
A variety of vaccines based on the F1 or V antigen have been reported to provide a high degree of protection against plague (2, 12, 19). In our previous work, to develop a safe and effective plague subunit vaccine, highly purified natural F1 antigen from Y. pestis EV76 was extracted by a new purification strategy (28), and a nontagged rV270 protein containing amino acids 1 to 270 of LcrV was prepared using thrombin digestion from recombinant Escherichia coli BL21 cells (29). The subunit vaccine, which comprises a dose of 20 μg F1 and 10 μg rV270 that were adsorbed to 25% (vol/vol) alhydrogel in phosphate-buffered saline (PBS) buffer (SV1), provides good protective efficacy against Y. pestis challenge in mice, guinea pigs, rabbits (15), and rhesus macaques (16). In addition, artificially passive immunization using polyclonal or monoclonal antibodies specific to F1 or LcrV protein has been demonstrated to be effective against plague in animals (1, 13, 15). However, passive transfer of maternal antibodies that can confer protection to newborn mice has not yet been demonstrated. Providing passive protection to infants is important within the first months of life, because infants have immature immune systems conducive to high susceptibility to infectious diseases (3). In the present study, the levels of passively acquired antibodies from mother mice immunized with SV1, the kinetics of maternal antibodies, the mode of transmission of maternal antibodies, and the protective efficacy against plague were evaluated in newborn mice.
MATERIALS AND METHODS
Vaccine and animals.
The native F1 and rV270 antigens were adsorbed to 25% (vol/vol) aluminum hydroxide in PBS buffer to give the SV1 containing 20 μg of F1 and 10 μg of rV270 in a final volume of 100 μl. BALB/c mice were obtained from Laboratory Animal Research Center, Academy of Military Medical Science, China, and bred in our laboratory. Food and water were given ad libitum, and all the animals were kept at a constant temperature (23 ± 1°C) and humidity (40% ± 10%) under a 12-h light/dark cycle. All animal experiments were conducted strictly in compliance with the Regulations of Good Laboratory Practice for nonclinical laboratory studies of drugs issued by the National Scientific and Technologic Committee of the People's Republic of China.
Mating, cross-fostering, and caesarean section.
Six-week-old BALB/c mice were immunized intramuscularly with a single dose of SV1, and control groups of mice were immunized with either PBS or alum adjuvant in PBS. After immunization, mice were housed as pairs of one male and one female for mating. Females showing physical signs of pregnancy were then housed separately. Upon parturition, the newborn mice (NM) from the mother mice immunized with SV1 (MM-S) were allowed to suckle on their natural mothers and weaned to the postpartum diet at about 4 weeks of age. To assess the relative effect of milk and prenatal immunity, some newborn mice were delivered from the parturient mother mice immunized with SV1 (PM-S) by caesarean section (these newborn mice were designated CSN in this study) and their sera were collected from the carotid artery during deep anesthesia, and other newborn mice delivered from the control pregnant mice immunized with PBS were cross-fostered to the lactating mice immunized with SV1 (LM-S) after natural birth (the newborn mice cross-fostered to LM-S were designated CFM in this study).
Antigen-specific antibody assays.
Sera collected from all animals were assayed for the presence of F1- or rV270-specific IgG by a modified enzyme-linked immunosorbent assay (ELISA) (17). Briefly, 96-well microtiter plates were coated with either F1 or rV270 antigens diluted to 500 ng/ml in 0.06 M sodium carbonate buffer (pH 9.6) and incubated overnight at 4°C. Nonspecific binding was blocked with 0.1% casein in 0.01 M phosphate-buffered saline. Serial samples were added to plates with serial dilution in 0.01 M PBS buffer containing 0.05% casein and incubated for 30 min at 37°C. After five washes with 0.01 M PBS buffer containing 0.05% Tween 20 (pH 7.2), 100 μl of goat anti-mouse IgG labeled with horseradish peroxidase (Dakewe Biotech Co., Ltd.) was added to each well and incubated for 20 min at 37°C. The plates were washed three times in 0.01 M PBS buffer containing 0.05% Tween 20 (pH 7.2), and 100 μl of 0.01% peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was added to each well. The reaction was stopped by the addition of 50 μl of 2.5 M H2SO4 per well, and then optical density (OD) was read at 450 nm with an ELISA plate reader (Bio-Rad). The titer of specific antibody was estimated as the maximum dilution of serum giving an OD reading 0.2 units over background. Background values were obtained from serum samples collected from the animals receiving only aluminum hydroxide. The antibody endpoint titer per immunization group is presented as the geometric mean endpoint titer to F1 or rV270 antigen.
