Abstract
Uridine 5′-diphosphate-glucose (UDP-Glc) is transported into the lumen of the Golgi cisternae, where is used for polysaccharide biosynthesis. When Golgi vesicles were incubated with UDP-[3H]Glc, [3H]Glc was rapidly transferred to endogenous acceptors and UDP-Glc was undetectable in Golgi vesicles. This result indicated that a uridine-containing nucleotide was rapidly formed in the Golgi vesicles. Since little is known about the fate of the nucleotide derived from UDP-Glc, we analyzed the metabolism of the nucleotide moiety of UDP-Glc by incubating Golgi vesicles with [α-32P]UDP-Glc, [β-32P]UDP-Glc, and [3H]UDP-Glc and identifying the resulting products. After incubation of Golgi vesicles with these radiolabeled substrates we could detect only uridine 5′-monophosphate (UMP) and inorganic phosphate (Pi). UDP could not be detected, suggesting a rapid hydrolysis of UDP by the Golgi UDPase. The by-products of UDP hydrolysis, UMP and Pi, did not accumulate in the lumen, indicating that they were able to exit the Golgi lumen. The exit of UMP was stimulated by UDP-Glc, suggesting the presence of a putative UDP-Glc/UMP antiporter in the Golgi membrane. However, the exit of Pi was not stimulated by UDP-Glc, suggesting that the exit of Pi occurs via an independent membrane transporter.
Glycosyltransferases, key enzymes in the biosynthesis of polysaccharides, utilize nucleotide sugars as substrates in this essential cellular process (Brett and Waldron, 1996). Different lines of evidence suggest that polysaccharide biosynthesis takes place in the lumen of the Golgi cisternae, and that transport of nucleotide sugars into the lumen is required to make the substrate available to the glycosyltransferases (Zhang and Staehelin, 1992; Muñoz et al., 1996). Once in the lumen, the sugar is transferred to endogenous acceptors, which can be polysaccharides at various stages of synthesis, glycoproteins, or glycolipids. However, in cells from elongating tissues, the activity of the Golgi apparatus is mainly dedicated to pectin and hemicellulose synthesis (Driouch et al., 1993). The other product of the transfer reaction is the nucleoside diphosphate, which is usually UDP, but it can be GDP or CDP as well (Brett and Waldron, 1996).
UDP and other nucleoside diphosphates have been described as inhibitors of glycosyltransferases (Ray et al., 1969; Fredrikson and Larsson, 1992) and therefore they need to be metabolized to avoid inhibition of polysaccharide biosynthesis. A Golgi nucleoside diphosphatase is located in the Golgi membrane with its active site facing the lumen of Golgi cisternae. For many years its activity has been correlated with polysaccharide biosynthesis, suggesting that it could play a role in this process (Dauwalder et al., 1969; Ray et al., 1969; Mitsui et al., 1994; Orellana et al., 1997). The products of the reaction catalyzed by this enzyme are UMP and Pi. According to our current hypothesis of the topology of reactions leading to polysaccharide biosynthesis in the Golgi apparatus, these metabolites should be produced in the lumen of the Golgi cisternae and then return to the cytoplasm, otherwise they would accumulate in this compartment, inhibiting the synthesis of additional polysaccharides. However, we do not know whether UMP is formed in the Golgi vesicles and then further metabolized to uridine and Pi. Previous studies from our laboratory showed that the incorporation of UDP-Glc into the lumen of Golgi vesicles was coupled to the exit of a uridine-containing nucleotide (Muñoz et al., 1996), suggesting an antiporter mechanism. In addition, these data also demonstrate that the nucleotide moiety exits the Golgi cisternae, however, the exact nature of this uridine-containing nucleotide was not known.
Since the metabolism of the nucleotide moiety of nucleotide sugars in the lumen of the Golgi cisternae may have an impact on the synthesis of polysacccharides in this organelle, we decided to analyze the metabolism of UDP-Glc in Golgi vesicles from the elongating region of etiolated pea (Pisum sativum L.) stems, focusing on the nucleotide moiety. The results in this study demonstrate that upon incorporation of UDP-Glc into Golgi vesicles, Glc is quickly transferred to endogenous acceptors, and UMP and Pi are formed in the lumen of Golgi vesicles as end products with no further hydrolysis of UMP. The results also show that UMP exits the vesicles by a mechanism that is stimulated by the entry of UDP-Glc, whereas Pi leaves the vesicles by a mechanism that is independent of the entry of UDP-Glc, suggesting that a putative UDP-Glc/UMP antiporter and Pi transporter could be localized in the Golgi membrane.
MATERIALS AND METHODS
Materials
[γ-32P]ATP (3000 Ci/mmol), [α-32P]UTP (3000 Ci/mmol), and [5,63H]UTP (37 Ci/mmol) were purchased from DuPont-NEN. UDP-[3H]Glc (4.5 Ci/mmol) was purchased from Amersham. Nonradioactive UDP, UDP-Glc, and buffer reagents were purchased from Sigma. Suc was high purity and obtained from ICN. Proteinase K was from Merck (Darmstadt, Germany). PEI-cellulose TLC plates were purchased from Aldrich.
