Abstract
A number of studies of highly exposed HIV-1-seronegative individuals (HESN) have found HIV-1-specific cellular responses. However, there is limited evidence that responses prevent infection or are linked to HIV-1 exposure. Peripheral blood mononuclear cells (PBMC) were isolated from HESN in HIV-1-discordant relationships and low-risk controls in Nairobi, Kenya. HIV-1-specific responses were detected using gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays stimulated by peptide pools spanning the subtype A HIV-1 genome. The HIV-1 incidence in this HESN cohort was 1.5 per 100 person years. Positive ELISpot responses were found in 34 (10%) of 331 HESN and 14 (13%) of 107 low-risk controls (odds ratio [OR] = 0.76; P = 0.476). The median immunodominant response was 18.9 spot-forming units (SFU)/106 peripheral blood mononuclear cells (PBMC). Among HESN, increasing age (OR = 1.24 per 5 years; P = 0.026) and longer cohabitation with the HIV-1-infected partner (OR = 5.88 per 5 years; P = 0.003) were associated with responses. These factors were not associated with responses in controls. Other exposure indicators, including the partner's HIV-1 load (OR = 0.99 per log10 copy/ml; P = 0.974) and CD4 count (OR = 1.09 per 100 cells/μl; P = 0.238), were not associated with responses in HESN. HIV-1-specific cellular responses may be less relevant to resistance to infection among HESN who are using risk reduction strategies that decrease their direct viral exposure.
INTRODUCTION
Conventional vaccines exploit natural immune responses that occur upon exposure to pathogens and confer protection. Several groups have demonstrated that a subset of HIV-1-exposed adults and infants have evidence of HIV-1-specific cellular activity yet remain HIV-1 seronegative (2, 4, 5, 9, 14, 19, 22–27, 32, 36, 39, 40, 43–46, 48). However, there is limited evidence that these immune responses prevent HIV-1 infection, and controversy exists about their detection and linkage to HIV-1 exposure.
Non-HIV-1-infected partners in HIV-1-discordant relationships represent one of the largest populations of highly HIV-1-exposed seronegative individuals (HESN) in sub-Saharan Africa, where more than 50% of all couples affected by HIV-1 are discordant (30, 50). There are conflicting data on the detection and prevalence of HIV-1-specific CD8+ T cell responses in this population; during the last decade, a number of studies in the United States, Europe, and Africa reported positive HIV-1-specific T cell responses at rates ranging from 10 to 75% among HESN in long-term relationships (2, 4, 5, 9, 14, 19, 22–27, 32, 36, 39, 40, 43–46, 48). In contrast, a recent study found no positive responses in an HIV-1-discordant couple cohort in Zambia after rigorous investigation (1). Many have suggested that discrepant results are due to cohort differences, especially variations in factors related to the level and timing of HIV-1 exposure (51), while others attribute them to differences in assay technique and sensitivity (7, 38, 49).
In this study, we hypothesized that examining factors associated with positive HIV-1-specific ELISpot responses could explain differences in results between cohorts, particularly when these factors are compared to a control population at low risk of exposure to HIV-1. Such analyses have not been routinely performed due to the large sample size required to examine correlates of immunity and the lack of a control population from the same region. In areas of high HIV prevalence, identifying an appropriate control population with characteristics similar to those of the study population can be challenging, because partner HIV-1 status is not always known or accurately reported and self-reporting of sexual activity may not be reliable. To address these limitations and to improve understanding of correlates of protective immune responses, we conducted a longitudinal cohort study of HIV-1-discordant couples and HIV-1-seronegative individuals in known HIV-1-negative relationships to investigate correlates of gamma interferon (IFN-γ) secretion following stimulation with HIV-1 peptide pools. HIV-1-specifc ELISpot assays were conducted in both HIV-1-discordant and -concordant HIV-1-negative couples, and sociodemographic, sexual behavior, medical history, and clinical data were collected from both partners in the couples.
MATERIALS AND METHODS
Study participants.
HIV-1-discordant couples were recruited from voluntary counseling and testing (VCT) centers in Nairobi, Kenya, between September 2007 and May 2009. Eligible couples reported sex ≥3 times in the 3 months prior to screening, were not pregnant, and planned to remain together for the duration of the study. At enrollment, HIV-1-infected participants did not have a history of clinical AIDS (WHO stage IV) and were not currently on antiretroviral therapy (ART). Eligibility screening and couple counseling, including risk reduction and condom counseling, preceded the enrollment visit.
During the same period, couples in which both partners tested HIV-1 seronegative were recruited from the same VCT centers as the discordant couples. Concordant negative couples also had to report having sex with their partner ≥3 times in the 3 months prior to screening and were not pregnant at enrollment. Couples were ineligible if either partner reported sexual relationships outside the primary partnership.
Written informed consent was obtained from all participants. The study received ethical approval from the institutional review boards of the University of Washington and the University of Nairobi and was conducted according to the guidelines set forth by the U.S. Department of Health and Human Services.
Clinical procedures.
At enrollment, clinical staff examined the participants, administered questionnaires to collect sociodemographic and sexual behavior data, and took a detailed medical history. Questions were presented in English or Kiswahili depending on participant preference, and interviews were conducted individually to ensure confidentiality. A self-reported number of sex acts with the study partner was recorded for both partners of the couple, and the mean number of acts reported by the couple was used for analysis. Urine pregnancy tests (Quick Vue One Step hCG Urine Pregnancy Kit; Quidel Corp., San Diego, CA) were done at enrollment and at each quarterly follow-up visit.
HIV-1, HSV-2, and syphilis diagnosis.