Challenge with Y. pestis.
The animals were challenged with virulent Y. pestis strain 141, which was isolated from Marmota himalayana on the Qinghai-Tibet plateau and has a median lethal dose (MLD) of 5.6 CFU for BALB/c mice, by the subcutaneous route and followed by observation for 14 days. All the surviving animals were killed humanely for a postmortem examination. Livers, spleens, and lungs of the challenged animals were removed to confirm if Y. pestis was present in these organs.
Statistical analysis.
The data among groups were compared by analysis of variance (ANOVA) with SARS 8.0 software, and probability values of <0.05 were taken as significant.
RESULTS
Kinetics of maternal antibodies.
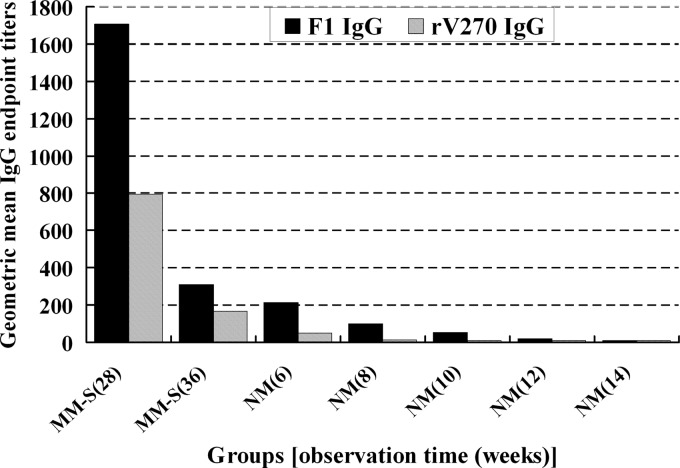
To further investigate the kinetics of maternal antibodies in NM, MM-S 18 weeks after immunization were used to produce offspring. Sera from NM at the age of 6, 8, 10, 12, or 14 weeks and from MM-S 28 weeks (at this time, the offspring are 6 weeks old) or 36 weeks (at this time offspring are 14 weeks old) after immunization were assayed for antibody levels. The rV270-specific IgG was undetectable in the sera of NM from the age of 10 weeks on, whereas F1-specific IgG was no longer detectable at the age of 14 weeks (Fig. 1).
Fig 1.
Antibody titer specific to F1 or rV270 in mother mice 28 or 36 weeks after immunization and their offspring at the age of 6, 8, 10, 12, or 14 weeks.
Mode of maternal antibody transfer.
To assess whether maternal antibodies are transferred through the placenta or colostrum, the sera of the PM-S and the CSN from the PM-S were assayed for antibody levels after 9 weeks of immunization. The results show that there is no F1- or rV270-specific IgG difference between CSN and PM-S (P > 0.05) (Fig. 2). The CFM were weaned to the postpartum diet at 4 weeks of age, and the titers of F1- or rV270-specific antibody in LM-S and CFM were determined during the 7 weeks of observation. The statistical results demonstrated that there was no significant F1- or rV270-specific IgG difference between LM-S and CFM during the first two time points (P > 0.05), whereas the titer of F1- or rV270-specific antibody in CFM was significantly lower than that in LM-S from the third time point (P < 0.05) (Fig. 3A and B).
Fig 2.
Antibody titer specific to F1 or rV270 in caesarean-section newborns (CSN) or parturient mice immunized with SV1 (PM-S) after 9 weeks of immunization.
Fig 3.
Antibody titer specific to F1 (A) and rV270 (B) in the CFM and the LM-S during the 7 weeks of observation. The newborn mice (CFM) delivered from the nonimmune mice were cross-fostered to the lactating mice (LM-S) immunized with SV1 after natural birth and weaned to the postpartum diet at 4 weeks of age.
Observation of protective efficacy.