Preparation of a Golgi-Enriched Vesicle Fraction
Pea (Pisum sativum var Alaska) seedlings were grown in moist vermiculite for 7 to 8 d in the dark at 25°C. Stem segments (1 cm) were excised from the elongating region of the hypocotyls. Vesicles were obtained as described by Muñoz et al. (1996) and Orellana et al. (1997). Pea stems were homogenized using razor blades in the presence of 1 volume of 0.5 m Suc, 0.1 m KH2PO4, pH 6.65, 5 mm MgCl2, and 1 mm DTT. The homogenate was filtered through Miracloth (Calbiochem) and centrifuged at 1,000g for 5 min. The supernatant was loaded onto a 1.3 m Suc cushion and centrifuged at 100,000g for 70 min. The upper phase was removed, leaving, and without disturbing, the interface fraction. On top of this, a discontinuous gradient was formed by adding 1.1 and 0.25 m Suc cushions and centrifuged for 90 min at 100,000g. The interface 0.25/1.1 m Suc fraction was collected, diluted in 1 volume of distilled water, and centrifuged at 100,000g for 35 min. The pellet was resuspended, using a Dounce homogenizer, in STM buffer containing 0.25 m Suc, 1 mm MgCl2, and 10 mm Tris-HCl, pH 7.5 (Suc-Tris-Mg buffer). The vesicles were kept at −70°C until use. All of the procedures were performed on ice or at 4°C. The UDPase latency of the vesicles varied from different preparations between 85 and 90%. The characterization of this fraction using marker enzymes has been described previously (Muñoz et al., 1996).
UDPase Activity Detected in Native Polyacrylamide Gels
Native PAGE and detection of UDPase activity was done as described by Bollag and Edelstein (1991). A 10% acrylamide gel containing the samples was run for 4 h at 80 V. Afterward, the gel was incubated for 15 min at 37°C in a solution containing 0.1 m Tris-Maleate, 1.5 mm Pb(NO3)2, 10 mm MgSO4, 5% Glc, and 3 mm UDP. Then the gel was rinsed with several changes of water and the active band was visualized with 1% (NH4)2S.
Synthesis and Purification of [α-32P]UDP-Glc, [β-32P]UDP-Glc, and [3H]UDP-Glc
Synthesis of [β-32P]UDP-Glc was done as described by Orellana et al. (1997). This method consists of the transfer of the γ-phosphate present in [γ-32P]ATP to Glc-1-P in a series of reactions catalyzed by hexokinase and phosphoglucomutase. Then the phosphate group present in Glc-1-32P is transferred to UDP-Glc through the reaction catalyzed by the UDP-Glc pyrophosphorylase coupled to inorganic pyrophosphatase. Synthesis of [α-32P]UDP-Glc and [3H]UDP-Glc was done by using [α-32P]UTP and [3H]UTP, respectively, in the reaction catalyzed by the UDP-Glc pyrophosphorylase coupled with the removal of pyrophosphate by the inorganic pyrophosphatase, exactly as described by Muñoz et al. (1996). The radioactive products were purified as described by Dhugga and Ray (1994) and Muñoz et al. (1996) using charcoal. Purity was checked by TLC using PEI-cellulose plates using 1 n acetic acid as the mobile phase for 3 cm, and then 1 n acetic acid-LiCl 3 m (90:10, v/v) for 15 cm. Purity was also verified by HPLC, demonstrating that the radioactive compounds exhibit the same retention time as standard UDP-Glc.
Metabolism of Nucleotide-Radiolabeled UDP-Glc
Golgi vesicles (100 μg of protein) were incubated with 1 μm [β-32P]UDP-Glc, [α-32P]UDP-Glc, or [3H]UDP-Glc in a medium containing 0.25 m Suc, 1 mm MgCl2, and 10 mm Tris-HCl, pH 7.5. After incubation the reaction was heat inactivated by placing the samples in a boiling water bath for 1 min and then on ice. The samples were analyzed by TLC using the same mobile system described above. When [3H]UDP-Glc was used, a control was carried on using vesicles that were previously boiled for 3 min. After the TLC separation the plate was cut in 1-cm strips and counted in a liquid-scintillation counter. When [β-32P]UDP-Glc or [α-32P]UDP-Glc was used, the TLC plate was exposed to autoradiography with an enhancing screen at −70°C. The migration of labeled compounds on TLC was compared with possible products derived from UDP-Glc hydrolysis.
UDP-Glc Incorporation into Golgi Vesicles
Incorporation of [β-32P]UDP-Glc, [α-32P]UDP-Glc, [3H]UDP-Glc, and UDP-[3H]Glc into Golgi vesicles was measured exactly as described by Muñoz et al. (1996). One hundred micrograms of protein was incubated with 1 μm radiolabeled UDP-Glc (1 × 106 cpm/mL) at 25°C. The reaction was stopped by diluting with 10 volumes of ice-cold STM buffer and filtering through 0.7-μm glass fiber filters in a filtration system (model FH225V, Hoeffer, Piscataway, NJ). Afterward, the filters were washed with an additional 10 volumes of ice-cold STM buffer and dried, and the radioactivity was determined by liquid-scintillation counting. The incorporation of [3H]Glc into 70% ethanol or 10% TCA-insoluble material was measured by incubating Golgi vesicles with UDP-[3H]Glc exactly as described above, but the reaction was stopped by adding ethanol or TCA to reach the appropriate concentration. The samples were kept on ice and then filtered through 0.7-μm glass fiber filters. The filters were washed and dried, and the radioactivity was determined by liquid-scintillation counting.