Participants were tested for HIV-1 at enrollment and quarterly by two rapid tests conducted in parallel using a Determine HIV-1/2 rapid test (Abbott Laboratories, Tokyo, Japan; now marketed by Inverness Medical as Alere Determine) and the Bioline HIV 1/2 rapid test (Standard Diagnostics Inc., Suwon, South Korea) and were eligible only if they had concordant rapid test results at screening. Plasma from HIV-1-infected partners collected at enrollment was assayed for HIV-1 RNA load using Gen-Probe Transcription Mediated Amplification (Gen-Probe, San Diego, CA). This assay detects the prevalent HIV-1 subtypes in Kenya, subtypes A, C, and D (10, 11). Serum samples were also collected at enrollment from both partners to test for herpes simplex virus 2 (HSV-2) and syphilis. Testing for HSV-2 was performed using Focus enzyme-linked immunosorbent assay (ELISA) kits (Cyprus, CA) with a cutoff of 3.5 (15), and testing for syphilis used a rapid plasma reagin (RPR) test (Becton, Dickinson and Company, Franklin Lakes, NJ) with confirmation by Treponema pallidum hemagglutination assay (TPHA) (Randox Laboratories, Crumlin, United Kingdom).
IFN-γ ELISpot assays.
IFN-γ ELISpot assays were used to assess cellular responses in partners in HIV-1-discordant relationships and in individuals in concordant HIV-1-seronegative partnerships. EDTA-anticoagulated blood samples were collected at enrollment, and peripheral blood mononuclear cells (PBMC) were isolated using density gradient separation. HIV-1-specific cellular responses were assessed using an IFN-γ ELISpot assay, as described previously, with modifications (28, 31). Briefly, 96-well MAIP45 nitrocellulose plates (Millipore Corporation, Billerica, MA) were coated with monoclonal antibody to IFN-γ (Mabtech, Nacka, Sweden) and blocked with RPMI 1640 supplemented with 10% fetal calf serum and 20 mM l-glutamine (R10) (all Sigma) before adding 1 × 105 PBMC to each well. Triplicate experimental wells were stimulated with pools of HIV-1 subtype A peptides comprised of 15-mers overlapping by 10 amino acids spanning the HIV-1 genome and reconstituted according to the manufacturer's instructions (PEPscreen; Sigma-Aldrich Corporation, St. Louis, MO). Twenty-two peptide pools were generated containing ∼40 sequential peptides per pool. Cells in 3 positive-control wells were stimulated with phytohemagglutinin (PHA) (Murex, Biotech Ltd., Dartford, United Kingdom), and cells in 9 negative-control wells were incubated with R10 alone. After overnight incubation, the cells were removed from the plates by washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20, followed in sequence by application of biotinylated anti-IFN-γ antibody, washing, and streptavidin-conjugated alkaline phosphatase (Mabtech). After the final washing, spot-forming units (SFU) were visualized by the addition of alkaline phosphatase substrate (Mabtech). The plates were dried overnight before being counted and quality controlled using ImmunoSpot software on a CTL ImmunoSpot S4 Core Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Statistical methods.
The mean number of SFU/106 PBMC across the triplicate stimulated wells was determined for each of the 22 peptide pools. The mean background was calculated using the 9 unstimulated negative-control wells. The number of peptide-specific SFU/106 PBMC was calculated by subtracting the mean background from the mean crude SFU/106 PBMC from the stimulated wells. Positive IFN-γ responses were defined as a mean of ≥50 peptide-specific SFU/106 PBMC and more than twice the mean SFU/106 PBMC across the negative-control wells. Participants were excluded from analysis if positive-control wells were <50 SFU/106 PBMC or if there was no difference in mean SFU/106 PBMC in positive- and negative-control wells. A participant was defined as a responder if he/she had ≥1 peptide pool with a positive response. The final analysis was restricted to those with low background response, defined as a mean of <50 SFU/106 PBMC across unstimulated negative-control wells. Sociodemographic, behavioral, and biological characteristics of the couple and the individuals within the couple were assessed as correlates of the presence of HIV-1-specific T cell IFN-γ responses. For univariate comparisons, odds ratios (OR), 95% confidence intervals (CI), and P values were calculated based on Fisher's exact test. Logistic regression was used for continuous variables. The partner's plasma viral load was log10 transformed, and all analyses were conducted using Stata statistical software (Stata Corp., College Station, TX).
RESULTS
Participant characteristics.
Among 408 HIV-1-discordant couples, there were 144 (35%) female HESN and 264 (65%) male HESN. The median age of HESN was 32 (interquartile range [IQR], 27 to 38) years, the median age at sexual debut was 18 (IQR, 16 to 20) years, and the median number of lifetime sexual partners was 4 (IQR, 2 to 6). Participants reported a median of 5 (IQR, 2.5 to 8) sex acts with their HIV-1-infected partner in the past month, and 100 (25%) reported unprotected sex in the past month. The median length of cohabitation was 5.0 (IQR, 2.0 to 10.0) years. Over the period of study follow-up, a total of 58 (14%) HIV-1-discordant couples experienced at least 1 pregnancy, and in 12 (3%) couples, the initially non-HIV-1-infected partner seroconverted. The seroconversion rate was 1.5 per 100 person years.
There were some relevant differences between male and female participants (Table 1). A larger proportion of males reported a history of sexually transmitted infection (STI) (42% versus 21%), and a smaller proportion were HSV-2 seropositive compared to female participants (41% versus 68%). Couples with a male non-HIV-1-infected partner reported slightly more sex acts in the past month (5.5 versus 4.0), and these couples tended to have an infected partner with higher CD4 counts (456.0 versus 359.0 cells/μl) and lower plasma RNA viral loads (4.6 versus 4.8 log10 RNA copies/ml).
Table 1.