The NM were weaned to the postpartum diet at 4 weeks of age and then separated from their mothers. NM at 6 weeks of age, MM-S 16 weeks after immunization, and normal mice (NOM) at 6 weeks of age were challenged with 4,800 CFU Y. pestis strain 141 by the subcutaneous route, followed by observation for 14 days. The NM had a 72% protection against 4,800 CFU Y. pestis strain 141 challenge, and complete protection was observed for MM-S when they were challenged with the same dose of Y. pestis, whereas NOM all succumbed to the same dose of Y. pestis (Table 1). When the NM were 14 weeks of age, the challenge test was carried out again. The results showed that the NM and NOM succumbed to 5,700 CFU of Y. pestis challenge, whereas complete protection against the same dose of Y. pestis challenge was observed in MM-S 36 weeks after immunization (Table 1). After 7 weeks of lactation, CFM and NOM were challenged with 5,000 CFU of Y. pestis strain 141 by the subcutaneous route and monitored by observation for 14 days. All NOM succumbed to 5,000 CFU of Y. pestis challenge, whereas an 84% protection against the same dose of Y. pestis challenge was observed for the CFM in this study (Table 1).
Table 1.
Protective efficacy of offspring from SV1-immunized mother mice and parental mice against a lethal subcutaneous challenge of Y. pestisa
| Group | Time of challenge | Challenge dose (CFU) | No. of survivors/total no. | Protection level (%) |
|---|---|---|---|---|
| MM-S | A | 4,800 | 12/12 | 100 |
| NM | B | 4,800 | 18/25 | 72 |
| MM-S | C | 5,700 | 12/12 | 100 |
| NM | D | 5,700 | 0/28 | 0 |
| CFM | E | 5,000 | 21/25 | 84 |
Abbreviations: MM-S, mother mice immunized with plague subunit vaccine SV1; NM, newborn mice delivered from mother mice immunized with plague subunit vaccine SV1; CFM, cross-fostered mice suckling on lactating mother mice immunized with plague subunit vaccine SV1; A, 16 weeks after immunization (at this time, offspring are 6 weeks old); B, at 6 weeks of age; C, 36 weeks after immunization (at this time, offspring are 14 weeks old); D, at 14 weeks of age; E, at 7 weeks after beginning of lactation.
Analysis of Y. pestis load in different organs.
A postmortem was carried out on the animals who succumbed to the challenge during the 14-day postchallenge observation period. Microbiological examination showed that Y. pestis organisms were isolated from the livers, spleens, and lungs of the dead animals, indicating that the death of the animals was caused by systemic infection by Y. pestis. The survivors were killed humanely and autopsied for microbiological analysis on day 14 postchallenge, and Y. pestis cells were not isolated from livers, spleens, and lungs of all the killed animals, suggesting that Y. pestis had been eliminated from the surviving animals.
DISCUSSION
At present, the recommended therapy for plague is antibiotic treatment. Antibiotics are used both to treat plague victims and as prophylaxis to control the spread of the disease (25). Although the incidence of antibiotic resistance is low, plasmid-borne antibiotic resistance has been identified in Y. pestis, thus limiting the utility of antibiotics (8, 16, 20, 30). Furthermore, side effects associated with continued antibiotic administration highlight the need for treatments that are free from side effects (23). Plague vaccines based on the F1 or V antigen provide a high degree of protection but must be administered several weeks before exposure to virulent Y. pestis (2, 12, 19). As an alternative to antibiotics, polyclonal or monoclonal antibody treatment has been demonstrated to be effective against plague (1, 10, 13–15, 22, 26). In this study, we first demonstrated that passive transfer of maternal antibodies can confer protection to newborn mice against Y. pestis infection.
To determine how long the maternal antibody response and effective protection can be sustained in NM, sera from NM at 6, 8, 10, 12, or 14 weeks of age and from MM-S 28 or 36 weeks after immunization were assayed for antibody level. The rV270-specific IgG was undetectable in the sera of NM from 10 weeks of age on, whereas F1-specific IgG was no longer detectable at 14 weeks of age. When the NM at 6 weeks of age and their mothers 16 weeks after immunization were challenged with 4,800 CFU Y. pestis strain 141 by the subcutaneous route, the NM had a 72% protection against 4,800 CFU Y. pestis strain 141 challenge, whereas complete protection was observed for MM-S after they were challenged with the same dose of Y. pestis. When challenged at 14 weeks of age, NM succumbed to 5,700 CFU of Y. pestis, whereas complete protection against the same dose of Y. pestis challenge was observed in MM-S 36 weeks after immunization. These results indicated that maternal antibodies could be sustained only for a short time in NM and thus provide only early protection to NM. In our previous study, we found that SV1 provided long-term protection against plague and antibody responses over a period of 518 days in a mouse model (30). However, in this study the passively acquired antibody in NM persisted for only about 3 months and provided only early protection, further indicating that antigen-specific long-lived antibody-secreting plasma cells or memory B cells are able to live for a long time in actively immunized mice, whereas maternal transfer of long-lived plasma cells or memory B cells might not occur through the placenta, although mother mice and their fetuses share a circulatory system. In addition, even though there is transmission of long-lived plasma cells or memory B cells, these cells might be ineffective in newborn mice because of their immature immune system. Thus, until the neonatal immune system is fully established, maternal immunization could protect the neonate against infections or at least modify the severity of infectious diseases for various periods of time. Therefore, antibodies can be recommended to treat plague patients or as prophylaxis to be given for people who stay temporarily in plague foci. In addition, several studies have reported that passively acquired antibodies can inhibit antibody responses to active immunization in early infancy (4–6, 25). Although the effects of maternal antibodies on active antibody response were not evaluated in this study, the time of maternal antibody disappearance might help determine when the active immunization should be performed in humans.