Analysis of Radiolabeled Metabolites Associated with the Golgi Vesicles or the Extravesicular Medium
Golgi vesicles (100 μg of protein) were incubated with [β-32P]UDP-Glc for 10 min at 25°C. The reaction was stopped by adding 10 volumes of STM buffer, and the vesicles were immediately separated from the incubation medium by filtration on 0.7-μm glass fiber filters using the filtration system described above. The material not retained by the filters was collected in tubes and an aliquot of this material was then analyzed by TLC and autoradiography as described above. The radiolabeled metabolites associated with the vesicles were extracted from them following the procedure described by Waldman and Rudnick (1990). The vesicles retained in the filters were boiled for 90 s in 1 mL of a solution containing 1% SDS, 100 mm NaCl, 0.5 μm UDP-Glc, 0.5 μm Pi, and 0.02% NaN3. The samples were put on ice and aliquots were immediately taken for analysis on TLC using PEI plates.
Enzyme and Protein Assays
UDPase in the presence and in the absence of 0.1% (v/v) Triton X-100 was measured as described by Briskin et al. (1987). Protein concentrations were measured by the BCA method (Pierce).
RESULTS
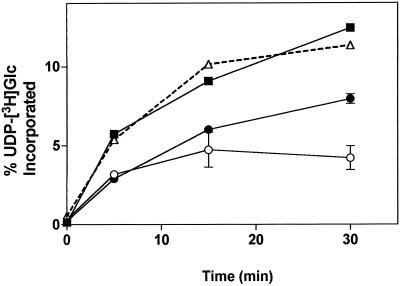
UDP-Glc is transported into the lumen of Golgi cisternae and utilized for the synthesis of polysaccharides (Muñoz et al., 1996). This poses the question of whether UDP-Glc is accumulated and concentrated in the lumen of the Golgi apparatus, as is the case in the mammalian Golgi apparatus (Hirschberg and Snider, 1987), or if it is immediately metabolized by glucosyltransferase(s). To address this question we studied the transfer of [3H]Glc onto endogenous acceptors after incubation of Golgi vesicles with UDP-[3H]Glc. At different incubation times most of the radioactivity incorporated from the transport of UDP-[3H]Glc into Golgi vesicles could be accounted for by the transfer of [3H]Glc into ethanol- and TCA-insoluble material (Fig. 1). This result indicates that once in the lumen of the Golgi apparatus, UDP-Glc is quickly metabolized and the concentration of soluble UDP-Glc in the Golgi lumen is undetectable.
Figure 1.
Incorporation of UDP-[3H]Glc into Golgi vesicles, and determination of radiolabeled products insoluble in 70% ethanol and 10% TCA. Golgi vesicles (100 μg of protein) were incubated with 1 μm (100,000 cpm) UDP-[3H]Glc for various times. The reaction was stopped by separating the vesicles from the incubation medium by filtration (▪), or by adding ethanol (○) or TCA (•) to a final concentration of 70 or 10%, respectively. The samples were put on ice and filtered, and the radioactivity was determined by liquid-scintillation counting. The total amount of insoluble radioactivity was determined by the summation of the 70% ethanol and 10% TCA-insoluble material (▵). The results are expressed as the percentage of the total radioactivity in UDP-[3H]Glc initially present in the assay. The experiment was repeated three times in duplicate, and the average values and their deviations are plotted. A representative experiment is shown.
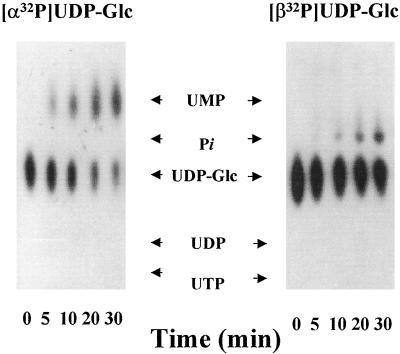
Since UDP-Glc was rapidly metabolized by the Golgi vesicles, our study focused on the fate of UDP, the other product of the transfer reaction. To study the metabolism of the nucleotide moiety of UDP-Glc we synthesized UDP-Glc radiolabeled with 32P in the α or β phosphate groups, and with 3H in the uridine ring, and with these substrates we could follow the formation of different metabolites generated during the metabolism of the nucleotide in the Golgi vesicles. The possible radiolabeled products that could be formed by the metabolism of these substrates are shown in Figure 2. When Golgi vesicles were incubated with [α-32P]UDP-Glc, the radiolabeled nucleotide sugar decreased and [α-32P]UMP increased, accumulating with time (Fig. 3). Neither [α-32P]UDP nor 32Pi was observed in this experiment. The absence of 32Pi in this experiment suggests that [α-32P]UMP was not further hydrolyzed to uridine plus Pi. When [β-32P]UDP-Glc was used, we observed a decrease in [β-32P]UDP-Glc and an increase and an accumulation of 32Pi with time (Fig. 3), corroborating our previous results (Orellana et al., 1997). Again, no [β-32P]UDP was observed under this condition. Moreover, in this work we could not detect Glc-1-32P, a possible breakdown product of UDP-Glc by organic pyrophosphatases, suggesting that the formation of Pi and UMP did not occur by a mechanism mediated by Glc-1-phosphate. This idea is also supported by the fact that we could not detect phosphatase activity in the Golgi vesicles when Glc-1-P was used as a substrate (Orellana et al., 1997).
Figure 2.
UDP-Glc radiolabeled at various positions and the potential resulting metabolic products for each. UDP-Glc radiolabeled in the uridine ring with tritium (a), or with 32P in the α (b) and β (c) phosphate groups, were prepared as described in Methods. The possible radiolabeled products derived from their metabolism are shown in each case.