Baseline characteristics of HIV-1-infected partners in HIV-1-discordant couples enrolled in the prospective cohort study compared to low-risk controls, by gender
| Characteristic | Median (IQR) or n (%) |
|||
|---|---|---|---|---|
| HESN |
Controls |
|||
| Male (n = 264) | Female (n = 144) | Male (n = 65) | Female (n = 64) | |
| Age (yr) | 34 (29, 40) | 29 (25, 35) | 30 (26, 33) | 25 (22, 30) |
| Less than primary education (n) | 40 (15.2) | 38 (26.4) | 6 (9.2) | 9 (14.1) |
| Age at 1st sex (yr) | 18 (16, 20) | 18 (16, 20) | 17 (15, 19) | 18 (16, 20) |
| Lifetime sexual partners (n) | 5 (3, 9) | 3 (2, 3) | 5 (34, 9) | 3 (2, 3) |
| Yr living together | 4.0 (2.0, 9.0) | 5.3 (2.0, 10.5) | 2.0 (0.0, 7.0) | 2.0 (0.0, 7.0) |
| Sex acts (n)a | 5.5 (3, 9) | 4 (2, 8) | 4.0 (2, 9) | 4.0 (2.0, 12.0) |
| Any unprotected sex (n)a | 68 (25.8) | 32 (22.2) | 50 (76.9) | 46 (71.9) |
| Married (n) | 252 (95.5) | 140 (97.2) | 40 (61.5) | 42 (66.6) |
| Desire future children (n) | 134 (51.0) | 59 (41.3) | 48 (73.8) | 50 (78.1) |
| History of STI (n) | 106 (41.6) | 29 (20.9) | 22 (33.8) | 15 (24.2) |
| Malaria (n)b | 19 (7.2) | 14 (9.7) | 4 (6.2) | 6 (9.4) |
| Genital sores (n)b | 17 (6.4) | 9 (6.3) | 1 (1.5) | 2 (3.1) |
| Circumcised (n) | 223 (84.5) | 57 (87.7) | ||
| HSV-2 seropositive (n) | 109 (41.3) | 98 (68.1) | 15 (23.8) | 19 (31.7) |
| Syphilis (n) | 4 (1.5) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| BV (n)c | 23 (16.1) | 14 (29.8) | ||
| Cervicitis (n) | 1 (0.7) | 0 (0.0) | ||
| Vaginitis (n) | 8 (5.6) | 3 (6.3) | ||
| Partner's CD4 count (no. of cells/μl) | 456.0 (303.0, 638.0) | 359.0 (254.0, 533.0) | ||
| Partner's plasma viral load (no. of log10 RNA copies/ml) | 4.6 (3.8, 5.2) | 4.8 (4.1, 5.4) | ||
With study partner in the past month.
Self-reported in the past 3 months.
BV, bacterial vaginosis.
Sixty-four HIV-1-seronegative females and 65 seronegative males in monogamous, concordant HIV-1-negative relationships were included as low-risk negative controls, and 27 HIV-1-infected partners in discordant relationships (26% male; 74% female) were included as positive controls. Low-risk controls were generally comparable to HESN, but the low-risk controls were younger (median age, 27 versus 32 years), reported a shorter time living together (median, 2.0 versus 5.0 years), and reported more unprotected sex in the past month (74% versus 25%). Compared to HESN, a smaller proportion of low-risk controls reported less than a primary education (12% versus 19%), were married (64% versus 96%), and were HSV-2 seropositive (28% versus 51%). A larger proportion of low-risk controls reported a desire for additional children (76% versus 48%).
As described elsewhere, the HIV-1-infected partners in the discordant-couple relationships were generally similar in demographic and behavioral characteristics to the uninfected partners (16, 17).
Prevalence and factors associated with positive ELISpot responses.
Sixty-two (15%) HESN and 23 (18%) low-risk controls had positive IFN-γ ELISpot responses detected following stimulation with HIV-1 peptides (OR = 0.83; 95% CI, 0.48 to 1.47; P = 0.49). When restricted to those with low background response (mean, <50 SFU/106 PBMC), positive responses were identified in 34 (10%) of 331 HESN and 14 (13%) of 107 negative controls (OR = 0.76; 95% CI, 0.38 to 1.60; P = 0.48). All subsequent analyses were restricted to those with low background response.
The maximum, or immunodominant, IFN-γ response was determined for each participant by pool. The median immunodominant pool response among HESN was 18.9 peptide-specific SFU/106 PBMC (IQR, 11.1 to 32.2 SFU/106 PBMC), and for low-risk controls it was 23.3 peptide-specific SFU/106 PBMC (IQR, 15.6 to 34.4 SFU/106 PBMC) (P = 0.015). Among HIV-1-infected partners assessed as positive controls, we found 23 (95.8%) of 24 had a positive ELISpot response. The median immunodominant pool response among HIV-1-infected positive controls was 513.3 SFU/106 PBMC (IQR, 211.7 to 1,010.6 SFU/106 PBMC).
