Abstract
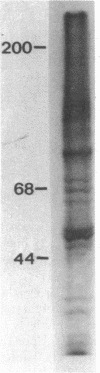
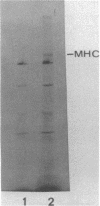
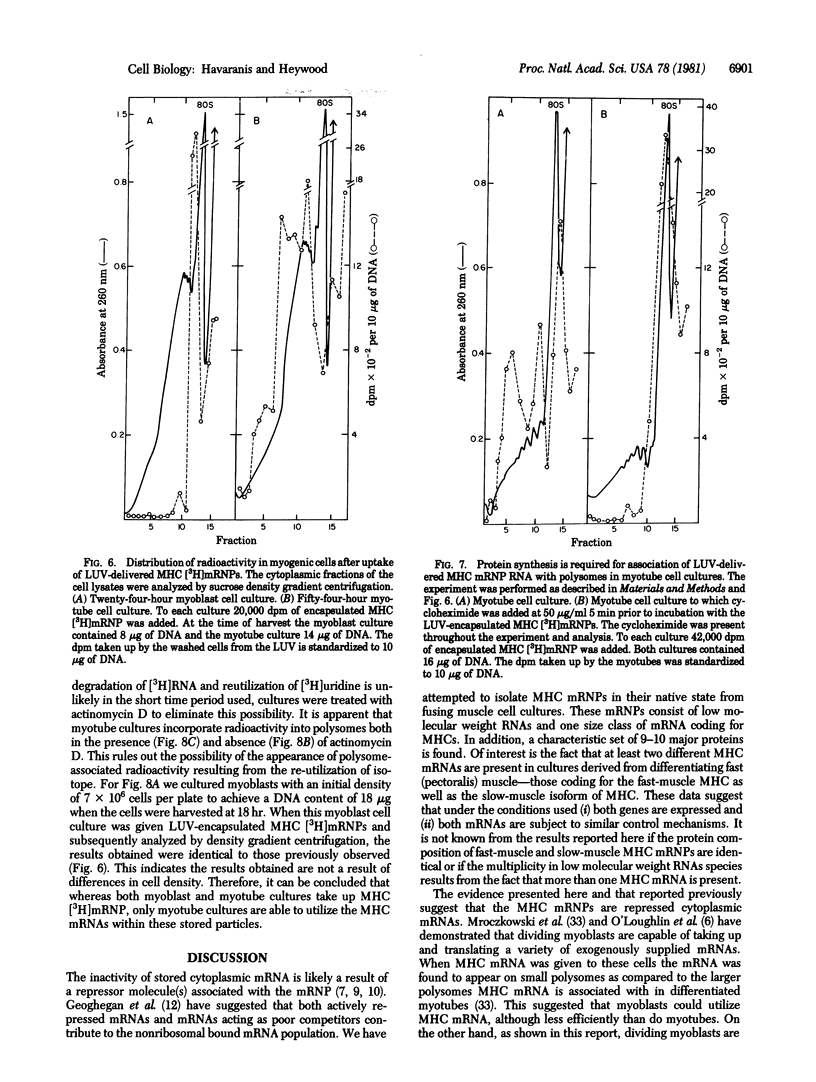
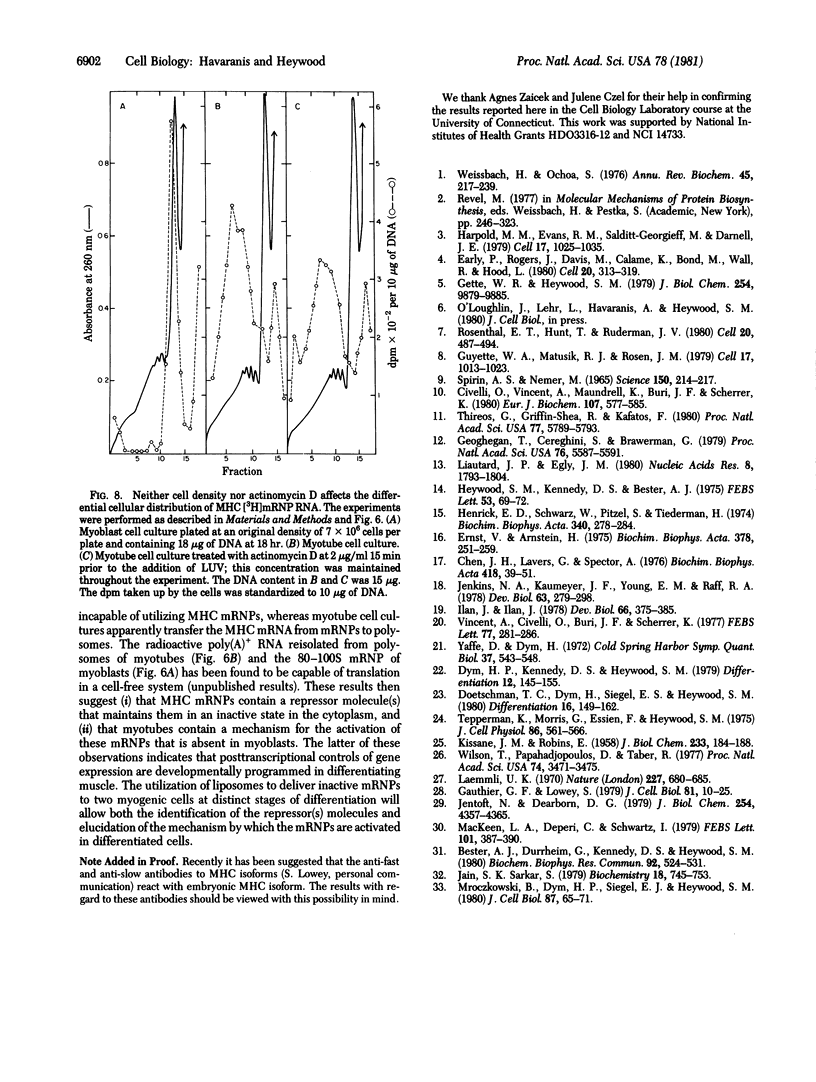
Myosin heavy chain messenger ribonucleoprotein particles (MHC mRNPs) have been isolated. Characterization of the RNA components revealed an mRNA of approximately the same size as tobacco mosaic virus RNA and three low molecular weight components. The protein consists of 9-10 major bands ranging in molecular weight between 22,000 and 130,000. The messenger contained in these mRNPs was found to direct the synthesis of both fast-muscle and slow-muscle MHC in a cell-free system. When MHC [3H]mRNPs were encapsulated into liposomes and subsequently delivered to myoblasts and myotubes, the mRNPs were taken up by the cells at both stages of differentiation. However, the MHC [3H]mRNPs taken up by the myoblasts remained as free cytoplasmic particles (80-120S), whereas in myotubes the incorporated mRNP RA was associated with polysomes. The results indicate that MHC mRNPs contain a repressor molecule(s) and that myotubes possess a mechanism for activating these mRNPs that is absent from myoblasts.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bester A. J., Durrheim G., Kennedy D. S., Heywood S. M. Isolation of myosin messenger ribonucleoprotein particles which contain a protein fraction affecting myosin synthesis. Biochem Biophys Res Commun. 1980 Jan 29;92(2):524–531. doi: 10.1016/0006-291x(80)90365-4. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Lavers G. C., Spector A. Calf lens messenger ribonucleoprotein complexes. Characterization and comparison of template activity with corresponding mRNAs. Biochim Biophys Acta. 1976 Jan 5;418(1):39–51. doi: 10.1016/0005-2787(76)90325-7. [DOI] [PubMed] [Google Scholar]