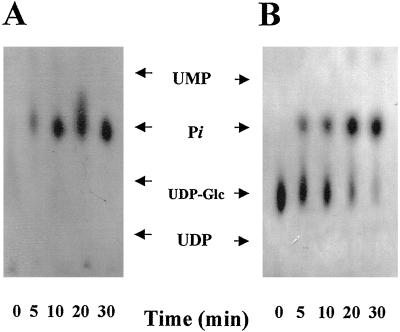
Figure 3.
Metabolism of [α-32P]UDP-Glc and [β-32P]UDP-Glc in pea stem Golgi vesicles (100 μg of protein) incubated with 1 μm (1 μCi/nmol) of [α-32P]UDP-Glc or [β-32P]UDP-Glc. After the indicated times an aliquot equivalent to 2 μg of protein was taken, boiled for 1 min, and loaded on a PEI-TLC plate. The TLC plate was run as described in “Materials and Methods,” air-dried, and exposed to −70°C with an enhancing screen. The migration of standards is shown with arrows. The uridine derivative standard was visualized by UV absorption and the Pi migration standard was visualized using 32Pi.
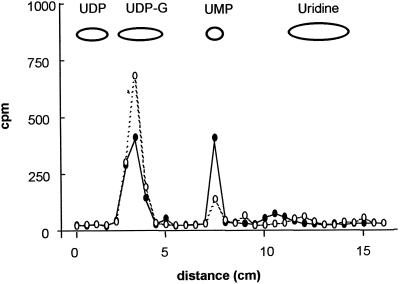
Finally, we incubated Golgi vesicles with UDP-Glc radiolabeled in the uridine ([3H]UDP-Glc). After a 10-min incubation the radiolabeled nucleotide decreased and [3H]UMP increased (Fig. 4). Different experiments did not show the appearance of [3H]UDP or [3H]uridine. Taking together all of the results described above, we conclude that upon incubation of Golgi vesicles with UDP-Glc, UDP-Glc is quickly metabolized, resulting in the transfer of the sugar onto endogenous acceptors and the generation of UMP and Pi as the end products of the metabolism of the nucleotide moiety by Golgi vesicles. The most likely explanation for the formation of these end products was the hydrolysis of UDP, one of the products of the Glc transfer reaction, to UMP plus Pi by the Golgi UDPase.
Figure 4.
Metabolism of [3H]UDP-Glc by pea stem Golgi vesicles. Golgi vesicles (100 μg of protein) were incubated with [3H]UDP-Glc (3 μCi/nmol) for 10 min (•). As a control, Golgi vesicles previously boiled for 3 min were incubated under the same conditions (○). After 10 min an aliquot of each sample equivalent to 2 μg of protein was taken, boiled for 1 min, and loaded onto a PEI-TLC plate. The plate was run, air-dried, and cut into 0.5-cm fragments. The radioactivity associated with each fragment was determined by liquid-scintillation counting. The migration of standards is depicted.
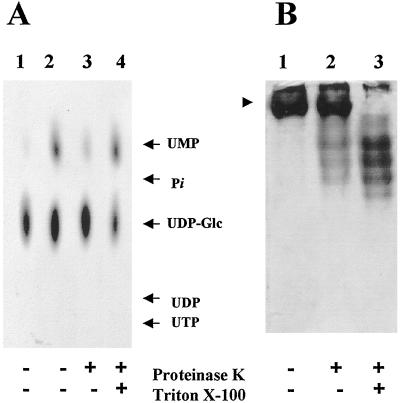
If UMP and Pi were generated by UDP hydrolysis due to the Golgi UDPase activity, these metabolites should be formed in the lumen of Golgi vesicles, where the catalytic site of the Golgi UDPase is located (Orellana et al., 1997). To test whether UMP was produced in the lumen of Golgi vesicles, we proteolytically blocked the transport of UDP-Glc into Golgi vesicles by a mild treatment with proteinase K under conditions that do not disrupt the vesicles or affect the activity of the enzymes located in the lumen (Muñoz et al., 1996; Orellana et al., 1997). When the formation of UMP in the proteolyzed vesicles was analyzed and compared with the control, we found a large decrease in the formation of this metabolite (Fig. 5A). To ensure that the decrease of the formation of UMP was due to a lack of UDP-Glc transport into the Golgi vesicles and not to the proteolytic inactivation of the enzymes involved in its formation, we permeabilized the protease-treated Golgi vesicles to bypass the transport step. When we did so, formation of UMP was restored, indicating that UMP was formed in the lumen of Golgi vesicles and that UDP-Glc transport was required. To demonstrate that proteolysis did not affect the activity of lumenal enzymes we analyzed the topology of Golgi UDP- ase, a lumenal enzyme, corroborating our previous results (Orellana et al., 1997) (Fig. 5B).
Figure 5.
Topology of UMP formation in Golgi vesicles. A, Golgi vesicles (100 μg of protein) were incubated in the presence (+) or absence (−) of proteinase K (20 μg) for 30 min at 30°C. The reaction was stopped with 1 mm PMSF, and then the vesicles were incubated on ice for 10 min in the presence or in the absence of 0.1% Triton X-100. The vesicles were then incubated with 1 μm (1.0 μCi/nmol) [α-32P]UDP-Glc for 10 min. The products formed during the incubation were analyzed by TLC using PEI plates and exposed to autoradiography at −70°C with an enhancing screen. Lanes: 1, Heat-inactivated Golgi vesicles; 2, normal Golgi vesicles; 3, proteolyzed Golgi vesicles; and 4, Golgi vesicles subjected to proteolysis and further permeabilization. Migration of standards is shown by arrows. B, Topology of Golgi UDPase. Sealed or Triton X-100-permeabilized Golgi vesicles were incubated in the presence of proteinase K using the same conditions as described in A. The samples were then separated in native gels and the UDPase activity was determined in situ as described in Methods. Lanes: 1, Untreated Golgi vesicles; 2, sealed Golgi vesicles treated with proteinase K; and 3, permeabilized Golgi vesicles treated with proteinase K. The arrowhead indicates the migration of Golgi UDPase in the native gel.