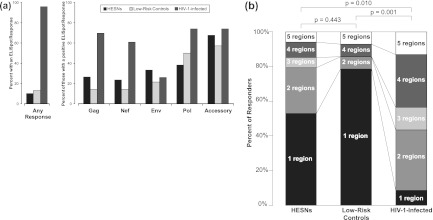
The relative distributions of responses by viral region were similar for HESN and low-risk controls, with the largest proportion of responses to accessory proteins (tat, rev, vif, vpu, and vpr) (68% of HESN responders and 57% of low-risk control responders), followed by pol (38% of HESN responders and 50% of low-risk control responders), and the smallest proportion of responses were against gag (26% of HESN responders and 14% of low-risk control responders) and nef (24% of HESN responders and 14% of low-risk control responders) (Fig. 1a). Although not significantly different from low-risk controls, HESN responded in higher proportions to gag, nef, env, and accessory proteins and in smaller proportions to pol. Among responders, the largest proportion of both HESN (53%) and low-risk controls (79%) responded to just 1 viral region, with a trend indicating that HESN may have been more likely to respond to 2 or more viral regions than low-risk controls (OR = 3.26; 95% CI, 0.67 to 20.94; P = 0.12) (Fig. 1b). In comparison, HIV-1-infected positive controls differed qualitatively from HESN and low-risk controls in the viral regions to which they responded. Approximately equal proportions of the 23 HIV-1-infected controls with a positive ELISpot response recognized gag (70%), nef (61%), pol (74%), and accessory proteins (74%), and a smaller proportion (26%) responded to env. Among HIV-1-infected controls with positive responses, nearly all (91%) responded to ≥2 viral regions and 43% responded to ≥4 viral regions.
Fig 1.
IFN-γ ELISpot assays were conducted on samples from HESN from HIV-1-discordant couples and low-risk controls from concordant HIV-negative couples using 1 × 105 cultured PBMC with 3 wells per peptide pool. Pools with ≥50 HIV SFU/106 PBMC and >2 times the background were defined as positive responses. A response to any peptide pool covering the viral region was counted as a region response (restricted to samples with a background of <50 SFU/106 PBMC). (a) T cell IFN-γ ELISpot responses by viral region. (b) Breadth of responses based on the number of viral regions to which participants mounted a response. The P values are for χ2 tests comparing the distributions between groups.
Correlates of positive ELISpot responses.
Among HESN, we observed that increasing age, longer time living with the HIV-1-infected partner, and having less than a primary education were significantly associated with having a cellular response to HIV-1 peptides (Table 2). For every 5-year increase in age, there was a 1.24-fold (95% CI, 1.03 to 1.51; P = 0.026) increased likelihood of a response, and for every 5-year increase in time living with their study partner, there was a 5.88-fold (95% CI, 1.80 to 19.16; P = 0.003) increased likelihood of a response. Those with less than a primary education were 2.45-fold (95% CI, 1.04 to 5.54; P = 0.024) more likely to have a response than those with at least a primary education. However, in comparison, among low-risk control participants, point estimates for age (OR = 0.64; 95% CI, 0.38 to 1.06; P = 0.083), duration living with their study partner (OR = 0.70; 95% CI, 0.38 to 1.28; P = 0.246), and low education (OR = 0.72; 95% CI, 0.02 to 6.02; P = 0.99) were not significant and showed a direction of association opposite that observed for HESN (Table 3).
Table 2.
Correlates of ELISpot responses among HESNa
| Characteristic | Median (IQR) or n (%) |
ORb | 95% CI | P value | |
|---|---|---|---|---|---|
| Responders (n = 34) | Non-responders (n = 297) | ||||
| Female (n) | 10 (29.4) | 106 (35.7) | 0.75 | 0.31–1.71 | 0.571 |
| Pregnancy during study (n) | 3 (8.8) | 42 (14.1) | 0.59 | 0.11–2.02 | 0.596 |
| Age (yr) | 32.5 (30, 46) | 32 (27, 38) | 1.24f | 1.03–1.51 | 0.026 |
| Less than primary education (n) | 12 (35.3) | 54 (18.2) | 2.45f | 1.04–5.54 | 0.024 |
| Yr living together | 6.5 (2.0, 13.0) | 4.0 (2.0, 9.0) | 5.88g | 1.80–19.16 | 0.003 |
| Lifetime partners (n) | 4.0 (3.0, 5.0) | 4.0 (2.0, 6.0) | 0.97 | 0.90–1.04 | 0.423 |
| Sex acts (n)c | 5.3 (2.0, 8.0) | 5.0 (2.5, 8.5) | 0.95 | 0.91–1.07 | 0.788 |
| Unprotected sex (n)c | 10 (29.4) | 82 (27.6) | 1.09 | 0.45–2.50 | 0.841 |
| History of STI (n) | 11 (33.3) | 105 (36.1) | 0.89 | 0.37–2.00 | 0.849 |
| Malaria (n)d | 2 (5.9) | 27 (9.1) | 0.63 | 0.07–2.70 | 0.752 |
| Genital sores (n)d | 0 (0.0) | 24 (8.1) | 0 | 0–1.30 | 0.152 |
| HSV-2 seropositive (n) | 17 (50.0) | 149 (50.2) | 0.99 | 0.46–2.16 | 0.999 |
| Circumcised (n)e | 22 (91.7) | 166 (86.9) | 1.66 | 0.37–15.37 | 0.746 |
| Partner's CD4 count (no. of cells/μl) | 476.0 (303.0, 617.0) | 400.5 (278.0, 617.0) | 1.09 | 0.94–1.26 | 0.238 |
| Partner's plasma viral load (no. of log10 RNA copies/ml) | 4.8 (3.9, 5.4) | 4.7 (4.0, 5.3) | 1.00 | 0.74–1.34 | 0.974 |
Analysis is restricted to samples with background of <50 SFU/106 PBMC. Odds ratios.
The OR for age and years living together is per 5 years. The OR for partner's CD4 count is per 100 cells/μl, and the OR for partner's plasma viral load is per log10 RNA copies/ml.
With study partner in the past month.
Self-reported in the past 3 months.
Males only.
P < 0.05.
P < 0.001.
Table 3.