To assess whether maternal antibodies are transferred through the placenta or colostrum, sera from PM-S, CSN, LM-S, and CFM were assayed for antibody levels. The results showed that maternal antibodies were acquired not only by placenta but also by breast-feeding. This mode of maternal antibody transmission is in accord with what has been reported for rats and mice (7). However, this transference does not occur through the placenta in pigs and cows, which makes colostrum and milk the only source of maternal antibodies and limits the use of these models for analyzing passive transfer of immunity (2). Several studies have demonstrated that maternal influenza-specific antibody is transferred mainly by breast-feeding in the mouse model (11, 18, 27), but in humans, maternal antibody to influenza virus vaccine is transferred transplacentally to human infants (26). Other studies also have demonstrated that the concentration of maternal antibodies in the murine neonate is only a small fraction of that in the mothers, but it rapidly reaches the maternal concentration after a few days of suckling, indicating that the transfer of IgG from mother to newborns seems to be more effective via the intestine than via the placenta (7, 8). The present study showed that there was no antibody titer difference between PM-S and CSN at the time of caesarean section or between LM-S and CFM during 3 weeks of lactation. These results indicate that transfer of maternal antibodies caused by the plague subunit vaccine in the mouse model is highly efficient either through transplacental transfer or via breast-feeding. However, the antibody acquired through breast milk declines very rapidly in CFM. From 5 weeks of age on, the titer of antibodies in CFM was significantly lower than that in LM-S. This result is similar to those from a previous study indicating that the transfer of IgG through the placenta decreased very rapidly in a Nigerian population of newborns (13). Although a low level of antibody relative to that found in maternal sera was detected in the sera of cross-fostered newborn mice, postnatally acquired immunity can provide significant protection for newborn mice against lethal challenge by Y. pestis in the first few weeks.
It has been reported that the transfer of maternal antibodies is mediated by receptors at the syncytiotrophoblast membrane that binds the Fc portion of the IgG molecules. Maternal antibodies are transferred from the maternal to the fetal circulation through the placenta or intestine, probably because of the presence of trophoblastic receptors that bind the Fc portion of the IgG molecules (22, 24). This theory may explain why maternal antibodies can be transferred through the placenta or intestine, but it cannot explain the differences in efficiency among the different transfer modes. The mode of maternal antibody transfer may be associated with antibody affinity and acceptor specificity.
ACKNOWLEDGMENT
Financial support for this study came from the National Natural Science Foundation of China (contract no. 81171529).