The results discussed above suggest that UMP and Pi are formed in the lumen of Golgi cisternae and that they are not metabolized further by Golgi vesicles. This situation could result in the accumulation of UMP and Pi in the lumen, unless they exit the vesicles. To address this point we analyzed both the kinetics of incorporation of the different radiolabeled substrates and the content of the Golgi vesicles incubated with UDP-Glc. Figure 6 shows that incorporation of [α-32P]UDP-Glc was rapid at the beginning but that it reached equilibrium by 5 min. This behavior was similar to the incorporation of [3H]UDP-Glc shown previously (Muñoz et al., 1996). However, when [β-32P]UDP-Glc was used, its incorporation also reached a rapid equilibrium, although there was a slight difference in the kinetic behavior compared with the other UDP-Glc-radiolabeled substrates. These results suggest that the metabolites of the nucleotide moiety of UDP-Glc generated in the lumen of the vesicles were not accumulated in that compartment and that they were transported out to the extravesicular medium. To further investigate this point we analyzed at different times the content of both the Golgi vesicles and the extravesicular medium after incubation with [β-32P]UDP-Glc. When the vesicle content was analyzed, we detected only Pi, whereas UDP-Glc was not detected at all (Fig. 7A). Radiolabeled 32Pi increased during the first 10 min and then remained constant. No accumulation of Pi was detected in the vesicles. When the extravesicular medium was analyzed, both UDP-Glc and Pi were detected. UDP-Glc decreased with time, whereas Pi increased (Fig. 7B). From this result and the result shown in Figure 5, we conclude that Golgi vesicles incubated with [β-32P]UDP-Glc produced Pi in the lumen of the vesicles, which was then exported out of the vesicular lumen.
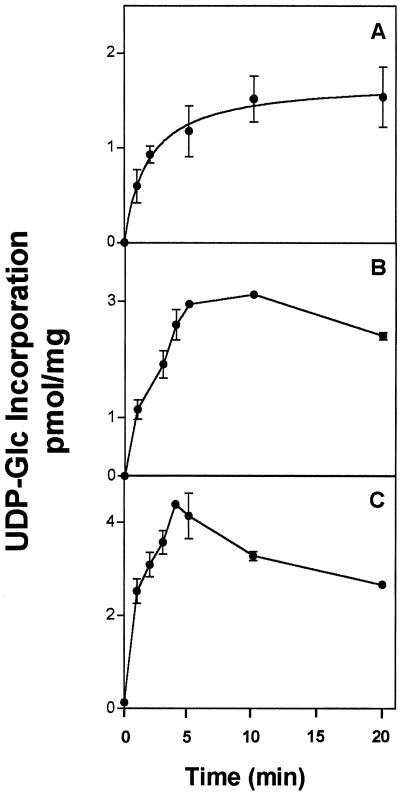
Figure 6.
Incorporation of [α-32P]UDP-Glc and [β-32P]UDP-Glc into pea stem Golgi vesicles. Golgi vesicles (200 μg of protein) were incubated with 1 μm (3.0 μCi/nmol) of [β-32P]UDP-Glc (A) or [α-32P]UDP-Glc (B) for different periods of time. The reaction was stopped by filtration through 0.7-μm glass fiber filters, and the amount of radioactivity associated with the filters was determined using liquid-scintillation counting. The measurements were done in triplicate, and the average and its deviation are depicted in the figure. C, Incorporation of [3H]UDP-Glc into pea stem Golgi vesicles from Muñoz et al. (1996).
Figure 7.
Metabolites associated with Golgi vesicles upon incubation with [β-32P]UDP-Glc. Golgi vesicles (100 μg of protein) were incubated with 1 μm (3.0 μCi/nmol) [β-32P]UDP-Glc for different periods of time. The incubation was stopped by filtration. A, The solutes associated with the vesicles were obtained by solubilizing the material associated with the filters and then analyzed by TLC on PEI plates. B, The solutes not associated with the vesicles were collected from the flow-through fraction of the filtration step and analyzed by TLC using PEI plates. In both cases the plates were exposed to autoradiography at −70°C with an enhancing screen; however, the exposure times were 8 d in A and 2 d in B. The migration of standards is shown by arrows.
The above results suggest that UMP and Pi are formed in the lumen of the Golgi vesicles and are exported out of the vesicles to the extravesicular medium. To investigate the mechanism by which UMP and Pi exit the vesicles we incubated Golgi vesicles with the different UDP-Glc nucleotide-radiolabeled substrates for 10 min, the time at which the incorporation reached equilibrium and the content of radiolabeled metabolites in the vesicles was UMP and Pi. To evaluate whether UDP-Glc promoted the exit of these metabolites we gave a pulse of unlabeled UDP-Glc and measured the radioactivity that remained associated with the vesicles 3 and 5 min after the pulse (Fig. 8). When we used either UDP-[3H]Glc or [α-32P]UDP-Glc, we observed a decrease in the radioactivity associated with the vesicles, suggesting that UDP-Glc stimulated the efflux of UMP. In contrast, when [β-32P]UDP-Glc was used as a substrate and 32Pi was in the lumen, we did not see a decrease in the radioactivity associated with the vesicles. A minor increase was observed but different experiments indicated that it was not significant. These results demonstrate that the radioactive metabolites derived from the nucleotide moiety of UDP-Glc exit the vesicles by different mechanisms. When the vesicles contained radiolabeled UMP, the efflux of this metabolite was stimulated by UDP-Glc, suggesting an exchange between UDP-Glc and UMP by an antiporter mechanism. In contrast, when the vesicles contained Pi, its efflux was not stimulated by UDP-Glc, suggesting that Pi exits the vesicles by a mechanism different from that of UMP.