Correlates of ELISpot responses among low-risk controlsa
| Characteristic | Median (IQR) or n (%) |
ORb | 95% CI | P value | |
|---|---|---|---|---|---|
| Responders (n = 14) | Nonresponders (n = 93) | ||||
| Female (n) | 11 (78.6) | 42 (45.2) | 4.45f | 1.07–26.12 | 0.023 |
| Age (yr) | 23.5 (21, 27) | 28 (25, 33) | 0.64 | 0.38–1.06 | 0.083 |
| Less than primary education (n) | 1 (7.1) | 9 (9.7) | 0.72 | 0.02–6.02 | 0.999 |
| Yr living together | 0.8 (0.0, 5.0) | 2.4 (0.0, 8.0) | 0.70 | 0.38–1.28 | 0.246 |
| Lifetime partners (n) | 3.0 (2.0, 5.0) | 3.0 (3.0, 6.0) | 0.98 | 0.91–1.05 | 0.533 |
| Sex acts (n)c | 8.0 (4.0, 9.0) | 4.0 (2.0, 12.0) | 0.99 | 0.92–1.08 | 0.978 |
| Unprotected sex (n)c | 13 (92.9) | 65 (69.9) | 5.60 | 0.76–246.31 | 0.106 |
| History of STI (n) | 7 (50.0) | 23 (25.3) | 2.96 | 0.78–10.95 | 0.108 |
| Malaria (n)d | 1 (7.1) | 6 (6.5) | 1.12 | 0.02–10.42 | 0.999 |
| Genital sores (n)d | 0 (0.0) | 2 (2.2) | 0 | 0–13.40 | 0.999 |
| HSV-2 seropositive (n) | 4 (30.8) | 23 (25.6) | 1.29 | 0.27–5.20 | 0.739 |
| Circumcised (n)e | 3 (100.0) | 44 (86.3) | NA | 0.11–∞ | 0.999 |
Analysis is restricted to samples with background of <50 SFU/106 PBMC.
OR for age and years living together are per 5 years. NA, not applicable.
With study partner in the past month.
Self-reported in the past 3 months.
Males only.
P < 0.05.
There was no significant association between IFN-γ responses and the infected partner's plasma viral load (OR = 0.99; 95% CI, 0.74 to 1.34; P = 0.974) or CD4 count (OR = 1.09 per 100 cells/μl; 95% CI, 0.94 to 1.26; P = 0.238). There were also no associations with HESN gender, number of reported sex acts, unprotected sex, HSV-2 serostatus, reports of genital ulcers in either partner, or male circumcision status. Among the low-risk controls, females were significantly more likely to have a positive response (OR = 4.45; 95% CI, 1.07 to 26.12; P = 0.042), but for all other potential correlates of HIV-1 exposure, there were no significant associations with positive ELISpot responses.
DISCUSSION
This is one of the largest studies of immune responses among HESN, with more than 400 ex vivo assays performed on samples collected from men and women in HIV-1-discordant couples. This study also presents data from an exceptionally large control population comprised of 127 HIV-1-negative individuals in monogamous relationships. Among HESN, 10% had positive IFN-γ ELISpot responses using standard criteria, and these responses were associated with older age and increased relationship duration. This is similar to what was found by Kaul and colleagues among commercial sex workers in Kenya: longer duration of sex work was associated with a positive response, and women who continued to engage in sex work without becoming infected were more likely to have responses than their younger counterparts (24). Such predictors are consistent with the hypothesis that ELISpot responses are more than a marker of exposure and may be part of a protective immune response, so that those individuals who do not seroconvert over time despite ongoing exposure are the ones most likely to have responses. While the failure of candidate vaccines that successfully elicit robust HIV-1-specific IFN-γ responses demonstrates that an IFN-γ response alone is not sufficient for protection (6, 34), there remains evidence that such responses, in the context of natural exposure, are at least a good correlate of protection (19, 21).
In this study, our intention was to evaluate whether HIV-1-specific cellular responses were protective; however, this was not possible, because there were too few HIV transmission events among enrolled couples. As in any discordant-couple cohort, there is major emphasis on HIV-1 prevention counseling and provision of condoms to couples, resulting in reduced exposure. This cohort had a low incidence rate of HIV-1 transmission at 2.1 per 100 person years for male-to-female and 1.1 per 100 person years for female-to-male transmission. This was a lower transmission rate than has been described in many previous studies of discordant couples (18) but is similar to the rates found in recent large clinical trials involving ongoing prevention counseling (8). From this, we can deduce that there was relatively low HIV-1 exposure. We have no reason to suspect that HIV-1-exposed, uninfected partners used postexposure (PEP) or preexposure (PrEP) prophylaxis that would have resulted in fewer HIV-1 infections despite high exposure. Questions regarding antiretroviral use were asked during exit interviews (data not shown), and this possibility has been assessed in similar cohorts in Nairobi with negative results. Low exposure levels, likely due to high levels of condom use, may explain the lack of observed associations with other markers of exposure, such as the HIV-1 load in the infected partner, frequency of sex, and genital ulcer disease. Our findings in this HESN cohort using overlapping peptide pools contrast with a previous study by our group among 161 HIV-exposed, uninfected infants in which we found a positive association between exposure to HIV-1 and HIV-1-specific ELISpot responses using defined CD8+ cytotoxic T lymphocyte (CTL) epitopes. We also found an association between infant ELISpot responses and protection against infection. Levels of exposure were likely to have been much greater in this previous cohort, which had a transmission rate of >15% overall (12, 19).