Footnotes
Published ahead of print 29 August 2012
REFERENCES
- 1. Du Y, Rosqvist R, Forsberg A. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elahi S, Buchanan RM, Babiuk LA, Gerdts V. 2006. Maternal immunity provides protection against pertussis in newborn piglets. Infect. Immun. 74:2619–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedlander AM, et al. 1995. Relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21(Suppl 2):S178–S181 [DOI] [PubMed] [Google Scholar]
- 4. Gans H, et al. 2003. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine 21:3398–3405 [DOI] [PubMed] [Google Scholar]
- 5. Gans H, et al. 2001. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J. Infect. Dis. 184:817–826 [DOI] [PubMed] [Google Scholar]
- 6. Gans HA, et al. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527–532 [DOI] [PubMed] [Google Scholar]
- 7. Gitlin JD, Gitlin D. 1974. Protein binding by specific receptors on human placenta, murine placenta, and suckling murine intestine in relation to protein transport across these tissues. J. Clin. Invest. 54:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guyer R, Koshland ME, Knopf PM. 1976. Immunoglobulin binding by mouse intestinal epithelial cell receptors. J. Immunol. 117:587–593 [PubMed] [Google Scholar]
- 9. Karlyshev AV, Galyov EE, Abramov VM, Zav'yalov VP. 1992. Caf1R gene and its role in the regulation of capsule formation of Y. pestis. FEBS Lett. 305:37–40 [DOI] [PubMed] [Google Scholar]
- 10. Leary SE, Griffin KF, Garmory HS, Williamson ED, Titball RW. 1997. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 23:167–179 [DOI] [PubMed] [Google Scholar]
- 11. Mbawuike IN, Six HR, Cate TR, Couch RB. 1990. Vaccination with inactivated influenza A virus during pregnancy protects neonatal mice against lethal challenge by influenza A viruses representing three subtypes. J. Virol. 64:1370–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Overheim KA, et al. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73:5152–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oyedele OO, Odemuyiwa SO, Ammerlaan W, Muller CP, Adu FD. 2005. Passive immunity to measles in the breastmilk and cord blood of some Nigerian subjects. J. Trop. Pediatr. 51:45–48 [DOI] [PubMed] [Google Scholar]
- 14. Perry RD, Fetherston JD. 1997. Yersinia pestis–etiologic agent of plague. Clin. Microbiol. Rev. 10:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qi Z, et al. 2010. Comparison of mouse, guinea pig and rabbit models for evaluation of plague subunit vaccine F1 + rV270. Vaccine 28:1655–1660 [DOI] [PubMed] [Google Scholar]
- 16. Qiu Y, et al. 2010. Comparison of immunological responses of plague vaccines F1 + rV270 and EV76 in Chinese-origin Rhesus macaque, Macaca mulatta. Scand. J. Immunol. 72:425–433 [DOI] [PubMed] [Google Scholar]
- 17. Rasoamanana B, et al. 1997. Field evaluation of an immunoglobulin G anti-F1 enzyme-linked immunosorbent assay for serodiagnosis of human plague in Madagascar. Clin. Diagn. Lab. Immunol. 4:587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reuman PD, Paganini CM, Ayoub EM, Small PA., Jr 1983. Maternal-infant transfer of influenza-specific immunity in the mouse. J. Immunol. 130:932–936 [PubMed] [Google Scholar]
- 19. Riedel S. 2005. Plague: from natural disease to bioterrorism. Proc. (Bayl. Univ. Med. Cent.) 18:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roggenkamp A, Geiger AM, Leitritz L, Kessler A, Heesemann J. 1997. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect. Immun. 65:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell P, et al. 1995. A comparison of Plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551–1556 [DOI] [PubMed] [Google Scholar]
- 22. Saji F, Samejima Y, Kamiura S, Koyama M. 1999. Dynamics of immunoglobulins at the feto-maternal interface. Rev. Reprod. 4:81–89 [DOI] [PubMed] [Google Scholar]
- 23. Shepard CW, et al. 2002. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerg. Infect. Dis. 8:1124–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidiropoulos D, Herrmann U, Morell A, von Muralt G, Barandun S. 1986. Transplacental passage of intravenous immunoglobulin in the last trimester of pregnancy. J. Pediatr. 109:505–508 [DOI] [PubMed] [Google Scholar]
- 25. Siegrist C. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406–3412 [DOI] [PubMed] [Google Scholar]
- 26. Sumaya CV, Gibbs RS. 1979. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J. Infect. Dis. 140:141–146 [DOI] [PubMed] [Google Scholar]
- 27. Sweet C, Bird RA, Jakeman K, Coates DM, Smith H. 1987. Production of passive immunity in neonatal ferrets following maternal vaccination with killed influenza A virus vaccines. Immunology 60:83–89 [PMC free article] [PubMed] [Google Scholar]
- 28. Wang T, et al. 2008. A new purification strategy for fraction 1 capsular antigen and its efficacy against Yersinia pestis virulent strain challenge. Protein Expr. Purif. 61:7–12 [DOI] [PubMed] [Google Scholar]
- 29. Wang T, et al. 2008. Cloning and expression of Yersinia pestis rV270 antigen and efficacy against Y. pestis virulent strain challenge. Lett. Biotechnol. 19:663–666 [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, et al. 2010. Long-term observation of subunit vaccine F1-rV270 against Yersinia pestis in mice. Clin. Vaccine Immunol. 17:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williamson ED. 2001. Plague vaccine research and development. J. Appl. Microbiol. 91:606–608 [DOI] [PubMed] [Google Scholar]