- Civelli O., Vincent A., Maundrell K., Buri J. F., Scherrer K. The translational repression of globin mRNA in free cytoplasmic ribonucleoprotein complexes. Eur J Biochem. 1980 Jun;107(2):577–585. doi: 10.1111/j.1432-1033.1980.tb06066.x. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Dym H. P., Siegel E. J., Heywood S. M. Myoblast stored myosin heavy chain transcripts are precursors to the myotube polysomal myosin heavy chain mRNAs. Differentiation. 1980 Jun;16(3):149–162. doi: 10.1111/j.1432-0436.1980.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Dym H. P., Kennedy D. S., Heywood S. M. Sub-cellular distribution of the cytoplasmic myosin heavy chain mRNA during myogenesis. Differentiation. 1979;12(3):145–155. doi: 10.1111/j.1432-0436.1979.tb01000.x. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Ernst V., Arnstein H. R. Synthesis of alpha- and beta-globin directed by messenger ribonucleoprotein from rabbit reticulocytes. Biochim Biophys Acta. 1975 Jan 20;378(2):251–259. doi: 10.1016/0005-2787(75)90113-6. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979 Apr;81(1):10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan T., Cereghini S., Brawerman G. Inactive mRNA-protein complexes from mouse sarcoma-180 ascites cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5587–5591. doi: 10.1073/pnas.76.11.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gette W. R., Heywood S. M. Translation of myosin heavy chain messenger ribonucleic acid in an eukaryotic initiation factor 3- and messenger-dependent muscle cell-free system. J Biol Chem. 1979 Oct 10;254(19):9879–9885. [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hendrick D., Schwarz W., Pitzel S., Tiedemann H. Messenger ribonucleoprotein-directed globin synthesis in an embryonic brain cell-free system. Biochim Biophys Acta. 1974 Mar 27;340(3):278–284. doi: 10.1016/0005-2787(74)90273-1. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Stored myosin messenger in embryonic chick muscle. FEBS Lett. 1975 Apr 15;53(1):69–72. doi: 10.1016/0014-5793(75)80684-3. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol. 1978 Oct;66(2):375–385. doi: 10.1016/0012-1606(78)90246-4. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Sarkar S. Poly(riboadenylate)-containing messenger ribonucleoprotein particles of chick embryonic muscles. Biochemistry. 