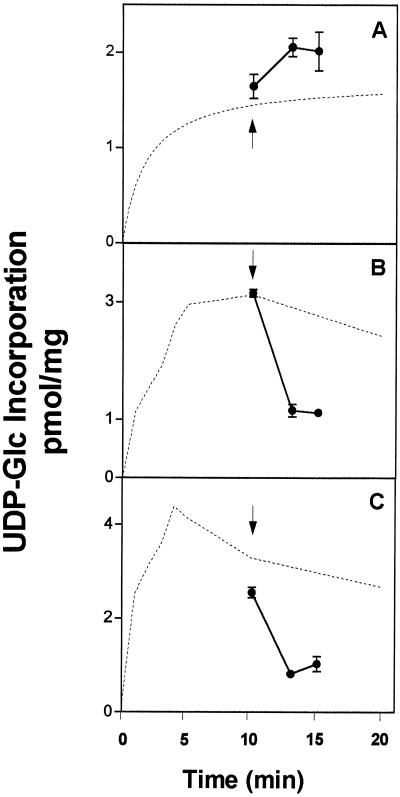
Figure 8.
UDP-Glc-induced efflux of radiolabeled solutes associated with Golgi vesicles. Golgi vesicles (300 μg of protein) were incubated for 10 min with 1 μm (3 μCi/nmol) [β-32P]UDP-Glc (A), [α-32P]UDP-Glc (B), or [3H]UDP-Glc (C). After the incubation a pulse of cold UDP-Glc (1 mm) was added to each sample (indicated with an arrow), and the incubation continued for 0, 3, or 5 min. Finally, the incubations were stopped by filtration, and the radioactivity that remained associated with the vesicles after the pulse of UDP-Glc was determined by liquid-scintillation counting. The amount of solutes associated with the vesicles is expressed in equivalents of UDP-Glc at the beginning of the incubation. The measurements were done in triplicate and the average and its deviation are depicted in the figure. The incorporation of the various substrates, obtained from Figure 6, is depicted with a broken line.
DISCUSSION
UDP-Glc is transported to the lumen of Golgi cisternae and Glc is transferred to endogenous acceptors, mainly polysaccharides, in a reaction(s) catalyzed by glucosyltransferase(s). Based on the data contained in this paper and information from previous work regarding the transport of UDP-Glc (Muñoz et al., 1996) and the function of Golgi UDPase (Orellana et al., 1997), our current working hypothesis on UDP-Glc metabolism and polysaccharide biosynthesis in the Golgi apparatus is depicted in Figure 9. According to this model, the mechanism of the biosynthesis of Glc-containing polysaccharides in elongating tissues can be envisioned as the following cycle: biosynthesis and further transport of the nucleotide sugar into the Golgi cisternae, resulting in the incorporation of Glc into macromolecules in the lumen of Golgi cisternae; the nucleotide moiety is then quickly converted into UMP and Pi, metabolites that return to the cytoplasm. One of the important concepts determined from this model is that glycosyltransferases are not the only elements involved in Glc-containing polysaccharide biosynthesis. The nucleotide sugar transporters themselves could play an essential role in polysaccharide biosynthesis by allowing the substrates to reach the compartment where the transferases for polysaccharide biosynthesis are located. Support for such a role has been provided in mammals, where Madin-Darby canine kidney cells, mutants in a Golgi UDP-Gal transporter, are unable to synthesize keratan sulfate, a galactosylated proteoglycan (Toma et al., 1996). Whether the nucleotide sugar transporter or the transferase is the limiting step for the rate of polymer formation in plants is a question that still remains to be answered.
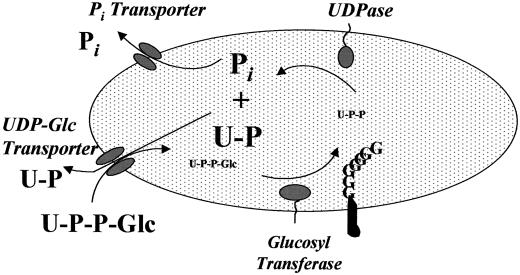
Figure 9.
Model for UDP-Glc metabolism in Golgi vesicles. UDP-Glc is transported into the lumen of Golgi cisternae, Glc is immediately transferred to polymers at different stages of growth, and UDP is released. However, this product is immediately hydrolyzed by Golgi UDPase, producing UMP and Pi. Although a pool of both metabolites can be observed in the Golgi cisternae, they do not accumulate and instead exit the Golgi cisternae. The exit of UMP is stimulated by UDP-Glc, suggesting an exchange mechanism, whereas Pi exits the Golgi cisternae by an independent mechanism.