Another important observation in our study is that a relatively high proportion of low-risk controls had positive responses. Among 23 publications we reviewed, 20 assessed responses in a control population (although not always from the same population used to recruit HESN), with a mean sample size of 13 individuals. This low number of controls greatly limited their power to detect low-level responses (Table 4). We found that 13% of our low-risk controls had positive responses compared to 10% of HESN, which may indicate nonspecificity of the ELISpot assay. This finding highlights the importance of using control populations that are both large enough to detect low proportions of responses and drawn from the same source population as the subjects of interest. Despite comparable proportions of responders among HESN and low-risk controls, it is interesting that responses among the low-risk controls were not correlated with any of the factors found to be correlates of responses among HESN. This may be due to chance, but it may also represent differences between the two populations in terms of factors influencing the specificity of the assay. In particular, the low-risk controls were far more likely to report unprotected sex, which may increase the likelihood of a recent genital infection. This may result in differences in immunologic responses that make the ELISpot assay less specific and could explain the larger proportion of female low-risk controls with ELISpot responses. In a large cohort of discordant couples, ∼30% of new infections were linked to external partnerships (37), and it is possible that the “low-risk” controls in our study in fact had other exposures to HIV-1. These findings emphasize the challenges of interpreting HIV-1-specific responses that occur at low levels in a small proportion of subjects.
Table 4.
Prevalence of HIV-1-specific cellular responses among HESN and low-risk controls in previously published studies ordered from lowest to highest prevalence in each category
| Study | HESN |
Low-risk controls |
Assay | ||
|---|---|---|---|---|---|
| n | Prevalence (%) | n | Prevalence (%) | ||
| Exposed, uninfected individuals | |||||
| Makedonas et al. (32) | 47 | 28 | 19 | 0 | Peptide CD8 IFN-γ ELISpot |
| Goh et al. (14) | 36 | 36 | 15 | 7 | Recombinant VV-CTLa precursor frequency |
| Bernard et al. (4) | 17 | 41 | 14 | 0 | Recombinant VV-CTL |
| Missale et al. (36) | 3 | 67 | NRb | 0 | Peptide CD8 IFN-γ Elispot and ICSc |
| Commercial sex workers | |||||
| Kaul et al. (25) | 39 | 21 | 0 | Recombinant VV-CTL | |
| Rowland-Jones et al. (43) | 9 | 33 | 14 | 0 | Peptide CTL |
| Alimonti et al. (2) | 13 | 39 | 0 | Peptide CD8 IFN-γ ICS | |
| Kaul et al. (24) | 91 | 47 | 18 | 0 | Peptide CD8 IFN-γ ELISpot |
| Rowland-Jones et al. (44) | 21 | 48 | 6 | 0 | Peptide CTL |
| Kaul et al. (23) | 16 | 69 | 7 | 0 | Peptide CD8 IFN-γ ELISpot |
| Discordant couples | |||||
| Addo et al. (1) | 28 | 0 | 15 | 0 | Peptide IFN-γ ICS |
| Ritchie et al. (42) | 16 | 0 | 21 | 0 | Peptide IFN-γ ELISpot |
| Perez et al. (39) | 23 | 13 | 13 | 0 | Peptide IFN-γ ELISpot and ICS |
| Kebba et al. (26) | 28 | 20 | 12 | 0 | Peptide IFN-γ ELISpot |
| Shacklett et al. (47) | 8 | 38 | 5 | 0 | Peptide IFN-γ ELISpot using dendritic cell presentation |
| Skurnick et al. (48) | 17 | 41 | 20 | NR | Recombinant VV-IFN-γ ELISpot |
| Bienzle et al. (5) | 11 | 45 | 6 | 0 | Peptide CTL |
| Promadej et al. (40) | 18 | 50 | 12 | 0 | Recombinant VV-IFN-γ ELISpot |
| Schenal et al. (45) | 15 | >50 | 8 | 12.5 | Peptide IFN-γ ICS |
| Ritchie et al. (42) | 24 | 53 to 61 | 27 | 26 to 44 | Cultured IFN-γ ELISpot |
| Kebba et al. (27) | 10 | 75 | 5 | 0 | Antigen CD4 ICS |
| Infants born to HIV-1-infected mothers | |||||
| John-Stewart et al. (19) | 217 | 12–22 | 20 | 0 | Peptide CD8 IFN-γ ELISpot |
| Schramm et al. (46) | 106 | 13 | 20 | 0 | Peptide IL-2d production ELISA |
| Clerici et al. (9) | 8 | 38 | 7 | 0 | Peptide CD8 IFN-γ ELISpot |
VV-CTL, vaccinia virus CTL.
NR, not reported.
ICS, intracellular cytokine staining.
IL-2, interleukin-2.
The high rate of weak positive responses among both HESN and low-risk controls raises questions about the utility of the ELISpot assay for measuring low-level responses among HESN. Similar to other studies among HIV-1-positive individuals, we found strong responses among approximately 96% of HIV-infected individuals, and these were directed equally against HIV-1 gag, pol, nef, and accessory-protein epitopes (24, 41). One hypothesis is that the ELISpot assay, when used in this population, is highly sensitive but less specific, which could contribute to increased rates of false-positive results in settings where true-positive results are infrequent. As with any test, when the prevalence of the outcome is lower (which would occur as studies move from HIV-1-infected individuals to HESN to candidate HIV vaccine trials to low-risk controls), there are proportionally more false-positive results, and the positive predictive value is lower.
This study had several strengths, including the large cohort size, the presence of large low-risk and positive-control populations, performance of assays on fresh samples to optimize sensitivity, and collection of detailed clinical histories and interviews for both partners in the relationship. The study was limited by the fact that we performed only one type of immune assay on unfractionated PBMC, making it difficult to distinguish CD8+ and CD4+ responses. CD4 responses to peptides restricted to multiple HLA-DR types have recently been shown to contribute to ELISpot responses observed in HESN and low-risk controls (42). We had limited ability to confirm responses using frozen samples due to limited numbers of PBMC. Stimulating peptide pools spanned the subtype A genome, and it is possible that a lack of cross-subtype reactivity resulted in lower response rates among those exposed to non-A subtypes; however, clade A accounts for >70% of HIV-1 infections in Kenya, and there is strong evidence of cross-clade ELISpot reactivity (3, 13, 20, 29, 33, 35, 52, 53). Finally, assays were performed in an unblinded manner. Despite these limitations, we believe this study contributes substantially to the existing literature on ELISpot responses among HESN.