1979 Mar 6;18(5):745–753. doi: 10.1021/bi00572a002. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Egly J. M. In vitro translation studies of the cytoplasmic nonpolysomal particles containing messenger RNA. Nucleic Acids Res. 1980 Apr 25;8(8):1793–1804. doi: 10.1093/nar/8.8.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKeen L. A., DiPeri C., Schwartz I. Reductive methylation of IF-3 and EFTu with [14C]formaldehyde and sodium cyanoborohydride. FEBS Lett. 1979 May 15;101(2):387–390. doi: 10.1016/0014-5793(79)81050-9. [DOI] [PubMed] [Google Scholar]
- Mroczkowski B., Dym H. P., Siegel E. J., Heywood S. M. Uptake and utilization of mRNA by myogenic cells in culture. J Cell Biol. 1980 Oct;87(1):65–71. doi: 10.1083/jcb.87.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Nemer M. Messenger RNA in early sea-urchin embryos: cytoplasmic particles. Science. 1965 Oct 8;150(3693):214–217. doi: 10.1126/science.150.3693.214. [DOI] [PubMed] [Google Scholar]
- Tepperman K., Morris G., Essien F., Heywood S. M. A mechanical dissociation method for preparation of muscle cell cultures. J Cell Physiol. 1975 Dec;86(3 Pt 1):561–565. doi: 10.1002/jcp.1040860313. [DOI] [PubMed] [Google Scholar]
- Thireos G., Griffin-Shea R., Kafatos F. C. Untranslated mRNA for a chorion protein of Drosophila melanogaster accumulates transiently at the onset of specific gene amplification. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5789–5793. doi: 10.1073/pnas.77.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Civelli O., Buri J. F., Scherrer K. Correlation of specific coding sequences with specific proteins associated in untranslated cytoplasmic messenger ribonucleoprotein complexes of duck erythroblasts. FEBS Lett. 1977 May 15;77(2):281–286. [PubMed] [Google Scholar]
- Wilson T., Papahadjopoulos D., Taber R. Biological properties of poliovirus encapsulated in lipid vesicles: antibody resistance and infectivity in virus-resistant cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3471–3475. doi: 10.1073/pnas.74.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]