UDP-Glc is neither accumulated nor concentrated in the lumen of Golgi vesicles, and Glc is rapidly transferred to endogenous acceptors, which are most likely cell wall polymers at different growing states. Given the rapid transfer of Glc onto acceptors, the process of sugar transfer must also be associated with the release of the nucleotide moeity from UDP-Glc. However, when we used UDP-Glc substrates radiolabeled in the nucleotide moiety, UDP was not detected and we only detected UMP and Pi. This poses the question of why UDP was not detected in the assays. Our possible explanation for this finding is that UDP would be generated and then quickly metabolized to form UMP and Pi in a reaction catalyzed by a Golgi-specific nucleoside diphosphatase (UDPase) localized in the lumen of the cisternae (Orellana et al., 1997). This is in agreement with the fact that UMP and Pi are indeed produced in the lumen of the Golgi vesicles. Moreover, UDPase is highly active in the elongating region of pea stems, correlating with a high rate of polysaccharide biosynthesis in the Golgi (Orellana et al., 1997).
However, a similar role for Golgi UDPase has been postulated in rat mammary glands during the synthesis of lactose. During this process, Gal is transferred from UDP-Gal and UMP is formed and accumulated without detecting accumulation of UDP (Khun and White, 1977). More evidence that supports a role for UDPase in sugar polymer biosynthesis comes from yeast, in which the deletion of the GDA-1 gene that codes for a Golgi GDPase produces an important decrease in mannosylation of proteins and glycolipids (Abeijon et al., 1993). An alternative explanation for the formation of UMP and Pi would be that Glc transfer is mediated by a Glc-phosphate intermediate, followed by its hydrolysis to release Pi. Although in previous work we have detected in some cases low levels of Glc-1-P, these results are not reproducible, and it changes from preparation to preparation. This is in contrast to UMP and Pi formation, which are absolutely reproducible. This suggests that the presence of Glc-1-P observed in some experiments could be due to contamination of organic pyrophosphatase, which breaks down UDP-Glc (Feingold and Avigad, 1980). More importantly, we could not detect any phosphatase activity toward Glc-1-P (Orellana et al., 1997), suggesting that production of Pi from UDP-Glc does not involve a Glc-phosphate intermediate. Taking all of this evidence together, we favored a process mediated by UDPase to explain the formation of UMP and Pi.
UMP and Pi do not accumulate in the lumen of Golgi vesicles. Instead, they reach an equilibrium that is maintained by the constant formation of UMP and Pi from UDP-Glc and the exit of both metabolites to the external medium. How do UMP and Pi leave the lumen of Golgi cisternae? Our results show that the exit of UMP is stimulated by the addition of UDP-Glc, supporting the hypothesis that UMP leaves the lumen by an exchange with incoming cytosolic UDP-Glc. Although we do not know whether UMP stimulates the entrance of UDP-Glc, based on the characteristic found in all nucleotide sugar transporters located in the Golgi membrane described to date (Hirschberg, 1996), we hypothesize that the UDP-Glc transporter would correspond to a UDP-Glc/UMP antiporter. Since UDP-Glc is rapidly metabolized, a constant influx of this substrate into the lumen is required. Therefore, it would be necessary to have a rapid and constant supply of UMP in the lumen. This would be provided by the hydrolysis of UDP catalyzed by UDPase. From this point of view, this enzyme plays a critical role in the mechanism because it drives the transfer reaction by consuming UDP, eliminates an inhibitor for glucosyltransferases, and provides UMP as the substrate for the putative antiporter, allowing the entrance of additional UDP-Glc. Thus, UDPase would play an important role in polysaccharide biosynthesis, explaining at the same time the high activity of this enzyme in the elongating region of a growing plant (Orellana et al., 1997).
Previous results from our laboratory indicated that the efflux of a [3H]uridine-containing nucleotide from Golgi vesicles was specifically driven by UDP-Glc and other UDP-sugars involved in polysaccharide biosynthesis, such as UDP-Gal and UDP-Xyl. Other nucleotide sugars such as GDP-Fuc and ADP-Glc did not promote this efflux (Muñoz et al., 1996). In this work we have demonstrated that the [3H]uridine-containing nucleotide corresponds to UMP; therefore, the efflux of UMP is specifically stimulated by uridine-derivative nucleotide sugars involved in polysaccharide biosynthesis in the Golgi. Since many of the substrates required for polysaccharide biosynthesis are uridine-derived nucleotide sugars, and since an active Golgi UDPase is found in the lumen, the supply of UMP and Pi to their respective lumenal pools may occur from a number of substrates used in both hemicellulose and pectin biosynthesis. Therefore, it is possible to postulate that many, if not all, nucleotide sugars that are transported into the lumen of Golgi cisternae may use an antiporter mechanism.
To our knowledge, this is the first time direct evidence has been provided for the formation of Pi and its further exit from Golgi vesicles when a nucleotide sugar is metabolized in the Golgi. The mechanism used by Pi to leave the Golgi cisternae is not directly stimulated by UDP-Glc, and a putative Pi transporter could be responsible for its exit. Whether the movement of phosphate through the Golgi membrane is driven by a concentration gradient of Pi is not yet known. Moreover, we do not know the size of the lumenal pool of Pi in the Golgi cisternae or the free concentration of Pi in the cytoplasm of meristematic cells. Therefore, it is difficult to predict whether a concentration gradient is the only driving force for the exit of phosphate. The fact that Pi is not accumulated in the lumen and is instead rapidly exported suggests that the concentration of Pi in the lumen must be kept low. In fact, preliminary data from our laboratory demonstrate that a concentration as low as 1 mm Pi can inhibit glucan synthase I, suggesting that the Pi concentration in the lumen has to be low.