In conclusion, we found that HIV-1-specific ELISpot responses in HESN were associated with the duration of HIV-1 exposure but that these responses did not occur in HESN at a level above what was found in low-risk controls. HIV-1-specific cellular immune responses have been found consistently in HESN who have persistent high levels of viral exposure. However, our findings, combined with other recent reports, indicate that among some HESN, these responses are reduced and may not contribute substantially to resistance to infection. This may be partially due to risk reduction strategies that decrease direct viral exposure, leading to new questions about the impact of such strategies on HIV-specific immune responses in HESN. Since weak positives also occurred in a relatively small proportion of control subjects in our study, the ELISpot assay was not sufficiently specific to investigate these rare and low-magnitude responses. Nonetheless, because the ELISpot assay can be readily conducted in large volume in resource-limited settings, it is likely to continue to play an important role in defining immune responses to HIV-1 and other pathogens. In future studies, adequate numbers of control subjects from the same source population as the HESN would enhance the validity and interpretability of results.
ACKNOWLEDGMENTS
This research was funded by U.S. National Institutes of Health (NIH) grant AI068431. B. L. Guthrie received support from the NIH training grant T32 AI07140. C. Farquhar received support from NIH grant K24 AI087399. R. Bosire was a scholar in the University of Washington (UW) AIDS International Training and Research Program supported by NIH Fogarty International Center grant D43 TW00007. Research support was also provided by the UW Center for AIDS Research (CFAR), an NIH program (P30 AI027757) that is funded by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, and NCCAM.
Footnotes
Published ahead of print 12 September 2012
REFERENCES
- 1. Addo MM, et al. 2011. Lack of detectable HIV-1-specific CD8(+) T cell responses in Zambian HIV-1-exposed seronegative partners of HIV-1-positive individuals. J. Infect. Dis. 203:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alimonti JB, et al. 2006. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol. Cell Biol. 84:482–485 [DOI] [PubMed] [Google Scholar]
- 3. Baeten JM, et al. 2007. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J. Infect. Dis. 195:1177–1180 [DOI] [PubMed] [Google Scholar]
- 4. Bernard NF, Yannakis CM, Lee JS, Tsoukas CM. 1999. Human immunodeficiency virus (HIV)-specific cytotoxic T lymphocyte activity in HIV-exposed seronegative persons. J. Infect. Dis. 179:538–547 [DOI] [PubMed] [Google Scholar]
- 5. Bienzle D, et al. 2000. Factors contributing to the lack of human immunodeficiency virus type 1 (HIV-1) transmission in HIV-1-discordant partners. J. Infect. Dis. 182:123–132 [DOI] [PubMed] [Google Scholar]
- 6. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bull M, et al. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Celum C, et al. 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N. Engl. J. Med. 362:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clerici M, et al. 2000. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 96:3866–3871 [PubMed] [Google Scholar]
- 10. DeVange Panteleeff D, et al. 2002. Validation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay with genital swabs and breast milk samples. J. Clin. Microbiol. 40:3929–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emery S, et al. 2000. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 38:2688–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farquhar C, et al. 2011. Breast milk HIV-1 RNA levels and female sex are associated with HIV-1-specific CD8+ T-cell responses in HIV-1-exposed, uninfected infants in Kenya. J. Infect. Dis. 204:1806–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geels MJ, et al. 2005. Broad cross-clade T-cell responses to gag in individuals infected with human immunodeficiency virus type 1 non-B clades (A to G): importance of HLA anchor residue conservation. J. Virol. 79:11247–11258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goh WC, et al. 1999. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J. Infect. Dis. 179:548–557 [DOI] [PubMed] [Google Scholar]
- 15. Golden MR, et al. 2005. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex. Transm. Dis. 32:771–777 [DOI] [PubMed] [Google Scholar]
- 16. Guthrie BL, et al. 2010. Predicting pregnancy in HIV-1-discordant couples. AIDS Behavior 14:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guthrie BL, et al. 2011. Barriers to antiretroviral initiation in HIV-1-discordant couples. J. Acquir. Immune Defic. Syndr. 58:e87–e93 doi:10.1097/QAI.0b013e31822f064e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guthrie BL, de Bruyn G, Farquhar C. 2007. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr. HIV Res. 5:416–429 [DOI] [PubMed] [Google Scholar]
- 19. John-Stewart GC, et al. 2009. HV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J. Infect. Dis. 199:889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kageha S, et al. 2012. HIV type 1 subtype surveillance in central Kenya. AIDS Res. Hum. Retrovir. 28:228–231 [DOI] [PubMed] [Google Scholar]
- 21. Kaul R, et al. 2001. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Invest. 107:1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaul R, et al. 2010. HIV viral set point and host immune control in individuals with HIV-specific CD8+ T-cell responses prior to HIV acquisition. AIDS 24:1449–1454 [DOI] [PubMed] [Google Scholar]
- 23. Kaul R, et al. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602–1611 [DOI] [PubMed] [Google Scholar]