To test this model it is important to characterize the different elements involved. Recently, the cloning of different Golgi nucleotide sugar transporters has been described (Abeijon et al., 1996; Eckhardt et al., 1996; Miura et al., 1996; Ma et al., 1997), as well as a Golgi GDPase in yeast (Abeijon et al., 1993); unfortunately, no homologous genes in plants have been described so far. The cloning of nucleotide sugar transporters and Golgi UDPase in plants will be an important tool to test this model.
ACKNOWLEDGMENTS
We would like to thank Lorena Norambuena for technical support, Dr. Caroline Clairmont for reading the manuscript, Dr. Mario Mera (Instituto de Investigaciones Agropecuarias-Carillanca, Temuco) for providing the seeds, and the people from the Laboratory of Membranes, Department of Biology, Faculty of Sciences, University of Chile, for helpful discussions.
Abbreviation:
- PEI
polyethylene imide
Footnotes
This work was supported by grant no. 1970494 from Fondecyt (Chile).
LITERATURE CITED
- Abeijon C, Robbins PW, Hirschberg CB. Molecular cloning of the Golgi apparatus uridine diphosphate-N-acetylglucosamine transporter from Kluyveromyces lactis. Proc Natl Acad Sci USA. 1996;93:5963–5968. doi: 10.1073/pnas.93.12.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeijon C, Yanagisawa K, Mandon EC, Häusler A, Moremen K, Hirschberg CB, Robbins PW. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J Cell Biol. 1993;122:307–323. doi: 10.1083/jcb.122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag DM, Edelstein SJ. Gel electrophoresis under nondenaturing conditions. In: Edelstein SJ, Bollag DM, editors. Protein Methods. Inc., New York: Wiley-Liss; 1991. pp. 143–160. [Google Scholar]
- Brett CT, Waldron KW. Cell wall formation. In: Brett C, Waldron K, editors. Physiology and Biochemistry of Plant Cell Walls, Ed 2. London: Chapman & Hall; 1996. , pp75–111. [Google Scholar]
- Briskin DP, Leonard RT, Hodges TK. Isolation of the plasma membrane: membrane markers and general principles. Methods Enzymol. 1987;148:542–558. [Google Scholar]
- Dauwalder M, Whaley WG, Kephart JE. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969;4:455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Dhugga KS, Ray PM. Purification of 1,3-β-d-glucan synthase activity from pea tissue. Eur J Biochem. 1994;220:943–953. doi: 10.1111/j.1432-1033.1994.tb18698.x. [DOI] [PubMed] [Google Scholar]
- Driouch A, Faye L, Staehelin A. The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci. 1993;18:210–214. doi: 10.1016/0968-0004(93)90191-o. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Mühlenhoff M, Bethe A, Gerardy-Schahn R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc Natl Acad Sci USA. 1996;93:7572–7576. doi: 10.1073/pnas.93.15.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold DS, Avigad G (1980) Sugar nucleotide transformations in plants. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 3. Academic Press, New York, pp 101–170
- Fredrikson K, Larsson C. Activators and inhibitors of the plant plasma membrane 1,3-β-glucan synthase. Biochem Soc Trans. 1992;20:710–713. doi: 10.1042/bst0200710. [DOI] [PubMed] [Google Scholar]
- Hirschberg CB (1996) Transporters of nucleotides and nucleotide derivatives in the endoplasmic reticulum and Golgi apparatus. In DE Clapham, BE Ehrlich, eds, Organellar Ion Channels and Transporters. Society of General Physiology Series 51, Rockefeller University Press, New York, pp 105–120 [PubMed]
- Hirschberg CB, Snider MD. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Kuhn N, White A. The role of nucleoside diphosphatase in a uridine associated cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977;168:423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Russell DG, Beverley SM, Turco SJ. Golgi GDP-mannose uptake requires Leishmania LPG2: a member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem. 1997;272:3799–3805. [PubMed] [Google Scholar]
- Mitsui T, Honma M, Kondo T, Hashimoto N, Kimura S, Igaue I. Structure and function of the Golgi complex in rice cells. Plant Physiol. 1994;106:119–125. doi: 10.1104/pp.106.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N, Ishida N, Hoshino M, Yamauchi M, Hara T, Ayusawa D, Kawakita M. Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J Biochem (Tokyo) 1996;120:236–241. doi: 10.1093/oxfordjournals.jbchem.a021404. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Norambuena L, Orellana A. Evidence for a UDP-glucose transporter in Golgi apparatus-derived vesicles from pea and its possible role in polysaccharide biosynthesis. Plant Physiol. 1996;112:1585–1594. doi: 10.1104/pp.112.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana A, Neckelmann G, Norambuena L. Topography and function of Golgi uridine-5′-diphosphatase from pea stems. Plant Physiol. 1997;114:99–107. doi: 10.1104/pp.114.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM, Shininger TL, Ray MM. Isolation of β-glucan synthase particles from plant cells and identification with Golgi membranes. Proc Natl Acad Sci USA. 1969;64:605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma L, Pinhal M, Dietrich C, Nader H, Hirschberg C. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J Biol Chem. 1996;271:3897–3901. doi: 10.1074/jbc.271.7.3897. [DOI] [PubMed] [Google Scholar]
- Waldman BC, Rudnick G. UDP-GlcNAc transport across the Golgi membrane: electroneutral exchange for dianionic UMP. Biochemistry. 1990;29:44–52. doi: 10.1021/bi00453a006. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA. Functional compartmentation of the Golgi apparatus of plant cells. Plant Physiol. 1992;99:1070–1083. doi: 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]