- 24. Kaul R, et al. 2001. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol. Lett. 79:3–13 [DOI] [PubMed] [Google Scholar]
- 25. Kaul R, et al. 2004. HIV-1 Env-specific cytotoxic T-lymphocyte responses in exposed, uninfected Kenyan sex workers: a prospective analysis. AIDS 18:2087–2089 [DOI] [PubMed] [Google Scholar]
- 26. Kebba A, et al. 2004. Distinct patterns of peripheral HIV-1-specific interferon-gamma responses in exposed HIV-1-seronegative individuals. J. Infect. Dis. 189:1705–1713 [DOI] [PubMed] [Google Scholar]
- 27. Kebba A, et al. 2004. HIV type 1 antigen-responsive CD4+ T-lymphocytes in exposed yet HIV type 1 seronegative Ugandans. AIDS Res. Hum. Retrovir. 20:67–75 [DOI] [PubMed] [Google Scholar]
- 28. Lalvani A, Pareek M. 2010. Interferon gamma release assays: principles and practice. Enferm. Infecc. Microbiol. Clín. 28:245–252 [DOI] [PubMed] [Google Scholar]
- 29. Lihana RW, Ssemwanga D, Abimiku A, Ndembi N. 2012. Update on HIV-1 diversity in Africa: a decade in review. AIDS Rev. 14:83–100 [PubMed] [Google Scholar]
- 30. Lingappa JR, et al. 2008. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS One 3:e1411 doi:10.1371/journal.pone.0001411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lohman BL, et al. 2005. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J. Virol. 79:8121–8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makedonas G, et al. 2005. Comparison of HIV-specific CD8 T-cell responses among uninfected individuals exposed to HIV parenterally and mucosally. AIDS 19:251–259 [PubMed] [Google Scholar]
- 33. Malhotra U, Nolin J, Mullins JI, McElrath MJ. 2007. Comprehensive epitope analysis of cross-clade Gag-specific T-cell responses in individuals with early HIV-1 infection in the US epidemic. Vaccine 25:381–390 [DOI] [PubMed] [Google Scholar]
- 34. McElrath MJ, et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKinnon LR, et al. 2005. Cross-clade CD8(+) T-cell responses with a preference for the predominant circulating clade. J. Acquir. Immune Defic. Syndr. 40:245–249 [DOI] [PubMed] [Google Scholar]
- 36. Missale G, et al. 2004. Parenteral exposure to high HIV viremia leads to virus-specific T cell priming without evidence of infection. Eur. J. Immunol. 34:3208–3215 [DOI] [PubMed] [Google Scholar]
- 37. Ndase P, et al. 2012. Outside sexual partnerships and risk of HIV acquisition for HIV uninfected partners in African HIV serodiscordant partnerships. J. Acquir. Immune Defic. Syndr. 59:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. 2003. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J. Immunol. Methods 282:103–115 [DOI] [PubMed] [Google Scholar]
- 39. Perez CL, Hasselrot K, Bratt G, Broliden K, Karlsson AC. 2010. Induction of systemic HIV-1-specific cellular immune responses by oral exposure in the uninfected partner of discordant couples. AIDS 24:969–974 [DOI] [PubMed] [Google Scholar]
- 40. Promadej N, et al. 2003. Broad human immunodeficiency virus (HIV)-specific T cell responses to conserved HIV proteins in HIV-seronegative women highly exposed to a single HIV-infected partner. J. Infect. Dis. 187:1053–1063 [DOI] [PubMed] [Google Scholar]
- 41. Ramduth D, et al. 2005. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J. Infect. Dis. 192:1588–1596 [DOI] [PubMed] [Google Scholar]
- 42. Ritchie AJ, et al. 2011. Differences in HIV-specific T cell responses between HIV-exposed and -unexposed HIV-seronegative individuals. J. Virol. 85:3507–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rowland-Jones S, et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59–64 [DOI] [PubMed] [Google Scholar]
- 44. Rowland-Jones SL, et al. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Invest. 102:1758–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schenal M, et al. 2005. Distinct patterns of HIV-specific memory T lymphocytes in HIV-exposed uninfected individuals and in HIV-infected patients. AIDS 19:653–661 [DOI] [PubMed] [Google Scholar]
- 46. Schramm DB, Meddows-Taylor S, Gray GE, Kuhn L, Tiemessen CT. 2007. Low maternal viral loads and reduced granulocyte-macrophage colony-stimulating factor levels characterize exposed, uninfected infants who develop protective human immunodeficiency virus type 1-specific responses. Clin. Vaccine Immunol. 14:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shacklett BL, et al. 2002. Dendritic cell amplification of HIV type 1-specific CD8+ T cell responses in exposed, seronegative heterosexual women. AIDS Res. Hum. Retrovir. 18:805–815 [DOI] [PubMed] [Google Scholar]
- 48. Skurnick JH, et al. 2002. Correlates of nontransmission in US women at high risk of human immunodeficiency virus type 1 infection through sexual exposure. J. Infect. Dis. 185:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinberg A, et al. 2010. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J. Immunol. Methods 363:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Were WA, et al. 2006. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J. Acquir. Immune Defic. Syndr. 43:91–95 [DOI] [PubMed] [Google Scholar]
- 51. Willberg CB, et al. 2008. Immunity to HIV-1 is influenced by continued natural exposure to exogenous virus. PLoS Pathog. 4:e1000185 doi:10.1371/journal.ppat.1000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu XG, et al. 2005. High degree of inter-clade cross-reactivity of HIV-1-specific T cell responses at the single peptide level. AIDS 19:1449–1456 [DOI] [PubMed] [Google Scholar]
- 53. Zembe L, et al. 2011. Intra- and inter-clade cross-reactivity by HIV-1 Gag specific T-cells reveals exclusive and commonly targeted regions: implications for current vaccine trials. PLoS One 6:e26096 doi:10.1371/journal.pone.0026096 [DOI] [PMC free article] [PubMed] [Google Scholar]